The Role of Tobacco Smoking in the Efficacy of Brief Alcohol Intervention: Results from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Interventions
2.3. Follow-Up
2.4. Measures
2.4.1. Outcome
2.4.2. Smoking
2.4.3. Covariates
2.5. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Moderator Smoking Status
3.3. Moderator Cigarettes per Day
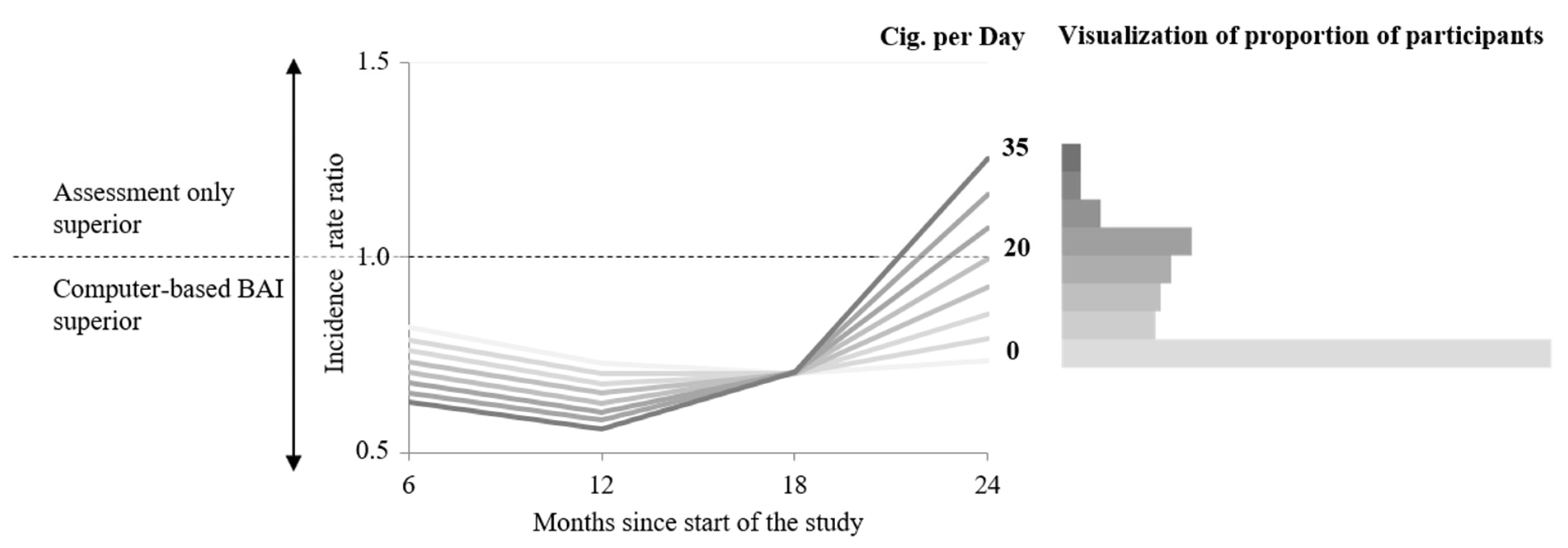
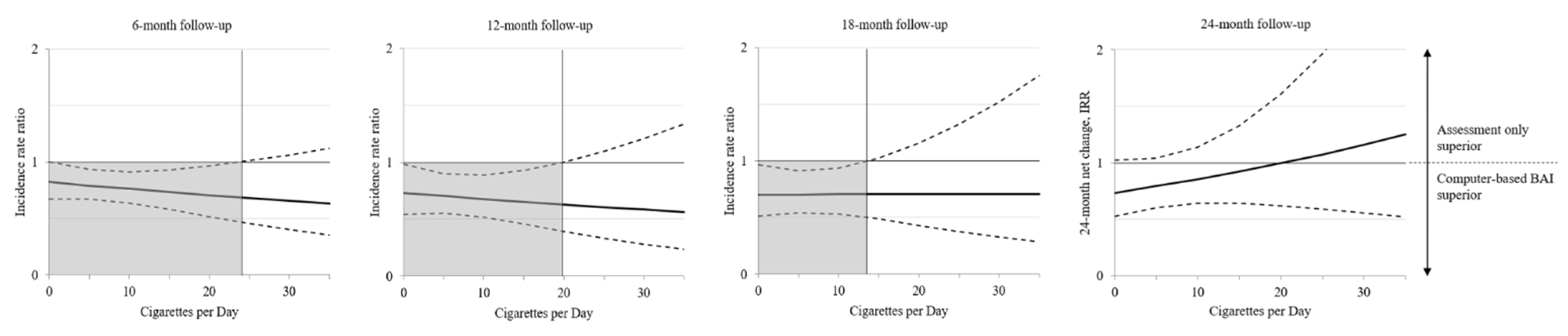
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health—2018; WHO Press: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. WHO Global Report: Mortality Attributable to Tobacco; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Wild, C.P.; Weiderpass, E.; Stuart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Kalman, D.; Kim, S.; DiGirolamo, G.; Smelson, D.; Ziedonis, D.M. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: The case for integrating smoking cessation services in substance use disorder treatment programs. Clin. Psychol. Rev. 2010, 30, 12–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maso, L.D.; Torelli, N.; Biancotto, E.; Di Maso, M.; Gini, A.; Franchin, G.; Levi, F.; La Vecchia, C.; Serraino, D.; Polesel, J. Combined effect of tobacco smoking and alcohol drinking in the risk of head and neck cancers: A re-analysis of case–control studies using bi-dimensional spline models. Eur. J. Epidemiol. 2016, 31, 385–393. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Hanke, M.; Freyer-Adam, J. Health Risk Behavior Patterns in a National Adult Population Survey. Int. J. Environ. Res. Public Health 2018, 15, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaner, E.F.; Beyer, F.R.; Muirhead, C.; Campbell, F.; Pienaar, E.D.; Bertholet, N.; Daeppen, J.B.; Saunders, J.B.; Burnand, B. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst. Rev. 2018, 2018, CD004148. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Bueno, C.; Rodríguez-Martín, B.; García-Ortiz, L.; Gómez-Marcos, M.; Martínez-Vizcaíno, V. Effectiveness of brief interventions in primary health care settings to decrease alcohol consumption by adult non-dependent drinkers: A systematic review of systematic reviews. Prev. Med. 2020, 76, S33–S38. [Google Scholar] [CrossRef]
- McKee, S.A.; Weinberger, A.H. How Can We Use Our Knowledge of Alcohol-Tobacco Interactions to Reduce Alcohol Use? Annu. Rev. Clin. Psychol. 2013, 9, 649–674. [Google Scholar] [CrossRef] [Green Version]
- Verplaetse, T.L.; McKee, S.A. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am. J. Drug Alcohol Abus. 2017, 43, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Bold, K.W.; Rosen, R.L.; Steinberg, M.L.; Epstein, E.E.; McCrady, B.S.; Williams, J.M. Smoking characteristics and alcohol use among women in treatment for alcohol use disorder. Addict. Behav. 2019, 101, 106137. [Google Scholar] [CrossRef]
- Hufnagel, A.; Frick, U.; Ridinger, M.; Wodarz, N. Recovery from alcohol dependence: Do smoking indicators predict abstinence? Am. J. Addict. 2017, 26, 366–373. [Google Scholar] [CrossRef]
- Eastwood, B.; Clare, T.; Dockrell, M.J.; Locker, J.; Chowdary, Q.; Jahr, S.; Jones, A.; Robson, D.; Marsden, J. Reciprocal influences of tobacco use on illicit opioid and alcohol use during the first six-months of specialist addiction treatment. Drug Alcohol Depend. 2021, 218, 108418. [Google Scholar] [CrossRef]
- Thurgood, S.L.; McNeill, A.; Clark-Carter, D.; Brose, L.S. A Systematic Review of Smoking Cessation Interventions for Adults in Substance Abuse Treatment or Recovery. Nicotine Tob. Res. 2016, 18, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Li, B.; Jiang, Y.; Guan, Y.; Wang, G.; Zhao, G. Smoking cessation mutually facilitates alcohol drinking cessation among tobacco and alcohol co-users: A cross-sectional study in a rural area of Shanghai, China. Tob. Induc. Dis. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Gaertner, B.; Haberecht, K.; Bischof, G.; John, U.; Freyer-Adam, J. How alcohol use problem severity affects the outcome of brief intervention delivered in-person versus through computer-generated feedback letters. Drug Alcohol Depend. 2018, 183, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Freyer-Adam, J.; Baumann, S.; Haberecht, K.; Tobschall, S.; Schnuerer, I.; Bruss, K.; Bandelin, E.; John, U.; Gaertner, B. In-person and computer-based alcohol interventions at general hospitals: Reach and retention. Eur. J. Public Health 2016, 26, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freyer-Adam, J.; Baumann, S.; Haberecht, K.; Tobschall, S.; Bischof, G.; John, U.; Gaertner, B. In-person alcohol counseling versus computer-generated feedback: Results from a randomized controlled trial. Health Psychol. 2018, 37, 70–80. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. 2012. Available online: www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 29 June 2021).
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT Alcohol Consumption Questions (AUDIT-C). Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Reinert, D.F.; Allen, J.P. The Alcohol Use Disorders Identification Test: An Update of Research Findings. Alcohol. Clin. Exp. Res. 2007, 31, 185–199. [Google Scholar] [CrossRef]
- Donovan, D.M.; Kivlahan, D.R.; Doyle, S.R.; Longabaugh, R.; Greenfield, S.F. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction 2006, 101, 1696–1704. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Moyer, A.; Finney, J.W.; Swearingen, C.E.; Vergun, P. Brief interventions for alcohol problems: A meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction 2002, 97, 279–292. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The Transtheoretical Model of Health Behavior Change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Noar, S.M.; Benac, C.N.; Harris, M.S. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol. Bull. 2007, 133, 673–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, T.L.; Joseph, J.; Yardley, L.; Michie, S. Using the Internet to Promote Health Behavior Change: A Systematic Review and Meta-analysis of the Impact of Theoretical Basis, Use of Behavior Change Techniques, and Mode of Delivery on Efficacy. J. Med Internet Res. 2010, 12, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.; Rollnick, S. Motivational Interviewing. Preparing People for Change; The Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Mchorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Berwick, D.M.; Murphy, J.M.; Goldman, P.A.; Ware, J.E.; Barsky, A.J.; Weinstein, M.C. Performance of a Five-Item Mental Health Screening Test. Med Care 1991, 29, 169–176. [Google Scholar] [CrossRef]
- Rumpf, H.-J.; Meyer, C.; Hapke, U.; John, U. Screening for mental health: Validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 2001, 105, 243–253. [Google Scholar] [CrossRef]
- DiClemente, C.C.; Prochaska, J.O.; Fairhurst, S.K.; Velicer, W.F.; Velasquez, M.M.; Rossi, J.S. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. J. Consult. Clin. Psychol. 1991, 59, 295–304. [Google Scholar] [CrossRef]
- Lippke, S.; Ziegelmann, J.P.; Schwarzer, R.; Velicer, W.F. Validity of stage assessment in the adoption and maintenance of physical activity and fruit and vegetable consumption. Health Psychol. 2009, 28, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X. Structural Equation Modeling: Applications Using Mplus; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Preacher, K.J. Latent growth curve models. In The Reviewer’s Guide to Quantitative Methods in the Social Sciences; Hancok, G.R., Mueller, R.O., Eds.; Taylor & Francis: New York, NY, USA, 2010. [Google Scholar]
- Johnson, P.O.; Neyman, J. Tests of certain linear hypotheses and their application to some educational problems. Stat. Res. Mem. 1936, 1, 57–93. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 6th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2011. [Google Scholar]
- Little, R.J.; Rubin, D.B. Statistical Analysis with Missing Data, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Marks, J.L.; Hill, E.M.; Pomerleau, C.S.; Mudd, S.A.; Blow, F.C. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J. Subst. Abus. Treat. 1997, 14, 521–527. [Google Scholar] [CrossRef]
- Weinberger, A.H.; Pacek, L.R.; Giovenco, D.; Galea, S.; Zvolensky, M.J.; Gbedemah, M.; Goodwin, R.D. Cigarette Use Among Individuals with Alcohol Use Disorders in the United States, 2002 to 2016: Trends Overall and by Race/Ethnicity. Alcohol. Clin. Exp. Res. 2019, 43, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tauchmann, H.; Lenz, S.; Requate, T.; Schmidt, C.M. Tobacco and alcohol: Complements or substitutes? Empir. Econ. 2012, 45, 539–566. [Google Scholar] [CrossRef]
- Baumann, S.; Gaertner, B.; Haberecht, K.; Meyer, C.; Rumpf, H.-J.; John, U.; Freyer-Adam, J. Does impaired mental health interfere with the outcome of brief alcohol intervention at general hospitals? J. Consult. Clin. Psychol. 2017, 85, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, L.R.; McClure, J.B.; Cofta-Woerpel, L.; Mazas, C.A.; Cao, Y.; Cinciripini, P.M.; Vidrine, J.I.; Li, Y.; Wetter, D. The Efficacy of Computer-Delivered Treatment for Smoking Cessation. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1555–1557. [Google Scholar] [CrossRef] [Green Version]
- Rooke, S.; Thorsteinsson, E.; Karpin, A.; Copeland, J.; Allsop, D. Computer-delivered interventions for alcohol and tobacco use: A meta-analysis. Addiction 2010, 105, 1381–1390. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, R.E.; Klesges, L.; Dzewaltowski, D.; Estabrooks, P.A.; Vogt, T.M. Evaluating the impact of health promotion programs: Using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ. Res. 2006, 21, 688–694. [Google Scholar] [CrossRef]
- Del Boca, F.K.; Darkes, J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction 2003, 98, 1–12. [Google Scholar] [CrossRef]
- Fagerström, K. Time to first cigarette; the best single indicator of tobacco dependence? Monaldi Arch. Chest Dis. 2003, 59, 91–94. [Google Scholar]

| Moderators | In-Person (n = 354) | Computer-Based (n = 386) | Assessment Only (n = 219) |
|---|---|---|---|
| Current smoking status | |||
| Non-smokers | 166 (46.9) | 179 (46.4) | 107 (48.9) |
| Never smoked | 76 (45.8) | 70 (39.1) | 38 (35.5) |
| Quit within the past 6 months | 15 (9.0) | 28 (15.6) | 10 (9.4) |
| Quit more than 6 months ago | 75 (45.2) | 81 (45.3) | 59 (55.1) |
| Smokers | 188 (53.1) | 207 (53.6) | 112 (51.1) |
| Daily | 158 (84.3) | 172 (83.1) | 87 (77.7) |
| Occasionally | 30 (15.7) | 35 (16.9) | 25 (22.3) |
| Number of cigarettes per day 0 | 171 (48.3) | 187 (48.5) | 113 (51.6) |
| 1–5 | 29 (8.2) | 35 (9.1) | 26 (11.9) |
| 6–10 | 41 (11.6) | 42 (10.9) | 12 (5.5) |
| 11–15 | 41 (11.6) | 43 (11.1) | 21 (9.6) |
| 16–20 | 44 (12.4) | 50 (13.0) | 31 (14.2) |
| 21–25 | 14 (3.9) | 15 (3.9) | 8 (3.7) |
| 26–30 | 8 (2.3) | 5 (1.3) | 5 (2.3) |
| 31–34 | 1 (0.3) | 0 (0) | 0 (0) |
| 35 | 5 (1.4) | 9 (2.3) | 3 (1.4) |
| Mean number of cigarettes per day, (SD) | 7.5 (9.5) | 7.6 (9.8) | 7.1 (9.9) |
| Month 6 | Month 12 | Month 18 | Month 24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Group | IRR | 95% CI | p | IRR | 95% CI | p | IRR | 95% CI | p | IRR | 95% CI | p |
| In-person BAI | ||||||||||||
| Smokers | 0.61 | 0.48–0.76 | <0.001 | 0.45 | 0.32–0.64 | <0.001 | 0.42 | 0.29–0.60 | <0.001 | 0.48 | 0.34–0.69 | <0.001 |
| Non-smokers | 0.57 | 0.44–0.72 | <0.001 | 0.41 | 0.28–0.59 | <0.001 | 0.41 | 0.28–0.61 | <0.001 | 0.58 | 0.40–0.85 | 0.005 |
| Computer-based BAI | ||||||||||||
| Smokers | 0.51 | 0.39–0.64 | <0.001 | 0.36 | 0.25–0.52 | <0.001 | 0.36 | 0.24–0.53 | <0.001 | 0.49 | 0.33–0.73 | <0.001 |
| Non-smokers | 0.49 | 0.38–0.64 | <0.001 | 0.35 | 0.23–0.51 | <0.001 | 0.34 | 0.23–0.52 | <0.001 | 0.48 | 0.32–0.73 | 0.001 |
| Assessment only | ||||||||||||
| Smokers | 0.61 | 0.46–0.81 | 0.001 | 0.48 | 0.32–0.73 | 0.001 | 0.49 | 0.32–0.76 | 0.001 | 0.65 | 0.41–1.03 | 0.068 |
| Non-smokers | 0.68 | 0.51–0.90 | 0.008 | 0.54 | 0.35–0.83 | 0.005 | 0.50 | 0.32–0.78 | 0.003 | 0.54 | 0.35–0.83 | 0.005 |
| Month 6 | Month 12 | Month 18 | Month 24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Group | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | p |
| PE versus AO | 1.24 | 0.35–1.70 | 1.24 | 0.78–2.00 | 1.02 | 0.65–1.67 | 0.69 | 0.40–1.13 | 0.156 |
| CO versus AO | 1.14 | 0.83–1.55 | 1.15 | 0.72–1.82 | 1.05 | 0.64–1.69 | 0.85 | 0.49–1.38 | 0.537 |
| PE versus CO | 1.09 | 0.82–1.45 | 1.08 | 0.72–1.67 | 0.98 | 0.64–1.55 | 0.81 | 0.52–1.29 | 0.363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krolo, F.; Baumann, S.; Tiede, A.; Bischof, G.; Krause, K.; Meyer, C.; John, U.; Gaertner, B.; Freyer-Adam, J. The Role of Tobacco Smoking in the Efficacy of Brief Alcohol Intervention: Results from a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 5847. https://doi.org/10.3390/ijerph19105847
Krolo F, Baumann S, Tiede A, Bischof G, Krause K, Meyer C, John U, Gaertner B, Freyer-Adam J. The Role of Tobacco Smoking in the Efficacy of Brief Alcohol Intervention: Results from a Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(10):5847. https://doi.org/10.3390/ijerph19105847
Chicago/Turabian StyleKrolo, Filipa, Sophie Baumann, Anika Tiede, Gallus Bischof, Kristian Krause, Christian Meyer, Ulrich John, Beate Gaertner, and Jennis Freyer-Adam. 2022. "The Role of Tobacco Smoking in the Efficacy of Brief Alcohol Intervention: Results from a Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 10: 5847. https://doi.org/10.3390/ijerph19105847






