Facial and Oral Manifestations Following COVID-19 Vaccination: A Survey-Based Study and a First Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
- -
- medical working staff (nurses, medical doctors, dentists);
- -
- have access to COVID-19 vaccines;
- -
- has been vaccinated with at least one dose.
2.2. Statistical Analysis
3. Results
3.1. Data
3.2. Correlations
3.3. Probability of Occurrence of Oral Symptoms
3.4. Probability of Occurrence of Facial Symptoms
3.5. Probability of Taking an Absence Day
3.6. Duration of Symptoms
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haynes, B.F.; Corey, L.; Fernandes, P.; Gilbert, P.B.; Hotez, P.J.; Rao, S.; Santos, M.R.; Schuitemaker, H.; Watson, M.; Arvin, A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020, 12, eabe0948. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; Normando, A.; Da Silva, R.C.; Acevedo, A.; Canto, G.D.L.; Sugaya, N.; Santos-Silva, A.; Guerra, E. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J. Dent. Res. 2021, 100, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Maciel, P.P.; Júnior, H.M.; Martelli, D.R.B.; Machado, R.A.; De Andrade, P.V.; Perez, D.E.D.C.; Bonan, P.R.F. Covid-19 pandemic: Oral repercussions and its possible impact on oral health. Pesqui. Bras. Odontopediatria Clin. Integr. 2020, 20 (Suppl. S1). [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns [published online ahead of print, 2021 Feb 1]. J. Oral Pathol. Med. 2021. [Google Scholar] [CrossRef]
- So, A.D.; Woo, J. Reserving coronavirus disease 2019 vaccines for global access: Cross sectional analysis. BMJ 2020, 371, m4750. [Google Scholar] [CrossRef] [PubMed]
- Riad, A. Oral side effects of COVID-19 vaccine. Br. Dent. J. 2021, 230, 59. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, B.; Ashok, N.; Alakeel, R.; Azzeghaibi, S.; Umair, A.; Darwish, S.; Mahmoud, R.; ElKhatat, E. Hepatitis B vaccination and associated oral manifestations: A non-systematic review of literature and case reports. Ann. Med. Health Sci. Res. 2014, 4, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muellenhoff, M.; Cukrowski, T.; Morgan, M.; Dorton, D. Oral pemphigus vulgaris after anthrax vaccine administration: Association or coincidence? J. Am. Acad. Dermatol. 2004, 50, 136–139. [Google Scholar] [CrossRef]
- De Simone, C.; Caldarola, G.; D’agostino, M.; Zampetti, A.; Amerio, P.; Feliciani, C. Exacerbation of pemphigus after influenza vaccination. Clin. Exp. Dermatol. 2008, 33, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A.; Rongioletti, F.; Drago, F.; Parodi, A. Lichen planus as a side effect of HBV vaccination [published correction appears in Dermatology 1999;198(2):222]. Dermatology 1999, 198, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Berman, A.H.; Izhar, M.; Hamilton, H.B.; Villano, D.; Vazquez, B.E.; Warrum, M.N.; Mahbob, M. Influenza vaccinations and chemosensory function. Am. J. Rhinol. Allergy 2014, 28, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Nhamo, G.; Chikodzi, D.; Kunene, H.P.; Mashula, N. COVID-19 vaccines and treatments nationalism: Challenges for low-income countries and the attainment of the SDGs. Glob. Public Health 2021, 16, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19) Vaccinations—Statistics and Research—Our World in Data, Ourworldindata. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 15 February 2021).
- Oral Side Effects of COVID-19 Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04706156 (accessed on 15 February 2021).
- Bordea, I.R.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.D.; Malcangi, G.; Pham, V.H.; Inchingolo, A.M.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, B.; Khalili, M.; Amiri, R.; Zartab, H.; Aflatoonian, M. Oral manifestations of COVID-19 disease: A review article. Dermatol. Ther. 2021, 34, e14578. [Google Scholar] [CrossRef] [PubMed]
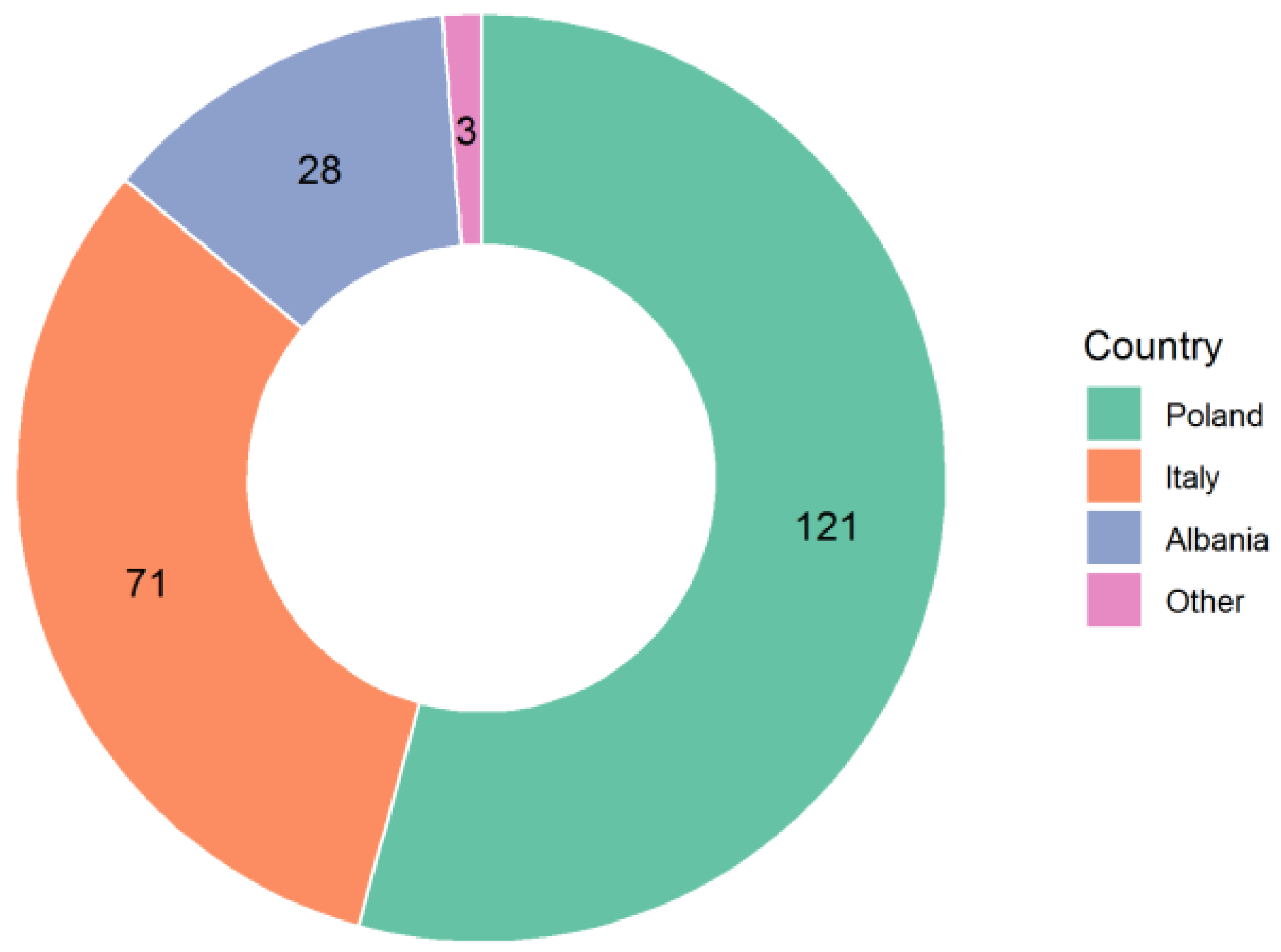

| Questionnaire Part A: Characteristics of Respondents | ||
|---|---|---|
| Q1: Country | Italy Poland France Germany Switzerland Albania Denmark Norway United Kingdom Spain Other | 71 (31.9%) 121 (54.3%) 28 (12.6%) 1 (0.4%) 1 (0.4%) 1 (0.4%) |
| Q2: City | ……. | |
| Q3: Gender | Female Male | 166 (74.4%) 57 (25.6%) |
| Q4: Age | 20–29 30–39 40–49 50–59 60–69 70–79 | 53 (23.8%) 47 (21.5%) 41 (17.9%) 47 (21.1%) 33 (14.8%) 2 (0.9%) |
| Q5: Profession | Student Medical Doctor Dentist Nurse Other | 13 (5.9%) 35 (15.3%) 69 (30.5%) 76 (34.7%) 30 (10.9%) |
| Questionnaire Part D—Underlying medical conditions and lifestyle factors | ||
| Q22: Are you a smoker? | Yes No | 30 (13.5%) 193 (76.7%) |
| Q23: Are you an allergic subject? | Yes No | 53 (23.8%) 170 (76.2%) |
| Q24: What are you allergic to? | ……. | |
| Q25: Do you have any general diseases? | Yes No | 47 (21.1%) 176 (78.9%) |
| Q26: Please list if any general diseases you have. | ……. | |
| Q27: Do you have any autoimmune disease? | Yes No | 18 (7.6%) 205 (92.4%) |
| Q28: Do you have diabetes type 1? | Yes No | 8 (3.6%) 215 (96.4%) |
| Q29: How would you describe your diet? | Omnivorous Vegetarian Flexitarian Vegan | 210 (94.2%) 11 (5) 2 (0.8%) |
| Q30: Do you have rapid mood swings? | Yes No | 43 (19.3%) 180 (80.7%) |
| Q31: Do you take any regular medication? | Yes No | 75 (33.6%) 148 (66.4%) |
| Q32: If you do take any regular medication, please list it here. | ……. | |
| Questionnaire Part B: COVID-19 Vaccine, Number of Doses, Date of Inoculation, Premedication, General Symptoms. | |||
|---|---|---|---|
| First or single dose | Second dose | ||
| Q6: Vaccine dose | First one Second one Single dose vaccine | 41 (18.4%) | 182 (81.6%) |
| Q7: Type / name of COVID-19 vaccine | Pfizer/BioNTech Moderna Astrazeneca Sputnik Other | 35 (15.7%) 1 (0.4%) 5 (2.3%) | 182 (81.6%) |
| Q8: Vaccination date (first dose) | ……. | ……. | ……. |
| Q9: Vaccination date (second dose) | ……. | ……. | ……. |
| Q10: Vaccination date (single dose) | ……. | ……. | ……. |
| Q11: In anticipation of the vaccine, did you take any medicine? | No Antipyretics Analgesics Anti-inflammatory | 203 (91.1%) 7 (3.1%) 9 (4%) 4 (1.8%) | 114 (62.6%) 35 (19.2%) 21 (11.5%) 12 (6.7%) |
| Q12: If you took any medicine in anticipation of the vaccine, can you describe the medication and the total dose. | ……. | ……. | ……. |
| Q13: What was the time between inoculation and onset of general symptoms? | No symptoms 1 h 2 h 3 h 6 h 12 h 24 h | 103 (45.9%) 11 (4.6%) 10 (4.6%) 14 (6.2%) 33 (15%) 34 (15.5%) 18 (8.2%) | 49 (13.9%) 8 (4.7%) 2 (1.2%) 18 (10.5%) 32 (18.6%) 50 (29%) 23 (22.1%) |
| Q14: Did your symptoms include fever? | No Yes, from 36.6 to 37.4 degree C Yes, from 37.5 to 38.0 degree C Yes, from 38.1 to 39.0 degree C | 185 (82.8%) 25 (11.2%) 8 (3.7%) 5 (2.3%) | 101 (54.7%) 47 (26.3%) 22 (12.3%) 12 (6.7%) |
| Q15: How long were the general symptoms present? | No symptoms 1 day 2 days 3 days 4 days 1 week | 137 (61.5%) 43 (19.3%) 23 (10.3%) 11 (4.9%) 6 (1.7%) 3 (1.3%) | 50 (27.5%) 62 (34.1%) 48 (26.4%) 18 (9.9%) 1 (0.5%) 3 (1.6%) |
| Q16: Did you experience loss of concentration? | Yes No | 26 (11.6%) 197 (88.4%) | 57 (32.4%) 125 (67.6%) |
| Q17: Did you need to take an absence day? | Yes No | 8 (3.6%) 215 (93.3%) | 53 (23.7%) 129(57.4%) |
| Questionnaire Part C—Facial and Oral Manifestations | |||
| Q18: Facial symptoms (changes in sensitivity, facial paresis, facial aesthetic paralysis) | Yes No | 6 (2.7%) 217 (97.3%) | 8 (4.2%) 174 (95.8%) |
| Q19: Oral symptoms | Yes No | 7 (3.1%) 216 (95.5%) | 9 (4%) 173 (76.6%) |
| Q20: Oral manifestations | No oral manifestations Burning sensation Oral aphthous-like lesions Taste alteration Xerostomia Tongue depapillation Pain Stomatitis/mucositis Commissural cheilitis Oral candidiasis | 216 (97%) 3 (1.3%) 3 (1.3%) 1 (0.4%) | 161 (88.7%) 5 (2.7%) 1 (0.5%) 4 (2.2%) 5 (2.7%) 1 (0.5%) 5 (2.7%) |
| Q21: If you experienced other oral manifestations, please list them below. | ……. | ……. | ……. |
| Variable | Yes | No | No Answer |
|---|---|---|---|
| Oral symptoms (dose 1) | 7 | 213 | 3 |
| Oral symptoms (dose 2) | 9 | 171 | 2 |
| Absence day (dose 1) | 8 | 208 | 7 |
| Absence day (dose 2) | 53 | 128 | 1 |
| Diabetes | 8 | 215 | – |
| Smoker | 30 | 171 | 22 |
| Allergic | 53 | 170 | – |
| Regular medication | 75 | 148 | – |
| General diseases | 47 | 176 | – |
| Symptoms-Related Characteristic | Age | Gender |
|---|---|---|
| Time between inoculation and general symptoms onset (first dose) | 0.135 (0.064) | 0.204 (0.005) |
| Time between inoculation and general symptoms onset (second dose) | 0.223 (0.003) | 0.232 (0.002) |
| Fever (first dose) | –0.079 (0.285) | –0.109 (0.138) |
| Fever (second dose) | –0.298 (<0.001) | –0.047 (0.537) |
| Symptoms Duration (first dose) | –0.152 (0.035) | –0.215 (0.003) |
| Symptoms Duration (second dose) | –0.207 (0.006) | –0.128 (0.094) |
| Concentration Loss (first dose) | –0.108 (0.114) | –0.021 (0.763) |
| Concentration Loss (second dose) | –0.333 (<0.001) | –0.001 (0.994) |
| Absence Day (first dose) | –0.077 (0.258) | –0.116 (0.089) |
| Absence Day (second dose) | –0.129 (0.083) | –0.099 (0.185) |
| Facial Symptoms (first dose) | –0.010 (0.896) | –0.028 (0.709) |
| Facial Symptoms (second dose) | –0.148 (0.079) | 0.017 (0.838) |
| Oral Symptoms (first dose) | 0.059 (0.386) | –0.107 (0.113) |
| Oral Symptoms (second dose) | –0.175 (0.019) | –0.028 (0.705) |
| Oral Symptoms, Second Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | z–Value | p-Value |
| (Intercept) | –0.111 | 1.181 | –0.094 | 0.925 |
| Age | –0.097 | 0.038 | –2.573 | 0.010 |
| General disease | 2.090 | 0.783 | 2.669 | 0.007 |
| Model characteristics | AIC: 64.31 McFadden’s R2 = 0.184 | |||
| Facial Symptoms, First Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | z-Value | p-Value |
| (Intercept) | –3.839 | 0.682 | –5.631 | <0.001 |
| Smoker | 1.424 | 0.979 | 1.455 | 0.146 |
| RegularMedication | –2.014 | 1.401 | –1.437 | 0.151 |
| GeneralDiseases | 2.021 | 1.167 | 1.733 | 0.083 |
| Model characteristics | AIC: 47.154 McFadden’s R2 = 0.142 | |||
| Facial Symptoms, Second Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | z–Value | p-Value |
| (Intercept) | –0.363 | 1.539 | –0.236 | 0.814 |
| Age | –0.091 | 0.048 | –1.894 | 0.059 |
| Autoimmune Pathologies | 1.577 | 0.507 | 3.112 | 0.002 |
| Model characteristics | AIC: 42.165 McFadden’s R2 = 0.273 | |||
| Taking an Absence Day, Second Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | z-Value | p-Value |
| (Intercept) | –0.586 | 0.227 | –2.584 | 0.010 |
| Gender—male | –0.676 | 0.408 | –1.655 | 0.098 |
| Smoker | 1.135 | 0.469 | 2.417 | 0.016 |
| Regular medication | –1.048 | 0.404 | –2.597 | 0.009 |
| Model characteristics | AIC: 211.52 McFadden’s R2 = 0.070 | |||
| Duration of Symptoms, First Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | t-Value | p-Value |
| (Intercept) | 1.4201 | 0.1413 | 10.048 | <0.001 |
| Gender—male | –0.618 | 0.237 | –2.602 | 0.010 |
| Regular medication | –0.627 | 0.222 | –2.820 | 0.005 |
| Model characteristics | R2 = 0.076 F[2189] = 7.75; p < 0.001 | |||
| Duration of Symptoms, First Dose | ||||
|---|---|---|---|---|
| Variable | Estimate | Std. Error | t-Value | p-Value |
| (Intercept) | 1.982 | 0.283 | 7.013 | <0.001 |
| Gender—male | –0.332 | 0.200 | –1.664 | 0.098 |
| Age | –0.0166 | 0.0065 | –2.552 | 0.012 |
| Smoker | 0.509 | 0.267 | 1.909 | 0.058 |
| General disease | 0.405 | 0.234 | 1.729 | 0.086 |
| Model characteristics | R2 = 0.076 F[4169] = 3.464; p = 0.009 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, M.; Duś-Ilnicka, I.; Jedliński, M.; Ndokaj, A.; Janiszewska-Olszowska, J.; Ardan, R.; Radwan-Oczko, M.; Guerra, F.; Luzzi, V.; Vozza, I.; et al. Facial and Oral Manifestations Following COVID-19 Vaccination: A Survey-Based Study and a First Perspective. Int. J. Environ. Res. Public Health 2021, 18, 4965. https://doi.org/10.3390/ijerph18094965
Mazur M, Duś-Ilnicka I, Jedliński M, Ndokaj A, Janiszewska-Olszowska J, Ardan R, Radwan-Oczko M, Guerra F, Luzzi V, Vozza I, et al. Facial and Oral Manifestations Following COVID-19 Vaccination: A Survey-Based Study and a First Perspective. International Journal of Environmental Research and Public Health. 2021; 18(9):4965. https://doi.org/10.3390/ijerph18094965
Chicago/Turabian StyleMazur, Marta, Irena Duś-Ilnicka, Maciej Jedliński, Artnora Ndokaj, Joanna Janiszewska-Olszowska, Roman Ardan, Malgorzata Radwan-Oczko, Fabrizio Guerra, Valeria Luzzi, Iole Vozza, and et al. 2021. "Facial and Oral Manifestations Following COVID-19 Vaccination: A Survey-Based Study and a First Perspective" International Journal of Environmental Research and Public Health 18, no. 9: 4965. https://doi.org/10.3390/ijerph18094965
APA StyleMazur, M., Duś-Ilnicka, I., Jedliński, M., Ndokaj, A., Janiszewska-Olszowska, J., Ardan, R., Radwan-Oczko, M., Guerra, F., Luzzi, V., Vozza, I., Marasca, R., Ottolenghi, L., & Polimeni, A. (2021). Facial and Oral Manifestations Following COVID-19 Vaccination: A Survey-Based Study and a First Perspective. International Journal of Environmental Research and Public Health, 18(9), 4965. https://doi.org/10.3390/ijerph18094965













