Invasive Weed Asystasia gangetica as a Potential Biomonitor and a Phytoremediator of Potentially Toxic Metals: A Case Study in Peninsular Malaysia
Abstract
:1. Introduction
- (a)
- (b)
- It has high abundance. It produces seeds in large quantities [7]. This causes the weed to be utilised as a cover crop in oil palm plantations [8,9]. It is an evergreen herb that forms mat-like structures that smother more desirable ground plants, hence affecting the biodiversity and agricultural aspects of a particular environment [6].
- (c)
- It is easy to grow and at a fast growth rate [10]. Under experimental polybags in greenhouse conditions, Kumalasari et al. [11] reported that the dry matter yields of both the leaf and stem of A. gangetica increased (p < 0.001) progressively with age and reached 11.6 g leaf dry matter, 19.0 g stem dry matter/plant, and 30.6 g total dry matter/plant at 90 days after transplanting. Even though the biomass is not substantial when compared with other plants, the fast growth rate of the weeds can still justify A. gangetica as a phytoremediator of PTMs.
- (d)
- It is adaptable to different environmental conditions [12], being high shade tolerant [10], it can even grow well under 90% shade [7]. It thrives best in full light and open areas [3,13]. It has high resistance towards metals stress and toxic effects, able to translocate metals from root to shoot, highly resistant towards pathogens and pests, easy adaptability to the climatic conditions of the growth area, and is not part of the food chain as it is not edible by nature [14,15,16,17].
- (e)
- (f)
- (g)
2. Materials and Methods
2.1. Sampling Site Descriptions and Soil Collection
2.2. Metal Analysis
2.2.1. Direct Aqua-Regia Method
2.2.2. Sequential Extraction Technique for Metals
2.3. Quality Control for Metal Analysis
2.4. Data Interpretation
2.4.1. Ecological Risk Index (ERI)
2.4.2. Calculation of Translocation Factor and Bioconcentration Factor
2.5. Data Analysis
3. Results
3.1. Potentially Toxic Metals in Asystasia gangetica
3.2. Potentially Toxic Metals in Habitat Topsoils
3.2.1. Total Metal Concentrations and EFLE
3.2.2. Ecological Risk Index (ERI)
3.3. Correlations of Potentially Toxic Metals between Topsoil and Plants Parts (Leaves, Stems, and Roots)
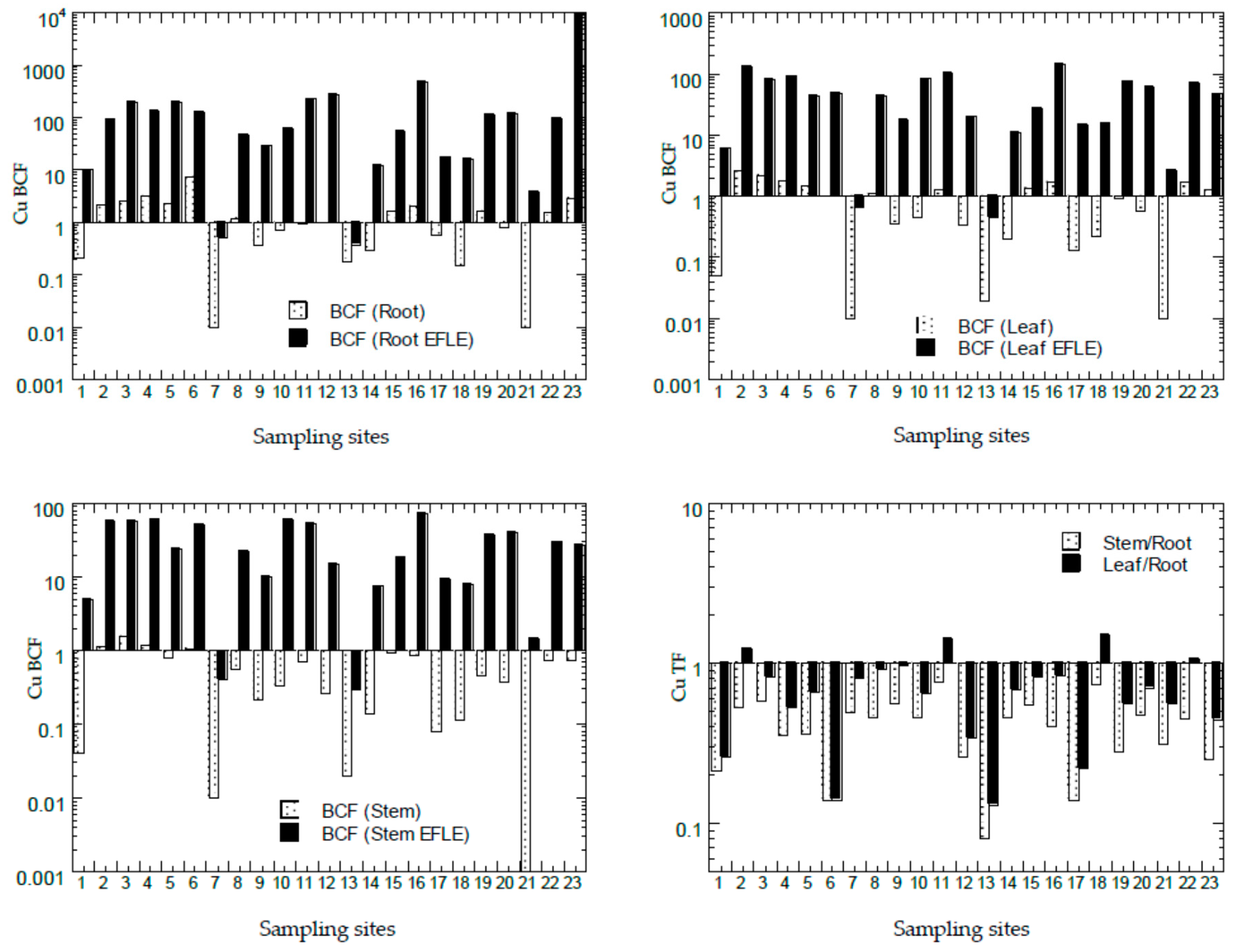
3.4. Bioconcentration Factors and Translocation Factors of Potentially Toxic Metals in Asystasia gangetica
4. Discussion
4.1. Biomonitoring of Potentially Toxic Metals
4.2. Asystasia as Phytoextractor of Cd and Ni
4.3. Asystasia as Phytostabiliser of Cu, Pb, and Zn
4.4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing Environmental Contamination through Phytoremediation by Invasive Plants: A Review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of South East Asia; Pelanduk Publications: Subang Jaya, Malaysia, 2000; ISBN 978-967-978-725-2. [Google Scholar]
- Kiew, R.; Vollesen, K. Asystasia (Acanthaceae) in Malaysia. Kew Bull. 1997, 52, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.-W.; Chiang, T.-Y.; Peng, J.-J. Asystasia Gangetica (L.) T. Anderson Subsp. Micrantha (Nees) Ensermu (Acanthaceae), A Newly Naturalized Plant in Taiwan. Taiwania 2005, 50, 117–122. [Google Scholar] [CrossRef]
- Skinner, J. The Invasive Weed Chinese Violet (Asystasia Gangetica Subspecies Micrantha) Now Threatens Northern Australia. Plant Prot. Q. 2015, 30, 126–132. [Google Scholar]
- Chandler, G.; Westaway, J.; Alford, L.; Schmid, M. Asystasia Gangetica Subsp. Micrantha, a New Record of an Exotic Plant in the Northern Territory. North. Territ. Nat. 2016, 27, 29–35. [Google Scholar]
- Adetula, O.A. Asystasia Gangetica (L.) T.Anderson. Available online: https://www.prota4u.org/database/protav8.asp?g=pe&p=Asystasia+gangetica+(L.)+T.Anderson (accessed on 9 February 2021).
- Asbur, Y.; Yahya, S.; Murtilaksono, K.; Sudradjat, R.; Sutarta, E.S. The Roles of Asystasia Gangetica (L.) T. Anderson and Ridge Terrace in Reducing Soil Erosion and Nutrient Losses in Oil Palm Plantation in South Lampung, Indonesia. J. Trop. Crop Sci. 2016, 3, 49–55. [Google Scholar] [CrossRef]
- Ariyanti, M.; Mubarok, S.; Asbur, Y. Study of Asystasia Gangetica (L.) T. Anderson as Cover Crop Against Soil Water Content in Mature Oil Palm Plantation. J. Agron. 2017, 16, 154–159. [Google Scholar] [CrossRef]
- Asbur, Y.; Yahya, S.; Murtilaksono, K.; Sudradjat; Sutarta, E. Study of Asystasia Gangetica (L.) Anderson Utilization as Cover Crop under Mature Oil Palm with Different Ages. Int. J. Sci. Basic Appl. Res. IJSBAR 2015, 19, 137–148. [Google Scholar]
- Kumalasari, N.R.; Abdullah, L.; Khotijah, L.; Wahyuni, L.; Indriyani, I.; Ilman, N.; Janato, F. Evaluation of Asystasia Gangetica as a Potential Forage in Terms of Growth, Yield and Nutrient Concentration at Different Harvest Ages. Trop. Grassl.-Forrajes Trop. 2020, 8, 153–157. [Google Scholar] [CrossRef]
- Sandoval, J.R.; Rodriguez, P.A. Asystasia Gangetica (Chinese Violet); Department of Botany-Smithsonian National Museum of Natural History: Washington, DC, USA, 2012. [Google Scholar]
- Samedani, B.; Juraimi, A.S.; Anwar, M.P.; Rafii, M.Y.; Sheikh Awadz, S.H.; Anuar, A.R. Competitive Interaction of Axonopus Compressus and Asystasia Gangetica under Contrasting Sunlight Intensity. Sci. World J. 2013, 2013, 308646. [Google Scholar] [CrossRef] [Green Version]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Chromium Bioaccumulation, Oxidative Stress Metabolism and Oil Content in Lemon Grass Cymbopogon Flexuosus (Nees Ex Steud.) W. Watson Grown in Chromium Rich over Burden Soil of Sukinda Chromite Mine, India. Chemosphere 2019, 218, 1082–1088. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.P.; Kneer, R.; Zhu, Y.G. Vacuolar Compartmentalization: A Second-Generation Approach to Engineering Plants for Phytoremediation. Trends Plant Sci. 2004, 9, 7–9. [Google Scholar] [CrossRef]
- Shabani, N.; Sayadi, M.H. Evaluation of Heavy Metals Accumulation by Two Emergent Macrophytes from the Polluted Soil: An Experimental Study. Environmentalist 2012, 32, 91–98. [Google Scholar] [CrossRef]
- Aksoy, A.; Şahin, U. Elaeagnus Angustifolia L. as a Biomonitor of Heavy Metal Pollution. Turk. J. Bot. 1999, 23, 83–88. [Google Scholar]
- Chakrabortty, S.; Paratkar, G.T. Biomonitoring of Trace Element Air Pollution Using Mosses. Aerosol Air Qual. Res. 2006, 6, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Asbur, Y.; Yahya, S.; Murtilaksono, K.; Sudradjat, S.E.S. Peran tanaman penutup tanah terhadap neraca hara di perkebunan kelapa sawit menghasilkan di Lampung Selatan. J. Penelit. Kelapa Sawit 2015, 23, 53–60. [Google Scholar]
- Asbur, Y.; Yahya, S.; Purwaningrum, Y.; Ariyanti, M. The Potentials of Asystasia Gangetica (L.) T. Anderson as Cover Crop under Mature Oil Palm Plantation. In Proceedings of the Multidisciplinary Knowledge for a Better Life; University Science of Malaysia (USM), Universitas Syiah Kuala (Unsyiah), and Universitas Islam Sumatera Utara (UISU): Medan, Indonesia, 2014; pp. 124–128.
- Chew, W.; Yap, C.K.; Ismail, A.; Zakaria, M.; Tan, S.G. Mercury Distribution in an Invasive Species (Asystasia Gangetica) from Peninsular Malaysia. Sains Malays. 2012, 41, 395–401. [Google Scholar]
- Yap, C.K.; Chew, W. The Invasive Weed, Asystasia gangetica as a Biomonitor of Heavy Metal Bioavailability and Pollution. In From Sources to Solution, Proceedings of the International Conference on Environmental Forensics 2013; Aris, A.Z., Tengku Ismail, T.H., Harun, R., Abdullah, A.M., Ishak, M.Y., Eds.; Springer: Singapore, 2014; pp. 519–523. [Google Scholar]
- Wittig, R. General aspects of biomonitoring heavy metals by plants. In Plants as Biomonitors; Markert, B., Ed.; VCH Publisher: Weinheim, Germany, 1993; pp. 3–28. ISBN 3-527-30001-5. [Google Scholar]
- Zhuang, P.; Ye, Z.H.; Lan, C.Y.; Xie, Z.W.; Shu, W.S. Chemically Assisted Phytoextraction of Heavy Metal Contaminated Soils Using Three Plant Species. Plant Soil 2005, 276, 153–162. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Florida Site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally Sustainable Way for Reclamation of Heavy Metal Polluted Soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Heavy Metals Accumulation in Plants Growing in Ex Tin Mining Catchment. Int. J. Environ. Sci. Technol. 2011, 8, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization Potential of Jatropha Curcas L. in Polymetallic Acid Mine Tailings. Int. J. Phytoremediation 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Ladislas, S.; El-Mufleh, A.; Gérente, C.; Chazarenc, F.; Andrès, Y.; Béchet, B. Potential of Aquatic Macrophytes as Bioindicators of Heavy Metal Pollution in Urban Stormwater Runoff. Water. Air. Soil Pollut. 2012, 223, 877–888. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-Accumulation Metals in Plants. Water. Air. Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Aksoy, A.; Demirezen, D. Fraxinus Excelsior as a Biomonitor of Heavy Metal Pollution. Pol. J. Environ. Stud. 2006, 15, 27–33. [Google Scholar]
- Akgüç, H.; Özy, L.; Yarci, C. Pyracantha Coccinea Roem. (Rosaceae) as a Biomonitor for Cd, Pb, and Zn in Mugla Province (Turkey). Pak. J. Bot. 2008, 40, 1767–1776. [Google Scholar]
- De Nicola, F.; Maisto, G.; Prati, M.V.; Alfani, A. Leaf Accumulation of Trace Elements and Polycyclic Aromatic Hydrocarbons (PAHs) in Quercus Ilex L. Environ. Pollut. 2008, 153, 376–383. [Google Scholar] [CrossRef]
- Khan, S.; Aijun, L.; Zhang, S.; Hu, Q.; Zhu, Y.-G. Accumulation of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Lettuce Grown in the Soils Contaminated with Long-Term Wastewater Irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef]
- Hoodaji, M.; Tahmourespour, A.; Amini, H. Assessment of Copper, Cobalt and Zinc Contaminations in Soils and Plants of Industrial Area in Esfahan City (in Iran). Environ. Earth Sci. 2010, 61, 1353–1360. [Google Scholar] [CrossRef]
- Divan, A.M.; de Oliveira, P.L.; Perry, C.T.; Atz, V.L.; Azzarini-Rostirola, L.N.; Raya-Rodriguez, M.T. Using Wild Plant Species as Indicators for the Accumulation of Emissions from a Thermal Power Plant, Candiota, South Brazil. Ecol. Indic. 2009, 9, 1156–1162. [Google Scholar] [CrossRef]
- Yildirim, D.; Sasmaz, A. Phytoremediation of As, Ag, and Pb in Contaminated Soils Using Terrestrial Plants Grown on Gumuskoy Mining Area (Kutahya Turkey). J. Geochem. Explor. 2017, 182, 228–234. [Google Scholar] [CrossRef]
- de Paula, P.H.M.; Mateus, V.L.; Araripe, D.R.; Duyck, C.B.; Saint’Pierre, T.D.; Gioda, A. Biomonitoring of Metals for Air Pollution Assessment Using a Hemiepiphyte Herb (Struthanthus Flexicaulis). Chemosphere 2015, 138, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.V.; Alagić, S.Č.; Milić, S.M.; Tošić, S.B.; Bugarin, M.M. Chemometric Characterization of Heavy Metals in Soils and Shoots of the Two Pioneer Species Sampled near the Polluted Water Bodies in the Close Vicinity of the Copper Mining and Metallurgical Complex in Bor (Serbia): Phytoextraction and Biomonitoring Contexts. Chemosphere 2021, 262, 127808. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Pang, B.H. Assessment of Cu, Pb, and Zn Contamination in Sediment of North Western Peninsular Malaysia by Using Sediment Quality Values and Different Geochemical Indices. Environ. Monit. Assess. 2011, 183, 23–39. [Google Scholar] [CrossRef]
- Yap, C.K.; Pang, B.H. Anthropogenic Concentrations of Cd, Ni and Zn in the Intertidal, River and Drainage Sediments Collected from North Western Peninsular Malaysia. Pertanika J. Sci. Technol. 2011, 19, 93–107. [Google Scholar]
- Halmi, M.I.E.; Gunasekaran, B.; Othman, A.R.; Kamaruddin, K.; Dahalan, F.A.; Ibrahim, N.; Shukor, M.Y. A Rapid Inhibitive Enzyme Assay for Monitoring Heavy Metals Pollution in the Juru Industrial Estate. Bioremediation Sci. Technol. Res. 2015, 3, 7–12. [Google Scholar]
- Yap, C.K.; Tan, S. Heavy Metal Pollution in the Juru River Basin Receiving Industrial Effluents: The Need for Biochemical and Molecular Studies in the Edible Cockles Anadara Granosa. Malays. Appl. Biol 2008, 37, 63–68. [Google Scholar]
- Yap, C.K.; Hatta, Y.; Edward, F.; Tan, S. Comparison of Heavy Metal Concentrations (Cd, Cu, Fe, Ni and Zn) in the Shells and Different Soft Tissues of Anadara Granosa Collected from Jeram, Kuala Juru and Kuala Kurau, Peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 2008, 31, 205–215. [Google Scholar]
- Yap, C.K.; Noorhaidah, A.; Azlan, A.; Nor Azwady, A.A.; Ismail, A.; Ismail, A.R.; Siraj, S.S.; Tan, S.G. Telescopium Telescopium as Potential Biomonitors of Cu, Zn, and Pb for the Tropical Intertidal Area. Ecotoxicol. Environ. Saf. 2009, 72, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Alshaebi, F.; Yaacob, W.Z.; Samsudin, A.; Alsabahi, E. Risk Assessment at Abandoned Tin Mine in Sungai Lembing, Pahang, Malaysia. Electron. J. Geotech. Eng. 2009, 14, 1–9. [Google Scholar]
- Mohd Zin, N.S.; Abdul Aziz, H.; Adlan, M.N.; Ariffin, A. Characterization of Leachate at Matang Landfill Site, Perak, Malaysia. Acad. J. Sci. 2012, 1, 317–322. [Google Scholar]
- Rashid, R.I.; Ibrahim, M.Z.; Abdullah, M.A.; Ishak, A.R. Characterization and Toxicity Study of Leachate from Closed Landfills in Selangor. Asia Pac. Environ. Occup. Health J. 2018, 4, 16–20. [Google Scholar]
- Kalantarifard, A.; Yang, G.S. Energy Potential from Municipal Solid Waste in Tanjung Langsat Landfill, Johor, Malaysia. Int. J. Eng. Sci. Technol. 2011, 3, 8560–8568. [Google Scholar]
- Yap, C.K.; Ismail, A.; Tan, S.G.; Omar, H. Correlations between Speciation of Cd, Cu, Pb and Zn in Sediment and Their Concentrations in Total Soft Tissue of Green-Lipped Mussel Perna Viridis from the West Coast of Peninsular Malaysia. Environ. Int. 2002, 28, 117–126. [Google Scholar] [CrossRef]
- Badri, M.A.; Aston, S.R. Observations on Heavy Metal Geochemical Associations in Polluted and Non-Polluted Estuarine Sediments. Environ. Pollut. Ser. B Chem. Phys. 1983, 6, 181–193. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The Composition of Earth’s Upper Crust, Natural Cycles of Elements, Natural Resources. In Elements and Their Compounds in the Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 2–16. ISBN 978-3-527-61963-4. [Google Scholar]
- Al-Farraj, A.S.; Al-Wabel, M.I.; Al-Shahrani, T.S.; El-Maghraby, S.E.; Al-Sewailem, M.A.S. Accumulation Coefficient and Translocation Factor of Heavy Metals through Rhazya Stricta Grown in the Mining Area of Mahad AD’Dahab, Saudi Arabia. In Proceedings of the Waste Management and the Environment, V; WIT Press: Tallinn, Estonia, 2010; pp. 325–336. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J. Multivariate Data Analysis: A Global Perspective; Pearson Education: Cranbury, New Jersey, USA, 2010; ISBN 978-0-13-515309-3. [Google Scholar]
- Byrne, B.M. Structural Equation Modeling With AMOS: Basic Concepts, Applications, and Programming, 2nd ed; Routledge: New York, NY, USA, 2010; ISBN 978-0-203-80553-4. [Google Scholar]
- Garson, G.D. Partial Least Squares: Regression and Path Modelling; Statistical Publishing Associates: Asheboro, NC, USA, 2012. [Google Scholar]
- Lum, A.F.; Ngwa, E.S.A.; Chikoye, D.; Suh, C.E. Phytoremediation Potential of Weeds in Heavy Metal Contaminated Soils of the Bassa Industrial Zone of Douala, Cameroon. Int. J. Phytoremediation 2014, 16, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Subha, M.; Srinivas, N. Phytoremediation Potential of Weedy Plants in Heavy Metal Contaminated Benthic Lake Sludge. Int. J. Appl. Eng. Res. 2017, 12, 4534–4538. [Google Scholar]
- Baker, A.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements, A Review of Their Distribution. Ecol. Phytochem Biorecovery 1989, 1, 81–126. [Google Scholar]
- Yap, C.K.; Omar, H.; Nulit, R.; Ong, G.H.; Bakhtiari, A.R.; Karami, A.; Al-Shami, S.A. Relationships of Zn Between Centella Asiatica and Geochemical Fractions of the Habitat Topsoils: Implications of Biomonitoring of Zn. Open Biol. Sci. J. 2017, 3, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, F.J.; Pechmann, I.; Bogden, J.D.; Grabosky, J.; Weis, P. Soil Metal Concentrations and Vegetative Assemblage Structure in an Urban Brownfield. Environ. Pollut. Barking Essex 1987 2008, 153, 351–361. [Google Scholar] [CrossRef]
- Cheng, S. Heavy Metal Pollution in China: Origin, Pattern and Control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I.; Kumar, P.N.; Dushenkov, S.; Salt, D.E. Bioconcentration of Heavy Metals by Plants. Curr. Opin. Biotechnol. 1994, 5, 285–290. [Google Scholar] [CrossRef]
- Vollenweider, P.; Bernasconi, P.; Gautschi, H.-P.; Menard, T.; Frey, B.; Günthardt-Goerg, M.S. Compartmentation of Metals in Foliage of Populus Tremula Grown on Soils with Mixed Contamination. II. Zinc Binding inside Leaf Cell Organelles. Environ. Pollut. 2011, 159, 337–347. [Google Scholar] [CrossRef]
- Wei, H.; Huang, M.; Quan, G.; Zhang, J.; Liu, Z.; Ma, R. Turn Bane into a Boon: Application of Invasive Plant Species to Remedy Soil Cadmium Contamination. Chemosphere 2018, 210, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.C.; Morais, I.; Campos, J.; Pratas, J. Nickel phytoextraction by a native population of Alyssum serpyllifolium subsp. lusitanicum on ultramafic soils (Portugal): Prospects for phytomining. Comun. Geol. 2020, 107, 115–117. [Google Scholar]
- Yu, G.; Jiang, P.; Fu, X.; Liu, J.; Sunahara, G.I.; Chen, Z.; Xiao, H.; Lin, F.; Wang, X. Phytoextraction of Cadmium-Contaminated Soil by Celosia Argentea Linn.: A Long-Term Field Study. Environ. Pollut. 2020, 266, 115408. [Google Scholar] [CrossRef] [PubMed]
- Atagana, H.I. Bioremediation of Co-Contamination of Crude Oil and Heavy Metals in Soil by Phytoremediation Using Chromolaena Odorata (L) King & H.E. Robinson. Water. Air. Soil Pollut. 2011, 215, 261–271. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive Species Based Efficient Green Technology for Phytoremediation of Fly Ash Deposits. J. Geochem. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Chinmayee, M.D.; Mahesh, B.; Pradesh, S.; Mini, I.; Swapna, T.S. The Assessment of Phytoremediation Potential of Invasive Weed Amaranthus Spinosus L. Appl. Biochem. Biotechnol. 2012, 167, 1550–1559. [Google Scholar] [CrossRef]
- Alshaal, T.; domokos-szabolcsy, E.; Marton, L.; Czako, M.; Katai, J.; Balogh, P.; Elhawat, N.; El-Ramady, H.; Fári, M. Phytoremediation of Bauxite-Derived Red Mud by Giant Reed. Environ. Chem. Lett. 2013, 11, 295–302. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N.; Singh, R.P.; Singh, D.P. Rhizoremediation Potential of Spontaneously Grown Typha Latifolia on Fly Ash Basins: Study from the Field. Ecol. Eng. 2014, 71, 722–727. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L. Comparative Analysis of Element Concentrations and Translocation in Three Wetland Congener Plants: Typha Domingensis, Typha Latifolia and Typha Angustifolia. Ecotoxicol. Environ. Saf. 2017, 143, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Z.; Zou, C.B.; Dai, Z.; Du, D.; Yan, C. The Mutual Restraint Effect between the Expansion of Alternanthera Philoxeroides (Mart.) Griseb and Cadmium Mobility in Aquatic Environment. Ecotoxicol. Environ. Saf. 2018, 148, 237–243. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Abbas, Q.; Ali, M.U.; Wang, R.; Ahmed, R.; Wang, C.; Al-Wabel, M.I.; Usman, A.R.A. Operational Control on Environmental Safety of Potentially Toxic Elements during Thermal Conversion of Metal-Accumulator Invasive Ragweed to Biochar. J. Clean. Prod. 2018, 195, 458–469. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Song, B.; Zheng, G.; Zhang, Z.; Zhao, J.; Okoli, C.P. Heavy Metal Contents and Enrichment Characteristics of Dominant Plants in Wasteland of the Downstream of a Lead-Zinc Mining Area in Guangxi, Southwest China. Ecotoxicol. Environ. Saf. 2018, 151, 266–271. [Google Scholar] [CrossRef]
- Banerjee, R.; Jana, A.; De, A.; Mukherjee, A. Phytoextraction of Heavy Metals from Coal Fly Ash for Restoration of Fly Ash Dumpsites. Bioremediation J. 2020, 24, 41–49. [Google Scholar] [CrossRef]
- Benavides, B.J.; Drohan, P.J.; Spargo, J.T.; Maximova, S.N.; Guiltinan, M.J.; Miller, D.A. Cadmium Phytoextraction by Helianthus Annuus (Sunflower), Brassica Napus Cv Wichita (Rapeseed), and Chyrsopogon Zizanioides (Vetiver). Chemosphere 2021, 265, 129086. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Najeeb, U.; Li, X.; Pan, J.; Huang, Q.; Zhou, W.; Liang, Z. Synergistic Effects of EDDS and ALA on Phytoextraction of Cadmium as Revealed by Biochemical and Ultrastructural Changes in Sunflower (Helianthus Annuus L.) Tissues. J. Hazard. Mater. 2021, 407, 124764. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Toxic Metal Decontamination by Phytoremediation Approach: Concept, Challenges, Opportunities and Future Perspectives. Environ. Technol. Innov. 2020, 18, 100672. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace Elements in Agroecosystems and Impacts on the Environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Barceló, J.; Poschenrieder, C. Phytoremediation: Principles and Perspectives. Contrib. Sci. 2003, 2, 333–344. [Google Scholar]
- Patra, D.K.; Pradhan, C.; Patra, H.K. An in Situ Study of Growth of Lemongrass Cymbopogon Flexuosus (Nees Ex Steud.) W. Watson on Varying Concentration of Chromium (Cr+6) on Soil and Its Bioaccumulation: Perspectives on Phytoremediation Potential and Phytostabilisation of Chromium Toxicity. Chemosphere 2018, 193, 793–799. [Google Scholar] [CrossRef]
- Patra, D.; Pradhan, C.; Patra, H. Chromium Stress Impact on Lemongrass Grown in Over Burden Soil of Sukinda Chromite Ore Mine (Odisha), India. Ann. Plant Sci. 2018, 7, 2394. [Google Scholar] [CrossRef]
- Robinson, B.H.; Leblanc, M.; Petit, D.; Brooks, R.R.; Kirkman, J.H.; Gregg, P.E.H. The Potential of Thlaspi Caerulescens for Phytoremediation of Contaminated Soils. Plant Soil 1998, 203, 47–56. [Google Scholar] [CrossRef]
- Ali, H.; Naseer, M.; Sajad, M.A. Phytoremediation of Heavy Metals by Trifolium Alexandrinum. Int. J. Environ. Sci. 2012, 2, 1459–1469. [Google Scholar]
- Malik, R.; Husain, S.; Nazir, I. Heavy Metal Contamination and Accumulation in Soil and Wild Plant Species from Industrial Area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Drozdova, I.; Alekseeva-Popova, N.; Dorofeyev, V.; Bech, J.; Belyaeva, A.; Roca, N. A Comparative Study of the Accumulation of Trace Elements in Brassicaceae Plant Species with Phytoremediation Potential. Appl. Geochem. 2019, 108, 104377. [Google Scholar] [CrossRef]
- Mataruga, Z.; Jarić, S.; Kostić, O.; Marković, M.; Jakovljević, K.; Mitrović, M.; Pavlović, P. The Potential of Elm Trees (Ulmus Glabra Huds.) for the Phytostabilisation of Potentially Toxic Elements in the Riparian Zone of the Sava River. Environ. Sci. Pollut. Res. 2020, 27, 4309–4324. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytostabilisation–An Appropriate Remediation Technique for Metals in Soils along Highways. In Construction for a Sustainable Environment; CRC Press: Boca Raton, FL, USA, 2009; pp. 265–270. [Google Scholar]
- Moreno-Jiménez, E.; Esteban, E.; Carpena-Ruiz, R.O.; Lobo, M.C.; Peñalosa, J.M. Phytostabilisation with Mediterranean Shrubs and Liming Improved Soil Quality in a Pot Experiment with a Pyrite Mine Soil. J. Hazard. Mater. 2012, 201–202, 52–59. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Rhizosphere Influence and Seasonal Impact on Phytostabilisation of Metals—A Field Study. Water Air Soil Pollut. 2012, 223, 107–124. [Google Scholar] [CrossRef]
- Boisson, S.; Stradic, S.L.; Collignon, J.; Séleck, M.; Malaisse, F.; Shutcha, M.N.; Faucon, M.-P.; Mahy, G. Potential of Copper-Tolerant Grasses to Implement Phytostabilisation Strategies on Polluted Soils in South D. R. Congo. Environ. Sci. Pollut. Res. 2016, 23, 13693–13705. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Peres, S.; Magalhães, M.C.F.; Leitão, S.; Pereira, A.S.; Cerejeira, M.J. Potential of Tamarix Africana and Other Halophyte Species for Phytostabilisation of Contaminated Salt Marsh Soils. J. Soils Sediments 2017, 17, 1459–1473. [Google Scholar] [CrossRef]
- Arco-Lázaro, E.; Martínez-Fernández, D.; Bernal, M.P.; Clemente, R. Response of Piptatherum Miliaceum to Co-Culture with a Legume Species for the Phytostabilisation of Trace Elements Contaminated Soils. J. Soils Sediments 2017, 17, 1349–1357. [Google Scholar] [CrossRef]
- Boisson, S.; Séleck, M.; Le Stradic, S.; Collignon, J.; Garin, O.; Malaisse, F.; Shutcha, M.N.; Mahy, G. Using Phytostabilisation to Conserve Threatened Endemic Species in Southeastern Democratic Republic of the Congo. Ecol. Res. 2018, 33, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.P.; Gómez, X.; Chang, R.; Arco-Lázaro, E.; Clemente, R. Strategies for the Use of Plant Biomass Obtained in the Phytostabilisation of Trace-Element-Contaminated Soils. Biomass Bioenergy 2019, 126, 220–230. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Li, X.; Xu, Y.; Sun, X.; Liang, Q. Formation of Iron Plaque in the Roots of Spartina Alterniflora and Its Effect on the Immobilization of Wastewater-Borne Pollutants. Ecotoxicol. Environ. Saf. 2019, 168, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Varun, M.; Jaggi, D.; D’Souza, R.; Paul, M.S.; Kumar, B. Abutilon Indicum L.: A Prospective Weed for Phytoremediation. Environ. Monit. Assess. 2015, 187, 527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, S. Status of Phytoremediation in World Scenario. Int. J. Environ. Bioremediation Biodegrad. 2014, 2, 178–191. [Google Scholar] [CrossRef]
- Erakhrumen, A.A. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation in Developing Countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Sousa, A.I.; Caçador, I.; Lillebø, A.I.; Pardal, M.A. Heavy Metal Accumulation in Halimione Portulacoides: Intra- and Extra-Cellular Metal Binding Sites. Chemosphere 2008, 70, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, L.-L.; Li, J.; Chen, M.; An, R.-D. Comparative Study on the Bioaccumulation of Lead, Cadmium and Nickel and Their Toxic Effects on the Growth and Enzyme Defence Strategies of a Heavy Metal Accumulator, Hydrilla Verticillata (L.f.) Royle. Environ. Sci. Pollut. Res. 2020, 27, 9853–9865. [Google Scholar] [CrossRef]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of Mineral Nutrition in Minimizing Cadmium Accumulation by Plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Lorestani, B.; Yousefi, N.; Cheraghi, M.; Farmany, A. Phytoextraction and Phytostabilization Potential of Plants Grown in the Vicinity of Heavy Metal-Contaminated Soils: A Case Study at an Industrial Town Site. Environ. Monit. Assess. 2013, 185, 10217–10223. [Google Scholar] [CrossRef]
- Chang Kee, J.; Gonzales, M.J.; Ponce, O.; Ramírez, L.; León, V.; Torres, A.; Corpus, M.; Loayza-Muro, R. Accumulation of Heavy Metals in Native Andean Plants: Potential Tools for Soil Phytoremediation in Ancash (Peru). Environ. Sci. Pollut. Res. Int. 2018, 25, 33957–33966. [Google Scholar] [CrossRef] [PubMed]
- Uka, U.N.; Chukwuka, K.S.; Afoke, C. Heavy Metal Accumulation by Telfairia Occidentalis Hook, F Grown on Waste Dumpsites in South-Eastern Nigeria. Res. J. Environ. Toxicol. 2013, 7, 47–53. [Google Scholar] [CrossRef] [Green Version]










| No. | Sampling Site | Date/Month/Year | Characteristics | N | PH (cm) | Leaf (WC; %) | Stem (WC; %) | Root (WC; %) |
|---|---|---|---|---|---|---|---|---|
| S1 | Kg. Bkt. Chandang | 8/6/2011 | Residential | 15 | 51.1 | 84.3 | 78.2 | 70.3 |
| S2 | Kg. Bkt. Rasa | 21/6/2011 | Residential | 14 | 93.0 | 84.7 | 83.5 | 69.9 |
| S3 | Ijok | 21/6/2011 | Residential | 16 | 59.1 | 73.0 | 74.8 | 28.9 |
| S4 | Kg. Ayer Hitam | 26/6/2011 | Plantation | 15 | 65.3 | 77.1 | 85.6 | 74.4 |
| S5 | Matang | 27/6/2011 | Landfill | 15 | 122.0 | 81.9 | 82.5 | 73.8 |
| S6 | Sepang | 2/7/2011 | Landfill | 14 | 44.4 | 78.3 | 84.7 | 74.9 |
| S7 | Sg. Kembung | 2/7/2011 | Landfill | 7 | 90.7 | 80.5 | 84.1 | 69.7 |
| S8 | Tanjung Piai | 9/7/2011 | Residential | 11 | 97.7 | 83.6 | 87.5 | 80.7 |
| S9 | Tanjung Langsat | 10/7/2011 | Landfill | 10 | 83.0 | 85.2 | 81.7 | 87.9 |
| S10 | Perah, Kuala Lipis | 15/7/2011 | Plantation | 13 | 65.0 | 84.3 | 83.1 | 75.0 |
| S11 | Kuala Krai | 15/7/2011 | Rubbish heap | 13 | 61.2 | 84.5 | 84.6 | 79.0 |
| S12 | Kota Bharu | 16/7/2011 | Residential | 11 | 63.6 | 88.6 | 88.5 | 84.0 |
| S13 | Sg. Lembing | 22/7/2011 | Abandoned mining | 13 | 44.8 | 74.5 | 76.2 | 64.8 |
| S14 | Kuantan | 22/7/2011 | Residential | 9 | 96.1 | 83.2 | 84.5 | 75.5 |
| S15 | Chukai/Kemaman | 23/7/2011 | Residential | 13 | 31.4 | 83.0 | 79.2 | 74.2 |
| S16 | Cheneh | 23/7/2011 | Residential | 12 | 133.0 | 85.5 | 79.8 | 75.1 |
| S17 | Nibong Tebal | 2/8/2011 | Rubbish heap | 10 | 67.5 | 85.3 | 84.2 | 83.3 |
| S18 | Juru | 2/8/2011 | Industrial | 11 | 54.5 | 82.7 | 84.2 | 77.2 |
| S19 | Alor Setar | 3/8/2011 | Plantation | 15 | 47.7 | 84.7 | 81.7 | 73.7 |
| S20 | Pendang | 3/8/2011 | Plantation | 7 | 38.6 | 84.4 | 89.6 | 73.2 |
| S21 | Kuala Terengganu | 16/11/2011 | Rubbish heap | 12 | 83.8 | 89.1 | 86.9 | 86.1 |
| S22 | Tg. Gemok | 17/11/2011 | Plantation | 10 | 107.5 | 84.4 | 83.2 | 66.3 |
| S23 | Pagoh | 17/1/2012 | Residential | 12 | 107.1 | 80.9 | 76.7 | 68.9 |
| CRM | Cd | Cu | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|---|
| NSC DC73319 Soil China | 110.7% | 85.0% | NA | NA | 99.8% | 99.7% |
| MESS-3 NRC | NA | 93.1% | NA | 102.0% | 115.6% | 82.8% |
| TH-1 sediment Canada | 102.4% | 92.9% | 95.6% | 112.3% | 100.0% | 110.2% |
| SRM 1547 | NA | NA | 105.6% | NA | NA | 114.9% |
| IAEA soil-5 | 156.3% | 91.3% | NA | 103.0% | 115.7% | 94.8% |
| Plant | Topsoils | TF-1 | TF-2 | BCF-1root | BCF-2root | BCF-1leaf | BCF-2leaf | BCF-1stem | BCF-2stem | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal | Root | Stem | Leaf | EFLE | AR | ERI | Stem/Root | Leaf/Root | Root/AR | Root/EFLE | Leaf/AR | Leaf/EFLE | Stem/AR | Stem/EFLE | |
| Cd | Min | 0.03 | 0.03 | 0.03 | 0.01 | 0.23 | 71.2 | 0.03 | 0.01 | 0.01 | 0.06 | 0.00 | 0.06 | 0.02 | 0.15 |
| Max | 2.18 | 1.25 | 1.16 | 0.51 | 12.4 | 3729 | 27.8 | 35.4 | 2.00 | 258 | 2.66 | 48.8 | 2.89 | 105 | |
| Mean | 0.40 | 0.55 | 0.29 | 0.16 | 1.94 | 583 | 9.91 | 5.30 | 0.35 | 16.4 | 0.34 | 4.67 | 0.68 | 12.4 | |
| SE | 0.13 | 0.07 | 0.08 | 0.03 | 0.58 | 173 | 2.03 | 1.96 | 0.12 | 11.1 | 0.13 | 2.17 | 0.14 | 5.27 | |
| Cu | Min | 9.22 | 5.57 | 7.94 | 0.11 | 4.66 | 0.93 | 0.08 | 0.13 | 0.01 | 0.37 | 0.01 | 0.46 | 0.00 | 0.29 |
| Max | 139 | 11.8 | 20.2 | 40.1 | 2363 | 473 | 0.75 | 1.50 | 7.52 | 9254 | 2.61 | 148 | 1.52 | 72.0 | |
| Mean | 27.0 | 7.71 | 12.9 | 3.41 | 242 | 43.3 | 0.40 | 0.70 | 1.49 | 504 | 0.90 | 50.0 | 0.53 | 29.2 | |
| SE | 5.68 | 0.30 | 0.71 | 1.99 | 128 | 23.1 | 0.04 | 0.08 | 0.34 | 398 | 0.16 | 8.88 | 0.09 | 4.85 | |
| Ni | Min | 0.63 | 0.23 | 0.03 | 0.02 | 2.38 | 0.21 | 0.14 | 0.01 | 0.02 | 1.27 | 0.01 | 0.05 | 0.01 | 0.52 |
| Max | 5.47 | 3.69 | 6.13 | 1.94 | 75.7 | 6.32 | 2.18 | 3.70 | 0.89 | 148 | 0.72 | 70.0 | 0.56 | 61.0 | |
| Mean | 2.14 | 1.48 | 2.18 | 0.39 | 16.1 | 1.42 | 0.83 | 1.18 | 0.25 | 23.6 | 0.20 | 9.78 | 0.14 | 10.6 | |
| SE | 0.26 | 0.18 | 0.35 | 0.09 | 3.59 | 0.31 | 0.11 | 0.21 | 0.05 | 8.70 | 0.04 | 2.97 | 0.03 | 3.25 | |
| Pb | Min | 2.43 | 0.01 | 2.10 | 0.59 | 7.22 | 2.41 | 0.00 | 0.41 | 0.01 | 0.73 | 0.01 | 2.00 | 0.00 | 0.01 |
| Max | 10.5 | 7.79 | 21.8 | 4.38 | 1004 | 323 | 1.14 | 4.02 | 0.37 | 86.1 | 1.35 | 14.6 | 0.38 | 5.10 | |
| Mean | 5.52 | 2.52 | 7.55 | 1.68 | 117 | 38.4 | 0.48 | 1.50 | 0.12 | 8.43 | 0.19 | 5.80 | 0.06 | 1.87 | |
| SE | 0.43 | 0.32 | 0.83 | 0.24 | 45.8 | 14.8 | 0.05 | 0.19 | 0.02 | 3.60 | 0.06 | 0.75 | 0.02 | 0.26 | |
| Zn | Min | 50.7 | 26.9 | 18.7 | 0.05 | 11.0 | 0.17 | 0.47 | 0.25 | 0.02 | 0.00 | 0.01 | 0.74 | 0.01 | 0.76 |
| Max | 300 | 246 | 160 | 130 | 3820 | 58.8 | 1.07 | 1.10 | 11.6 | 2931 | 3.33 | 481 | 7.99 | 674 | |
| Mean | 121 | 86.5 | 61.7 | 15.3 | 514 | 7.88 | 0.73 | 0.54 | 1.98 | 390 | 0.89 | 89.3 | 1.44 | 135 | |
| SE | 14.6 | 11.1 | 7.93 | 6.40 | 217 | 3.34 | 0.04 | 0.04 | 0.54 | 175 | 0.20 | 28.7 | 0.41 | 43.6 | |
| EFLE | AR | ||
|---|---|---|---|
| Cd | Root | 0.25 ns | 0.17 ns |
| Stem | 0.09 ns | 0.03 ns | |
| Leaf | 0.04 ns | 0.03 ns | |
| Cu | Root | 0.48 * | 0.41 ns |
| Stem | 0.54 * | 0.48 * | |
| Leaf | 0.30 ns | 0.26 ns | |
| Ni | Root | 0.03 ns | 0.02 ns |
| Stem | 0.33 ns | 0.48 * | |
| Leaf | 0.40 ns | 0.36 ns | |
| Pb | Root | 0.58 * | 0.53 * |
| Stem | 0.29 ns | 0.05 ns | |
| Leaf | 0.42 * | 0.28 ns | |
| Zn | Root | 0.63 * | 0.50 * |
| Stem | 0.56 * | 0.44 * | |
| Leaf | 0.69 * | 0.60 * |
| No. | Plants | Type | Contaminant (s) | Country | References |
|---|---|---|---|---|---|
| 1 | Chromolaena odorata | Invasive | Crude oil and Cd, Ni, Zn | South Africa | [71] |
| 2 | Ipomoea carnea | Invasive | Cd, Pb, Cu, Cr, Mn, and Ni | India | [72] * |
| 3 | Amaranthus spinosus | Invasive | Cu, Zn, Cr, Pb, and Cd | India | [73] |
| 4 | Arundo donax | Invasive | Improved pH, EC, OC, microbial counts, and soil enzyme activities and uptake Cd, Pb, Co, Ni, and Fe | Hungary | [74] ** |
| 5 | Typha latifolia | Invasive | Zn, Mn, Cu, Pb, Cd, Cr, and Ni | India | [75] |
| 6 | Typha latifolia | Invasive | Al, As, Cd, Cr, Cu, Hg, Mn, Ni, Pb, and Zn | Italy | [76] |
| 7 | Alternanthera philoxeroides | Invasive | Cd | China | [77] |
| 8 | Ambrosia artemisiifolia | Invasive | As, Cd, Cr, Cu, Mn, Ni, Pb, V, and Zn | China | [78] |
| 9 | Ageratum conyzoides, Bidens pilosa, Senecio scandens, Imperata cylindrical, Buddleja davidii | Invasive | Cd, Pb, and Zn | China | [79] |
| 10 | Chromolaena odorata, Bidens pilosa, and Praxelis clematidea | Invasive | Cd | China | [68] |
| 11 | Alyssum serpyllifolium sp. Lusitanicum | Non-invasive | Ni | Portugal | [69] |
| 12 | Celosia argentea | Non-invasive | Cd | Field experiment | [70] |
| 13 | Saccharum spontaneum and Saccharum munja | Non-invasive | Zn, Pb, Cu, Ni, Cd, and As | Pot experiments | [80] *** |
| 14 | Euphorbia helioscopia and Urtica dioica | Non-invasive | As, Cd, Pb, Cu, and Zn | Bor (Serbia) | [40] |
| 15 | Helianthus annuus, Brassica napus, and Chyrsopogon zizanioides | Non-invasive | Cd | Greenhouse pot experiments | [81] |
| 16 | Helianthus annuus | Non-invasive | Cd | Experimental | [82] |
| No. | Non-Invasive Plant (s) | Type | Metals | Country | References |
|---|---|---|---|---|---|
| 1 | Phyla nodiflora | Non-invasive | Cu and Zn | Field; North Florida, USA | [26] |
| 2 | Gentiana pennelliana | Non-invasive | Pb, Cu, and Zn | Field; North Florida, USA | [26] |
| 3 | Festuca rubra | Non-invasive | Pb and Mn | Field experiment | [93] |
| 4 | Tamarix gallica | Indigenous to Saudi Arabia and the Sinai Peninsula | Trace elements | pot experiment | [94] |
| 5 | Lolium perenne, | Native to Europe, Asia, and northern Africa | Cu, Pb, Mn, and Zn | highway soil in southwest British Columbia, Canada | [95] |
| 6 | Loudetia simplex | Native to Southern Africa and Madagascar | Cu | South D. R. Congo | [96] |
| 7 | Tamarix africana | Non-invasive | As, Cd, Cr, Cu, Pb, and Zn | Coina River | [97] * |
| 8 | Piptatherum miliaceum | Native to Eurasia | Trace elements | Sierra Minera of La Unión-Cartagena (SE Spain) | [98] |
| 9 | Microchloa altera | Non-invasive | Heavy metals | Democratic Republic of the Congo (DRC) | [99] |
| 10 | Silybum marianum, Piptatherum miliaceum, Nicotiana glauca and Helianthus annuus | Non-invasive | Trace metals | Pot experiment | [100] |
| 11 | Spartina alterniflora | Invasive | Cu, Zn, Pb, and Cr | China | [101] ** |
| 12 | Brassica campestris | Non-invasive | Cd, Cu, Ni, Pb, and Zn | Botanical Garden of Komarov Botanical Institute, Russia | [91] *** |
| 13 | Saccharum spontaneum and Saccharum munja | Non-invasive | Zn, Pb, Cu, Ni, Cd, and As | Pot experiments | [80] *** |
| 14 | Ulmus glabra | Non-invasive | As, Cd, Cr, Cu, Ni, Pb, and Zn | Sava River | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Chew, W.; Al-Mutairi, K.A.; Al-Shami, S.A.; Nulit, R.; Ibrahim, M.H.; Wong, K.W.; Bakhtiari, A.R.; Sharifinia, M.; Cheng, W.H.; et al. Invasive Weed Asystasia gangetica as a Potential Biomonitor and a Phytoremediator of Potentially Toxic Metals: A Case Study in Peninsular Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 4682. https://doi.org/10.3390/ijerph18094682
Yap CK, Chew W, Al-Mutairi KA, Al-Shami SA, Nulit R, Ibrahim MH, Wong KW, Bakhtiari AR, Sharifinia M, Cheng WH, et al. Invasive Weed Asystasia gangetica as a Potential Biomonitor and a Phytoremediator of Potentially Toxic Metals: A Case Study in Peninsular Malaysia. International Journal of Environmental Research and Public Health. 2021; 18(9):4682. https://doi.org/10.3390/ijerph18094682
Chicago/Turabian StyleYap, Chee Kong, Weiyun Chew, Khalid Awadh Al-Mutairi, Salman Abdo Al-Shami, Rosimah Nulit, Mohd Hafiz Ibrahim, Koe Wei Wong, Alireza Riyahi Bakhtiari, Moslem Sharifinia, Wan Hee Cheng, and et al. 2021. "Invasive Weed Asystasia gangetica as a Potential Biomonitor and a Phytoremediator of Potentially Toxic Metals: A Case Study in Peninsular Malaysia" International Journal of Environmental Research and Public Health 18, no. 9: 4682. https://doi.org/10.3390/ijerph18094682
APA StyleYap, C. K., Chew, W., Al-Mutairi, K. A., Al-Shami, S. A., Nulit, R., Ibrahim, M. H., Wong, K. W., Bakhtiari, A. R., Sharifinia, M., Cheng, W. H., Okamura, H., Ismail, M. S., & Saleem, M. (2021). Invasive Weed Asystasia gangetica as a Potential Biomonitor and a Phytoremediator of Potentially Toxic Metals: A Case Study in Peninsular Malaysia. International Journal of Environmental Research and Public Health, 18(9), 4682. https://doi.org/10.3390/ijerph18094682







