Significantly Reduced Alanine Aminotransferase Level Increases All-Cause Mortality Rate in the Elderly after Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Data Preprocessing
2.4. Statistical Analyses
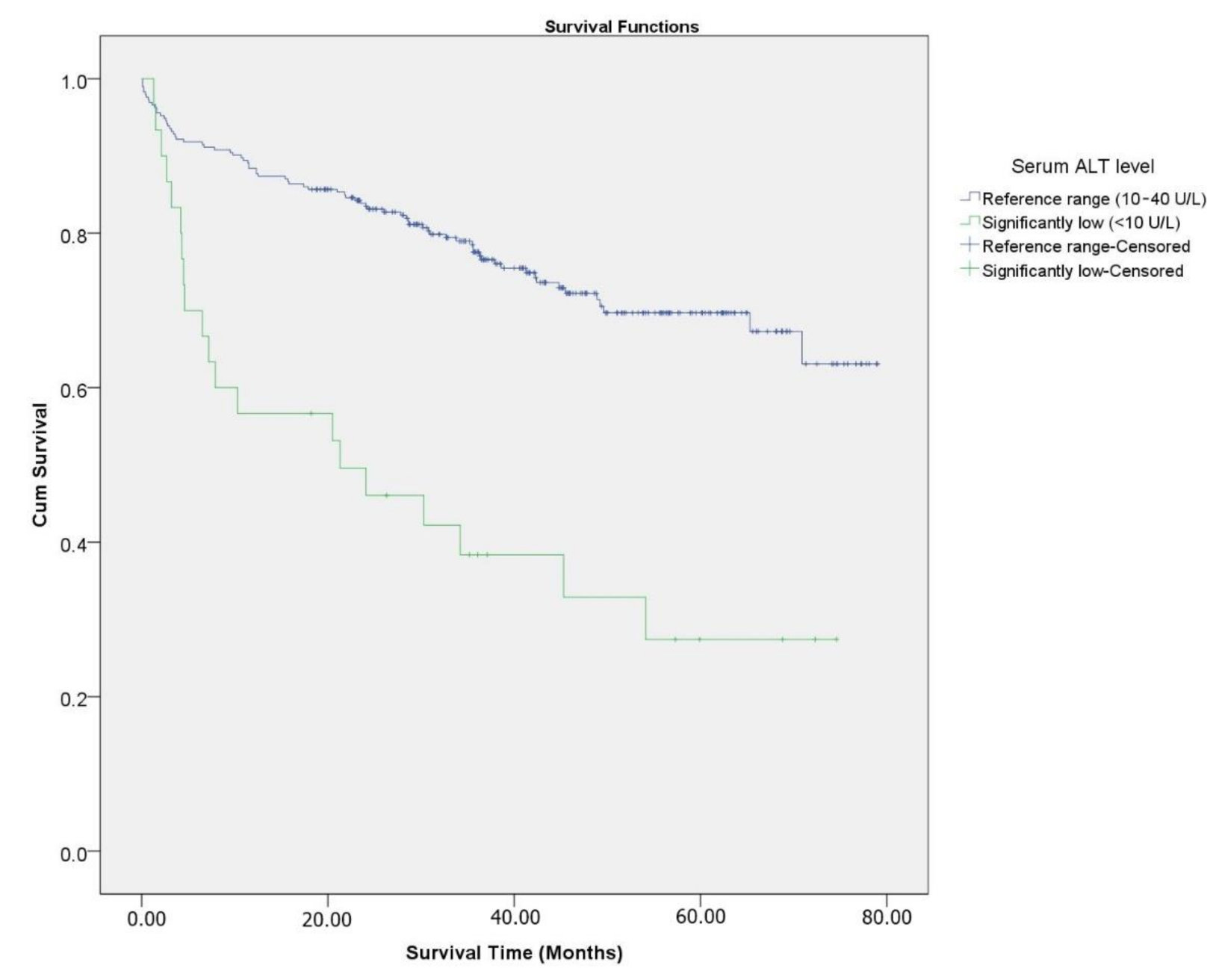
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Blyth, F.M.; Creasey, H.M.; Handelsman, D.J.; Naganathan, V.; Sambrook, P.N.; Seibel, M.J.; Waite, L.M.; Cumming, R.G. The association of alanine transaminase with aging, frailty, and mortality. J. Gerontol. Ser. A Biomed. Sci. 2010, 65, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-E.; Goh, B.-B.G.; Ekstrom, V.; Ong, M.-L.; Tan, C.-K. Low serum albumin predicts early mortality in patients with severe hypoxic hepatitis. World J. Hepatol. 2017, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ning, H.; Que, S.; Wang, L.; Qin, X.; Peng, T. Complex association between alanine aminotransferase activity and mortality in general population: A systematic review and meta-analysis of prospective studies. PLoS ONE 2014, 9, e91410. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.H.; Bettencourt, R.; Brenner, D.A.; Barrett–Connor, E.; Loomba, R. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 285–290.e1. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.-C.; Guralnik, J.M.; Salive, M.E.; Sorkin, J.D. Serum albumin level and physical disability as predictors of mortality in older persons. J. JAMA 1994, 272, 1036–1042. [Google Scholar] [CrossRef]
- Okamura, T.; Hayakawa, T.; Hozawa, A.; Kadowaki, T.; Murakami, Y.; Kita, Y.; Abbott, R.D.; Okayama, A.; Ueshima, H.; NIPPON DATA80 Research Group. Lower levels of serum albumin and total cholesterol associated with decline in activities of daily living and excess mortality in a 12-year cohort study of elderly Japanese. J. Am. Geriatr. Soc. 2008, 56, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Ackerman, Z.; Maaravi, Y.; Ben-Dov, I.Z.; Ein-Mor, E.; Stessman, J. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J. Am. Geriatr. Soc. 2006, 54, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. Ser. A Biol. Sci. 2004, 59, M255–M263. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group: Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Baztán, J.J.; Gálvez, C.P.; Socorro, A. Recovery of functional impairment after acute illness and mortality: One-year follow-up study. Gerontology 2009, 55, 269–274. [Google Scholar]
- Ruban, A.; Daya, N.; Schneider, A.L.C.; Gottesman, R.; Selvin, E.; Coresh, J.; Lazo, M.; Koton, S. Liver Enzymes and Risk of Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. J. Stroke 2020, 22, 357–368. [Google Scholar] [CrossRef]
- Stahon, K.E.; Bastian, C.; Griffith, S.; Kidd, G.J.; Brunet, S.; Baltan, S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J. Neurosci. 2016, 36, 9990–10001. [Google Scholar] [CrossRef]
- Bhatia, R.S.; Garg, R.K.; Gaur, S.P.; Kar, A.M.; Shukla, R.; Agarwal, A.; Verma, R. Predictive value of routine hematological and biochemical parameters on 30-day fatality in acute stroke. Neurol. India 2004, 52, 220. [Google Scholar]
- Collaboration, P.S. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar]
- Ayis, S.A.; Coker, B.; Rudd, A.G.; Dennis, M.S.; Wolfe, C.D. Predicting independent survival after stroke: A European study for the development and validation of standardised stroke scales and prediction models of outcome. J. Neurol. Neurosurg. Psychiatry 2013, 84, 288–296. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Paul, N.L.; Gray, A.M.; Pendlebury, S.T.; Bull, L.M.; Welch, S.J.; Cuthbertson, F.C.; Rothwell, P.M.; Oxford Vascular Study. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013, 44, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, V.; Milionis, H.; Michel, P.; Makaritsis, K.; Vemmou, A.; Koroboki, E.; Manios, E.; Vemmos, K.; Ntaios, G. ASTRAL score predicts 5-year dependence and mortality in acute ischemic stroke. Stroke 2013, 44, 1616–1620. [Google Scholar] [CrossRef]
- de Souza, S.P.; Matos, R.S.; Barros, L.L.; Rocha, P.N. Inverse association between serum creatinine and mortality in acute kidney injury. J. Bras. Nefrol. 2014, 36, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.D.; Kurrle, S.E. Frailty and Rehabilitation. Interdiscip. Top. Gerontol. Geriatr. 2015, 41, 137–150. [Google Scholar] [PubMed]
- Dziedzic, T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev. Neurother. 2015, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, D.T.; Ebben, J.P.; Foley, R.N.; Weinhandl, E.D.; Bradbury, B.D.; Collins, A. Hemoglobin level variability: Associations with mortality. J Clin. J. Am. Soc. Nephrol. 2008, 3, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Ovbiagele, B. The impact of body mass index on mortality after stroke. Stroke 2009, 40, 2704–2708. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.; Han, E.S.; Ryu, M.; Cho, Y.; Chae, S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology 2014, 60, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yang, X.; Sui, R.B.; Yang, B. The Prognostic Value of the THRIVE Score, the iScore Score and the ASTRAL Score in Chinese Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2018, 27, 2877–2886. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Zoico, E.; Harris, T.B.; Meigs, J.B.; Di Francesco, V.; Fantin, F.; Bissoli, L.; Bosello, O. Health consequences of obesity in the elderly: A review of four unresolved questions. Int. J. Obes. 2005, 29, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- McAuley, P.; Myers, J.; Abella, J.; Froelicher, V. Body mass, fitness and survival in veteran patients: Another obesity paradox? Am. J. Med. 2007, 120, 518–524. [Google Scholar] [CrossRef]
- Ajčević, M.; Furlanis, G.; Stella, A.B.; Cillotto, T.; Caruso, P.; Ridolfi, M.; Lugnan, C.; Miladinović, A.; Ukmar, M.; Cova, M.A.; et al. A CT perfusion based model predicts outcome in wake-up stroke patients treated with recombinant tissue plasminogen activator. Physiol. Meas. 2020, 41, 075011. [Google Scholar] [CrossRef]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. J. Am. Med. Assoc. 2013, 309, 2480–2488. [Google Scholar] [CrossRef]
- Bennett, A.E.; Wilder, M.J.; McNally, J.S.; Wold, J.J.; Stoddard, G.J.; Majersik, J.J.; Ansari, S.; de Havenon, A. Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J. Neurointerv. Surg. 2018, 10, 823–827. [Google Scholar] [CrossRef]
- Andalib, S.; Lattanzi, S.; Di Napoli, M.; Petersen, A.; Biller, J.; Kulik, T.; Macri, E.; Girotra, T.; Torbey, M.T.; Divani, A.A. Blood pressure variability: A new predicting factor for clinical outcomes of intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 105340. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.S.; Rothwell, P.M.; Potter, J.F.; Robinson, T.G. Prognostic significance of short-term blood pressure variability in acute stroke: Systematic review. Stroke 2015, 46, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Świtońska, M.; Piekuś-Słomka, N.; Słomka, A.; Sokal, P.; Żekanowska, E.; Lattanzi, S. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sci. 2020, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, Q.; Song, Y.; He, M.; Zeng, Y.; Liu, Z.; Xu, J. Prognostic role of neutrophil–lymphocyte ratio in patients with acute ischemic stroke. Medicine 2017, 96, e8624. [Google Scholar] [CrossRef]
- Zangari, R.; Zanier, E.R.; Torgano, G.; Bersano, A.; Beretta, S.; Beghi, E.; Casolla, B.; Checcarelli, N.; Lanfranconi, S.; Maino, A.; et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J. Neuroinflamm. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Lattanzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Glycosylated hemoglobin and functional outcome after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1786–1791. [Google Scholar] [CrossRef]
- Bortz, W.M., 2nd. A conceptual framework of frailty: A review. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M283–M288. [Google Scholar] [CrossRef]
- Alter, G.; Riley, J.C. Frailty, sickness, and death: Models of morbidity and mortality in historical populations. Popul. Stud. 1989, 43, 25–45. [Google Scholar] [CrossRef]

| Covariates | Total (n = 323) | Expired (n = 96) | Surviving (n = 227) | p Value |
|---|---|---|---|---|
| Age (years) | 76.5 ± 6.6 | 79.3 ± 6.9 | 75.3 ± 6.1 | <0.001 * |
| Sex, n (%) | 0.314 | |||
| Female | 167 (51.7%) | 45 (46.9%) | 122 (53.7%) | |
| Male | 156 (48.3%) | 51 (53.1%) | 105 (46.3%) | |
| Initial NIHSS at admission | 5.4 ± 6.5 | 8.7 ± 8.4 | 3.9 ± 4.8 | <0.001 * |
| IVtPA treatment, n (%) | 13 (4.0%) | 5 (5.2%) | 8 (3.5%) | 0.538 |
| Stent Retriever treatment, n (%) | 20 (6.2%) | 6 (6.2%) | 14 (6.2%) | 1.000 |
| IVtPA with Stent Retriever treatment | 3 (0.9%) | 1 (1.0%) | 2 (0.9%) | 1.000 |
| Time to IVtPA (minute) | 68.8 ± 30.5 | 76.8 ± 16.9 | 63.9 ± 38.7 | 0.500 |
| Time to Stent Retriever (minute) | 132.7 ± 60.0 | 141.3 ± 78.1 | 129.0 ± 56.0 | 0.693 |
| SBP at admission | 144.2 ± 24.0 | 145.5 ± 25.4 | 143.6 ± 23.5 | 0.529 |
| SBPV during first 24 h | 42.3 ± 17.3 | 45.5 ± 18.7 | 40.9 ± 16.5 | 0.031 * |
| Survival Time (month) | 37.1 ± 20.4 | 18.4 ± 17.0 | 45.0 ± 16.2 | <0.001 * |
| TOAST, n (%) | 0.643 | |||
| Large-artery atherosclerosis | 162 (50.2%) | 53 (55.2%) | 109 (48.0%) | |
| Cardioembolism | 85 (26.3%) | 25 (26.0%) | 60 (26.4%) | |
| Small-vessel occlusion | 45 (13.9%) | 10 (10.4%) | 35 (15.4%) | |
| Stroke of other determined etiology | 1 (0.3%) | 0 (0.0%) | 1 (0.4%) | |
| Stroke of undetermined etiology | 30 (9.3%) | 8 (8.3%) | 22 (9.7%) | |
| Location, n (%) | 0.197 | |||
| Both | 23 (7.1%) | 8 (8.3%) | 15 (6.6%) | |
| Infratentorial | 67 (20.8%) | 14 (14.6%) | 53 (23.3%) | |
| Supratentorial | 233 (72.1%) | 74 (77.1%) | 159 (70.0%) | |
| Hemispheric localization, n (%) | 0.036 * | |||
| Both | 57 (17.7%) | 25 (26.0%) | 32 (14.1%) | |
| Left | 129 (39.9%) | 34 (35.4%) | 95 (41.9%) | |
| Right | 137 (42.4%) | 37 (38.5%) | 100 (44.1%) | |
| Laboratory findings at diagnosis | ||||
| Extremely low ALT (<10 U/L), n (%) | 30 (9.3%) | 20 (20.8%) | 10 (4.4%) | <0.001 * |
| Low albumin (<3.5 g/dL), n (%) | 60 (18.6%) | 32 (33.3%) | 28 (12.3%) | <0.001 * |
| Low Hb (<11 g/dL), n (%) | 64 (19.8%) | 33 (34.4%) | 31 (13.7%) | <0.001 * |
| Random Glucose, n (%) | 0.298 | |||
| Hyperglycemia (>7.3 mmol/L) | 72 (22.3%) | 22 (22.9%) | 50 (22.0%) | |
| Hypoglycemia (<3.7 mmol/L) | 1 (0.3%) | 1 (1.0%) | 0 (0.0%) | |
| Reference (3.7~7.3 mmol/L) | 250 (77.4%) | 73 (76.0%) | 177 (78.0%) | |
| Creatinine (mg/dL) | 0.8 ± 0.5 | 1.0 ± 0.8 | 0.8 ± 0.3 | 0.048 * |
| ESR (mm/h) | 23.1 ± 20.4 | 27.2 ± 22.2 | 21.3 ± 19.5 | 0.017 * |
| NLR | 3.5 ± 3.6 | 4.0 ± 4.5 | 3.3 ± 3.2 | 0.094 |
| Total cholesterol (mg/dL) | 174.4 ± 42.3 | 170.6 ± 41.8 | 176.0 ± 42.7 | 0.289 |
| BMI, n (%) | <0.001 * | |||
| Reference (18.5–24.9) | 196 (60.7%) | 65 (67.7%) | 131 (57.7%) | |
| Underweight (<18.5) | 16 (4.9%) | 10 (10.4%) | 6 (2.6%) | |
| Overweight (25.0–29.9) | 99 (30.7) | 16 (16.7%) | 83 (36.6%) | |
| Obese (≥30) | 12 (3.7%) | 5 (5.2%) | 7 (3.1%) | |
| Atrial fibrillation, n (%) | 82 (25.4%) | 24 (25.0%) | 58 (25.6%) | 1.000 |
| DM, n (%) | 103 (31.9%) | 32 (33.3%) | 71 (31.3%) | 0.817 |
| Hypertension, n (%) | 227 (70.3%) | 64 (66.7%) | 163 (71.8%) | 0.429 |
| Coronary artery occlusive disease, n (%) | 46 (14.2%) | 15 (15.6%) | 31 (13.7%) | 0.773 |
| Cancer history, n (%) | 14 (4.3%) | 6 (6.2%) | 8 (3.5%) | 0.423 |
| Smoking history, n (%) | 53 (16.4%) | 17 (17.7%) | 36 (15.9%) | 0.806 |
| Alcohol consumption, n (%) | 53 (16.4%) | 14 (14.6%) | 39 (17.2%) | 0.681 |
| Covariates | Unadjusted HR | (95% CI) | Adjusted HR | (95% CI) |
|---|---|---|---|---|
| 10–40 U/L ALT level | 1.00 | (Reference) | 1.00 | (Reference) |
| <10 U/L ALT level | 2.73 | (1.50–4.98) | 3.24 | (1.95–5.41) |
| Age (years) | 1.08 | (1.04–1.11) | ||
| Initial NIHSS at admission | 1.10 | (1.07–1.13) | ||
| Creatinine (mg/dL) | 1.50 | (1.16–1.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.J.; Yang, Y.-J.; Jeon, N.-m.; Hong, Y.-P.; Kim, Y.I.; Kim, D.-Y. Significantly Reduced Alanine Aminotransferase Level Increases All-Cause Mortality Rate in the Elderly after Ischemic Stroke. Int. J. Environ. Res. Public Health 2021, 18, 4915. https://doi.org/10.3390/ijerph18094915
An SJ, Yang Y-J, Jeon N-m, Hong Y-P, Kim YI, Kim D-Y. Significantly Reduced Alanine Aminotransferase Level Increases All-Cause Mortality Rate in the Elderly after Ischemic Stroke. International Journal of Environmental Research and Public Health. 2021; 18(9):4915. https://doi.org/10.3390/ijerph18094915
Chicago/Turabian StyleAn, Sang Joon, Yun-Jung Yang, Na-mo Jeon, Yeon-Pyo Hong, Yeong In Kim, and Doo-Young Kim. 2021. "Significantly Reduced Alanine Aminotransferase Level Increases All-Cause Mortality Rate in the Elderly after Ischemic Stroke" International Journal of Environmental Research and Public Health 18, no. 9: 4915. https://doi.org/10.3390/ijerph18094915
APA StyleAn, S. J., Yang, Y.-J., Jeon, N.-m., Hong, Y.-P., Kim, Y. I., & Kim, D.-Y. (2021). Significantly Reduced Alanine Aminotransferase Level Increases All-Cause Mortality Rate in the Elderly after Ischemic Stroke. International Journal of Environmental Research and Public Health, 18(9), 4915. https://doi.org/10.3390/ijerph18094915






