Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults
Abstract
:1. Introduction
2. Methods
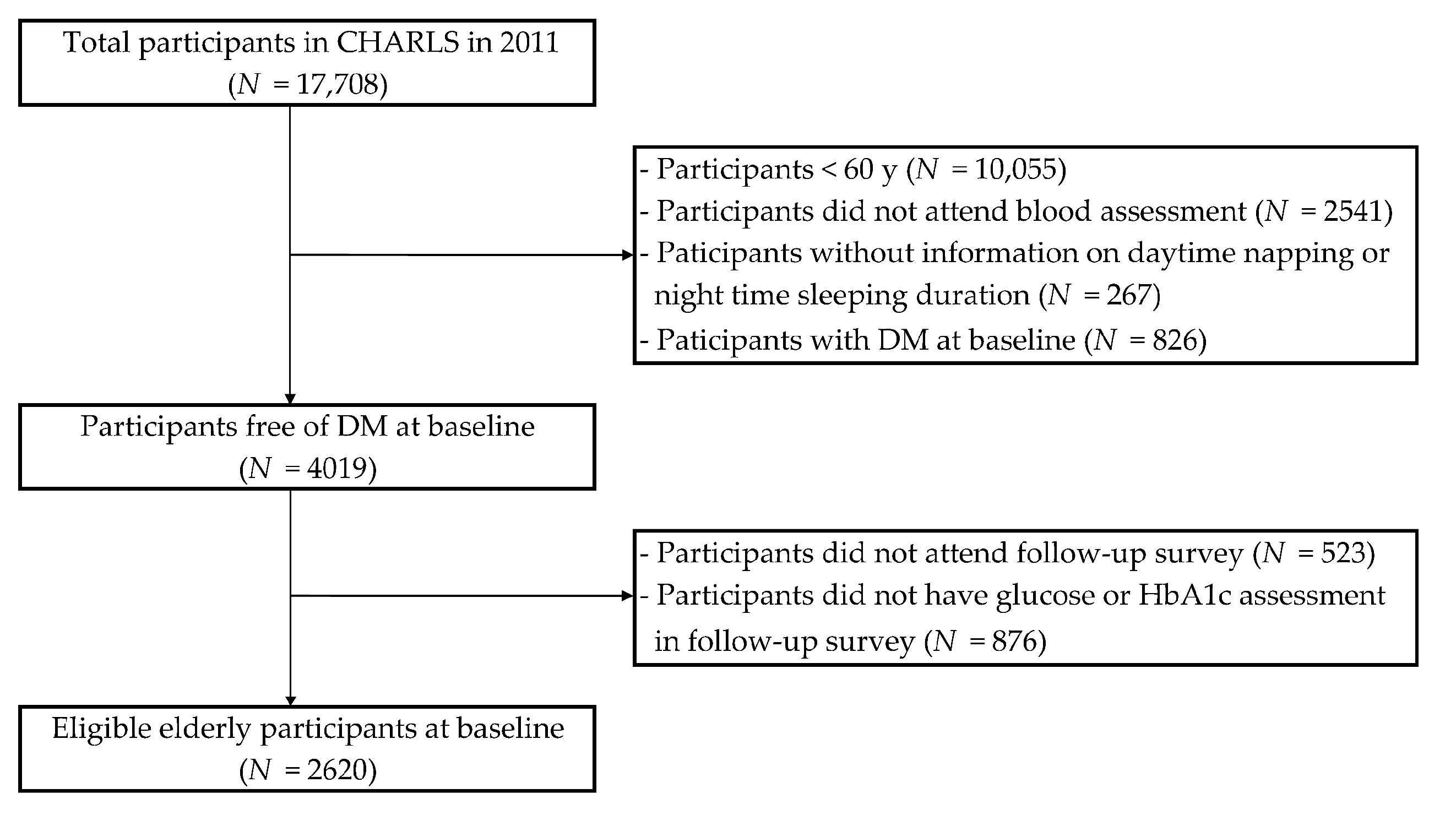
2.1. Study Design and Population
2.2. Ascertainment of DM
2.3. Daytime Napping and Nighttime Sleep Duration
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef]
- Bragg, F.; Holmes, M.V.; Iona, A.; Guo, Y.; Du, H.; Chen, Y.; Bian, Z.; Yang, L.; Herrington, W.; Bennett, D.; et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA 2017, 317, 280–289. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Song, Y.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 2010, 33, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, V.Y.; Cao, B.; Wong, C.K.H.; Yu, E.Y.T. The association between daytime napping and risk of diabetes: A systematic review and meta-analysis of observational studies. Sleep Med. 2017, 37, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Q.; Wei, J.; Meng, X.; Jia, C. Association of daytime napping with prediabetes and diabetes in a Chinese population: Results from the baseline survey of the China health and retirement longitudinal study. J. Diabetes 2018, 10, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Cappuccio, F.P.; Surtees, P.G.; Luben, R.; Brayne, C.; Khaw, K.T. Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 996–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Shojima, N.; Yamauchi, T.; Kadowaki, T. J-curve relation between daytime nap duration and type 2 diabetes or metabolic syndrome: A dose-response meta-analysis. Sci. Rep. 2016, 6, 38075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowall, B.; Lehnich, A.T.; Strucksberg, K.H.; Führer, D.; Erbel, R.; Jankovic, N.; Moebus, S.; Jöckel, K.H.; Stang, A. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: The heinz nixdorf recall study. Sleep Med. 2016, 21, 35–41. [Google Scholar] [CrossRef]
- Han, X.; Liu, B.; Wang, J.; Pan, A.; Li, Y.; Hu, H.; Li, X.; Yang, K.; Yuan, J.; Yao, P.; et al. Long sleep duration and afternoon napping are associated with higher risk of incident diabetes in middle-aged and older Chinese: The Dongfeng-Tongji cohort study. Ann. Med. 2016, 48, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hublin, C.; Lehtovirta, M.; Partinen, M.; Koskenvuo, M.; Kaprio, J. Napping and the risk of type 2 diabetes: A population-based prospective study. Sleep Med. 2016, 17, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The china health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Crimmins, E.; Hu, P.P.; Kim, J.K.; Meng, Q.; Strauss, J.; Wang, Y.; Zeng, J.; Zhang, Y.; Zhao, Y. Venous blood-based biomarkers in the china health and retirement longitudinal study: Rationale, design, and results from the 2015 wave. Am. J. Epidemiol. 2019, 188, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [CrossRef] [Green Version]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- National Health and Family Planning Commission of the People’s Republic of China. WST 428-2013 Criteria of Weight for Adults; Standards Press of China: Beijing, China, 2013.
- Zhao, X.; Cheng, L.; Zhu, C.; Cen, S.; Lin, W.; Zheng, W.; Yang, M.; Yang, F.; Zhu, S. A double-edged sword: The association of daytime napping duration and metabolism related diseases in a Chinese population. Eur. J. Clin. Nutr. 2021, 75, 291–298. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, J. Association of daytime napping and diagnosed diabetes in middle-aged premenopausal, middle-aged postmenopausal, and older postmenopausal chinese women. Am. J. Health Promot. AJHP 2019, 33, 1107–1114. [Google Scholar] [CrossRef]
- Schwartz, J.; Allison, M.A.; Ancoli-Israel, S.; Hovell, M.F.; Patterson, R.E.; Natarajan, L.; Marshall, S.J.; Grant, I. Sleep, type 2 diabetes, dyslipidemia, and hypertension in elderly Alzheimer’s caregivers. Arch. Gerontol. Geriatr. 2013, 57, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, C.; Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Long daytime napping is associated with increased adiposity and type 2 diabetes in an elderly population with metabolic syndrome. J. Clin. Med. 2019, 8, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhorter, K.L.; Park, Y.M.; Gaston, S.A.; Fang, K.B.; Sandler, D.P.; Jackson, C.L. Multiple sleep dimensions and type 2 diabetes risk among women in the Sister Study: Differences by race/ethnicity. BMJ Open Diabetes Res. Care 2019, 7, e000652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Li, Y.; Mao, Z.; Liu, X.; Zhang, H.; Yang, K.; Zhang, H.; Tu, R.; Qian, X.; Jiang, J.; et al. Gender-specific independent and combined dose-response association of napping and night sleep duration with type 2 diabetes mellitus in rural Chinese adults: The RuralDiab study. Sleep Med. 2018, 45, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Castriotta, R.J.; Portaluppi, F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007, 8, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Dodt, C.; Mölle, M.; Born, J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology 2007, 32, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 2009, 94, 2692–2701. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, I.; Maloney, T.; March, J.D. Precise conservation of NREM period 1 (NREMP1) delta across naps and nocturnal sleep: Implications for REM latency and NREM/REM alternation. Sleep 1992, 15, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Van Cauter, E.; Blackman, J.D.; Roland, D.; Spire, J.P.; Refetoff, S.; Polonsky, K.S. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J. Clin. Investig. 1991, 88, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masa, J.F.; Rubio, M.; Pérez, P.; Mota, M.; de Cos, J.S.; Montserrat, J.M. Association between habitual naps and sleep apnea. Sleep 2006, 29, 1463–1468. [Google Scholar] [CrossRef]
- Borel, A.L. Sleep apnea and sleep habits: Relationships with metabolic syndrome. Nutrients 2019, 11, 2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, M.S.; Lam, B.; Ng, M.M.; Lam, W.K.; Tsang, K.W.; Lam, K.S. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002, 165, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.L.; Tamisier, R.; Böhme, P.; Priou, P.; Avignon, A.; Benhamou, P.Y.; Hanaire, H.; Pépin, J.L.; Kessler, L.; Valensi, P.; et al. Obstructive sleep apnoea syndrome in patients living with diabetes: Which patients should be screened? Diabetes Metab. 2019, 45, 91–101. [Google Scholar] [CrossRef]
- Jakubowski, K.P.; Boylan, J.M.; Cundiff, J.M.; Matthews, K.A. Poor sleep moderates the relationship between daytime napping and inflammation in Black and White men. Sleep Health 2017, 3, 328–335. [Google Scholar] [CrossRef]
- Leng, Y.; Ahmadi-Abhari, S.; Wainwright, N.W.; Cappuccio, F.P.; Surtees, P.G.; Luben, R.; Brayne, C.; Khaw, K.T. Daytime napping, sleep duration and serum C reactive protein: A population-based cohort study. BMJ Open 2014, 4, e006071. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Lucassen, E.A.; Rother, K.I.; Cizza, G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann. N. Y. Acad. Sci. 2012, 1264, 110–134. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Chaput, J.P.; Després, J.P.; Bouchard, C.; Tremblay, A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity 2007, 15, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Seixas, A.; Shetty, S.; Shenoy, S. Sleep duration and diabetes risk: Population trends and potential mechanisms. Curr. Diabetes Rep. 2016, 16, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Brownson, R.C. Sleep duration and obesity-related risk factors in the rural Midwest. Prev. Med. 2008, 46, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.M.; Melanson, E.L.; Frydendall, E.J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 2011, 589, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Lassen, A.; Mahnke, C.; Schultes, B.; Schiöth, H.B.; Born, J.; Lange, T. Acute sleep deprivation reduces energy expenditure in healthy men. Am. J. Clin. Nutr. 2011, 93, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- McHill, A.W.; Wright, K.P., Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18 (Suppl. 1), 15–24. [Google Scholar] [CrossRef]
- Dowd, J.B.; Goldman, N.; Weinstein, M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann. Epidemiol. 2011, 21, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.R.; Zhu, X.; Storfer-Isser, A.; Mehra, R.; Jenny, N.S.; Tracy, R.; Redline, S. Sleep duration and biomarkers of inflammation. Sleep 2009, 32, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandner, M.A.; Drummond, S.P. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med. Rev. 2007, 11, 341–360. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Van Cauter, E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Chapman, C.D.; Cedernaes, J.; Benedict, C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med. Rev. 2018, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Mokhlesi, B. Obstructive sleep apnea and diabetes: A state of the art review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- Cai, H.; Shu, X.O.; Xiang, Y.B.; Yang, G.; Li, H.; Ji, B.T.; Gao, J.; Gao, Y.T.; Zheng, W. Sleep duration and mortality: A prospective study of 113,138 middle-aged and elderly Chinese men and women. Sleep 2015, 38, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Kronholm, E.; Härmä, M.; Hublin, C.; Aro, A.R.; Partonen, T. Self-reported sleep duration in Finnish general population. J. Sleep Res. 2006, 15, 276–290. [Google Scholar] [CrossRef]
- Kanady, J.C.; Drummond, S.P.; Mednick, S.C. Actigraphic assessment of a polysomnographic-recorded nap: A validation study. J. Sleep Res. 2011, 20, 214–222. [Google Scholar] [CrossRef]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L. Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Annis, A.M.; Caulder, M.S.; Cook, M.L.; Duquette, D. Family history, diabetes, and other demographic and risk factors among participants of the National Health and Nutrition Examination Survey 1999–2002. Prev. Chronic Dis. 2005, 2, A19. [Google Scholar] [PubMed]
- Guo, V.Y.; Yu, E.Y.; Wong, C.K.; Sit, R.W.; Wang, J.H.; Lam, C.L. Hypertriglyceridaemic-waist phenotype and risk of diabetes in people with impaired fasting glucose in primary care: A cohort study. Diabet. Med. A J. Br. Diabet. Assoc. 2018, 35, 576–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, V.Y.; Cao, B.; Cai, C.; Cheng, K.K.; Cheung, B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018, 55, 87–98. [Google Scholar] [CrossRef]
- Guo, V.Y.; Yu, E.Y.; Wong, C.; Sit, R.W.; Wang, J.H.; Ho, S.Y.; Lam, C.L. Validation of a nomogram for predicting regression from impaired fasting glucose to normoglycaemia to facilitate clinical decision making. Fam. Pract. 2016, 33, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.B.; Jiang, C.Q.; Thomas, G.N.; Arora, T.; Zhang, W.S.; Taheri, S.; Adab, P.; Lam, T.H.; Cheng, K.K. Napping is associated with increased risk of type 2 diabetes: The Guangzhou biobank cohort study. Sleep 2010, 33, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [Green Version]

| Baseline Characteristics | Daytime Napping (h/day) | ||
|---|---|---|---|
| None | ≤1 | >1 | |
| N | 1234 | 904 | 482 |
| Demographic and lifestyle factors | |||
| Mean age (years) | 66.6 (5.7) | 67.1 (5.9) | 67.2 (5.8) |
| Gender, n (%) * | |||
| Male | 530 (42.9%) | 501 (55.4%) | 306 (63.5%) |
| Female | 704 (57.1%) | 403 (44.6%) | 176 (36.5%) |
| Education, n (%) * | |||
| Illiterate/no formal education | 778 (63.1%) | 487 (53.9%) | 248 (51.5%) |
| Primary school | 299 (24.2%) | 249 (27.5%) | 138 (28.6%) |
| Middle school or above | 156 (12.7%) | 168 (18.6%) | 96 (19.9%) |
| Race, n (%) * | |||
| Han ethnicity | 1101 (92.8%) | 841 (96.1%) | 437 (94.8%) |
| Other minorities | 85 (7.2%) | 34 (3.9%) | 24 (5.2%) |
| Area of residence, n (%) * | |||
| Urban | 866 (70.2%) | 592 (65.5%) | 340 (70.5%) |
| Rural | 368 (29.8%) | 312 (34.5%) | 142 (29.5%) |
| Current marital status, n (%) | |||
| Not married | 224 (18.2%) | 146 (16.2%) | 82 (17.0%) |
| Married or cohabitated | 1010 (81.8%) | 758 (83.8%) | 400 (83.0%) |
| Ever smoker, n (%) * | 489 (39.6%) | 398 (44.0%) | 255 (52.9%) |
| Ever drinker, n (%) * | 484 (39.3%) | 420 (46.5%) | 253 (52.5%) |
| Mean sleep duration (hour) * | 5.9 (2.1) | 6.2 (1.9) | 6.7 (1.9) |
| Sleep duration groups, n (%) * | |||
| ≤4 h | 389 (31.5%) | 347 (38.4%) | 221 (45.9%) |
| 4–6 h | 426 (34.5%) | 329 (36.4%) | 142 (29.5%) |
| 6–8 h | 316 (25.6%) | 166 (18.4%) | 62 (12.9%) |
| >8 h | 103 (8.3%) | 62 (6.9%) | 57 (11.8%) |
| Clinical/biochemical measures | |||
| BMI (kg/m2) * | 22.5 (3.6) | 22.9 (3.7) | 23.2 (3.6) |
| Obesity, n (%) | 79 (7.0%) | 75 (9.0%) | 38 (8.7%) |
| Waist circumference (cm) * | |||
| Male | 82.9 (9.4) | 83.6 (9.3) | 85.7 (9.6) |
| Female | 84.3 (9.9) | 86.6 (10.5) | 86.7 (11.1) |
| Central obesity, n (%) | 415 (37.3%) | 333 (40.4%) | 188 (43.1%) |
| Systolic BP (mmHg) | 133.5 (22.6) | 132.4 (21.9) | 134.6 (22.4) |
| Diastolic BP (mmHg) | 74.9 (11.9) | 73.8 (11.7) | 75.0 (11.2) |
| Plasma glucose (mmol/L) | 5.7 (0.8) | 5.7 (0.7) | 5.7 (0.8) |
| HbA1c (%) * | 5.1 (0.4) | 5.1 (0.4) | 5.2 (0.4) |
| Total cholesterol (mmol/L) | 5.0 (1.0) | 5.0 (1.0) | 4.9 (1.0) |
| Triglycerides (mmol/L) | 1.34 (0.8) | 1.32 (0.8) | 1.34 (0.8) |
| HDL-cholesterol (mmol/L) * | 1.4 (0.4) | 1.4 (0.4) | 1.3 (0.4) |
| LDL-cholesterol (mmol/L) | 3.1 (0.9) | 3.1 (0.9) | 3.0 (0.9) |
| Outcomes | |||
| DM, n (%) | 155 (12.6%) | 123 (13.6%) | 80 (16.6%) |
| Baseline Characteristics | Nighttime Sleep Duration (h/day) | |||
|---|---|---|---|---|
| ≤4 | 4–6 | 6–8 | >8 | |
| N | 544 | 897 | 957 | 222 |
| Demographic and lifestyle factors | ||||
| Mean age (years) * | 67.4 (5.9) | 66.8 (5.9) | 66.5 (5.5) | 67.7 (6.0) |
| Gender, n (%) * | ||||
| Male | 228 (41.9%) | 469 (52.3%) | 521 (54.4%) | 119 (53.6%) |
| Female | 316 (58.1%) | 428 (47.7%) | 436 (45.6%) | 103 (46.4%) |
| Education, n (%) * | ||||
| Illiterate/no formal education | 384 (70.6%) | 479 (53.4%) | 513 (53.7%) | 137 (61.7%) |
| Primary school | 109 (20.0%) | 238 (26.5%) | 282 (29.5%) | 57 (25.7%) |
| Middle school or above | 51 (9.4%) | 180 (20.1%) | 161 (16.8%) | 28 (12.6%) |
| Race, n (%) * | ||||
| Han ethnicity | 496 (95.2%) | 822 (95.1%) | 869 (94.0%) | 192 (90.1%) |
| Other minorities | 25 (4.8%) | 42 (4.9%) | 55 (6.0%) | 21 (9.9%) |
| Area of residence, n (%) * | ||||
| Urban | 401 (73.7%) | 572 (63.8%) | 653 (68.2%) | 172 (77.5%) |
| Rural | 143 (26.3%) | 325 (36.2%) | 304 (31.8%) | 50 (22.5%) |
| Current marital status, n (%) * | ||||
| Not married | 110 (20.2%) | 143 (15.9%) | 141 (14.7%) | 58 (26.1%) |
| Married or cohabitated | 434 (79.8%) | 754 (84.1%) | 816 (85.3%) | 164 (73.9%) |
| Ever smoker, n (%) * | 213 (39.2%) | 383 (42.7%) | 445 (46.5%) | 101 (45.5%) |
| Ever drinker, n (%) * | 219 (40.3%) | 408 (45.5%) | 424 (44.4%) | 106 (47.7%) |
| Mean daytime napping (hour) * | 0.49 (0.03) | 0.63 (0.02) | 0.77 (0.03) | 0.79 (0.06) |
| Clinical/biochemical measures | ||||
| BMI (kg/m2) | 22.5 (3.7) | 22.8 (3.8) | 23.0 (3.5) | 22.6 (3.6) |
| Obesity, n (%) | 36 (7.3%) | 68 (8.3%) | 73 (8.3%) | 15 (7.5%) |
| Waist circumference (cm) | ||||
| Male * | 81.9 (9.0) | 83.8 (9.7) | 84.4 (9.4) | 84.5 (9.3) |
| Female | 84.6 (10.1) | 85.8 (10.5) | 85.7 (10.4) | 85.2 (9.9) |
| Central obesity, n (%) | 179 (36.2%) | 321 (39.8%) | 353 (40.5%) | 83 (41.1%) |
| Systolic BP (mmHg) | 133.7 (23.4) | 133.0 (22.7) | 133.1 (21.3) | 134.7 (22.6) |
| Diastolic BP (mmHg) | 75.0 (12.3) | 74.1 (11.4) | 74.4 (11.6) | 75.5 (12.4) |
| Plasma glucose (mmol/L) | 5.7 (0.8) | 5.7 (0.7) | 5.7 (0.8) | 5.7 (0.9) |
| HbA1c (%) | 5.1 (0.4) | 5.1 (0.4) | 5.1 (0.4) | 5.1 (0.4) |
| Total cholesterol (mmol/L) | 5.0 (1.0) | 5.0 (1.0) | 5.0 (1.0) | 5.1 (1.0) |
| Triglycerides (mmol/L) | 1.36 (0.9) | 1.28 (0.7) | 1.37 (0.8) | 1.30 (0.8) |
| HDL-cholesterol (mmol/L) * | 1.4 (0.4) | 1.4 (0.4) | 1.3 (0.4) | 1.4 (0.4) |
| LDL-cholesterol (mmol/L) | 3.1 (0.9) | 3.1 (0.9) | 3.1 (0.9) | 3.1 (0.9) |
| Outcomes | ||||
| DM, n (%) | 87 (16.0%) | 109 (12.2%) | 125 (13.1%) | 37 (16.7%) |
| Independent Variable | Crude Model | Adjusted Model 1 † | Adjusted Model 2 § | Adjusted Model 3 ξ |
|---|---|---|---|---|
| RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | |
| Daytime napping (h/day) | ||||
| None | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| ≤1 | 1.10 (0.85, 1.41) | 1.09 (0.83, 1.43) | 1.12 (0.85, 1.47) | 1.07 (0.81, 1.42) |
| >1 | 1.39 (1.03, 1.86) * | 1.60 (1.17, 2.19) * | 1.63 (1.18, 2.24) * | 1.52 (1.10, 2.10) * |
| Nighttime sleep duration (h/day) | ||||
| ≤4 | 1.27 (0.94, 1.70) | 1.28 (0.93, 1.76) | 1.35 (0.98, 1.87) | 1.45 (1.04, 2.01) * |
| 4–6 | 0.92 (0.70, 1.21) | 0.92 (0.69, 1.24) | 0.96 (0.71, 1.29) | 0.97 (0.72, 1.31) |
| 6–8 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| >8 | 1.33 (0.89, 1.99) | 1.52 (1.00, 2.32) * | 1.52 (1.00, 2.31) * | 1.55 (1.01, 2.38) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Lu, C.; Chen, W.; Guo, V.Y. Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 5012. https://doi.org/10.3390/ijerph18095012
Lin L, Lu C, Chen W, Guo VY. Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults. International Journal of Environmental Research and Public Health. 2021; 18(9):5012. https://doi.org/10.3390/ijerph18095012
Chicago/Turabian StyleLin, Li, Ciyong Lu, Weiqing Chen, and Vivian Yawei Guo. 2021. "Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults" International Journal of Environmental Research and Public Health 18, no. 9: 5012. https://doi.org/10.3390/ijerph18095012
APA StyleLin, L., Lu, C., Chen, W., & Guo, V. Y. (2021). Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults. International Journal of Environmental Research and Public Health, 18(9), 5012. https://doi.org/10.3390/ijerph18095012





