An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
2.3. In-Depth Interviews
3. Results
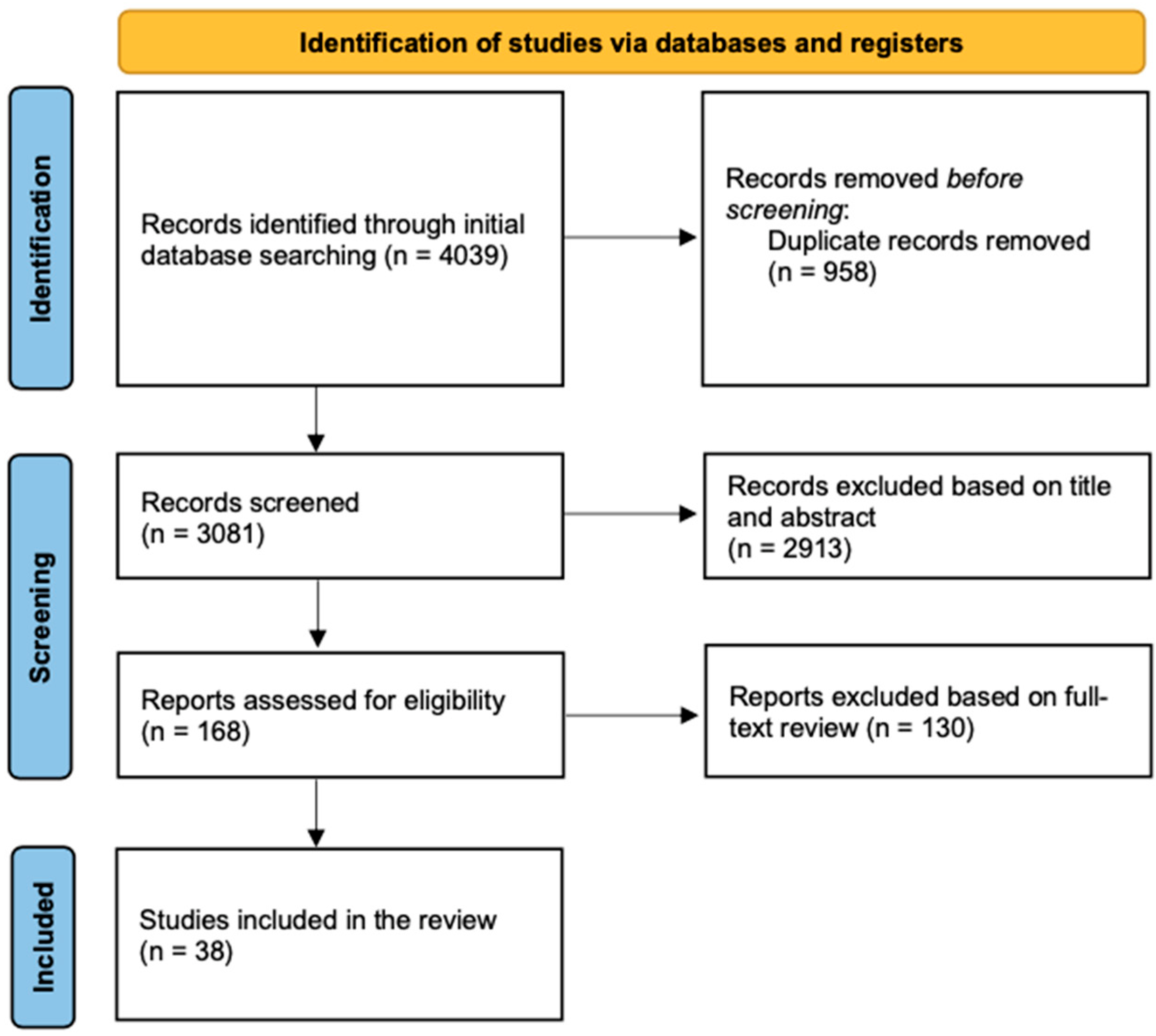
3.1. Studies Included
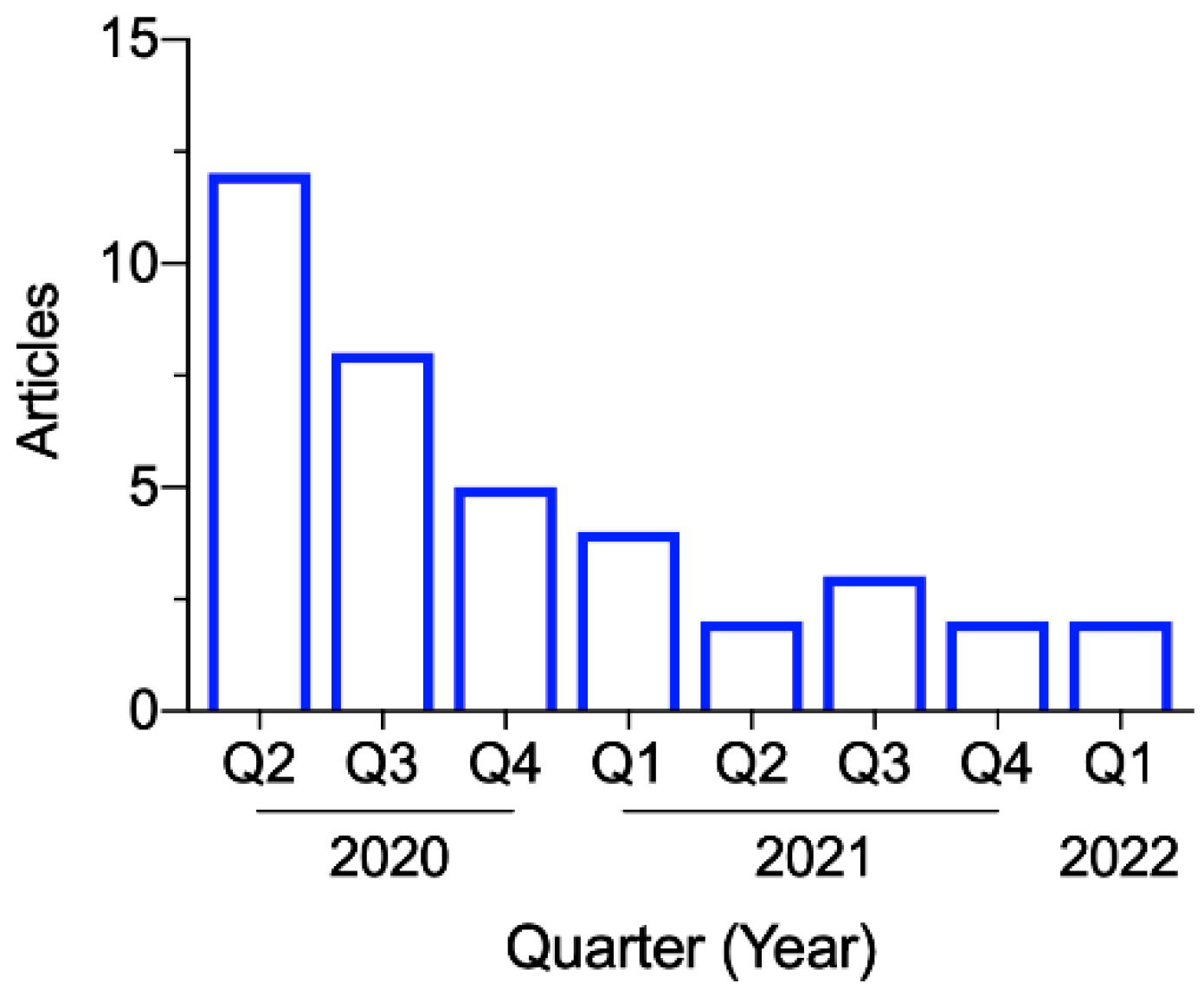
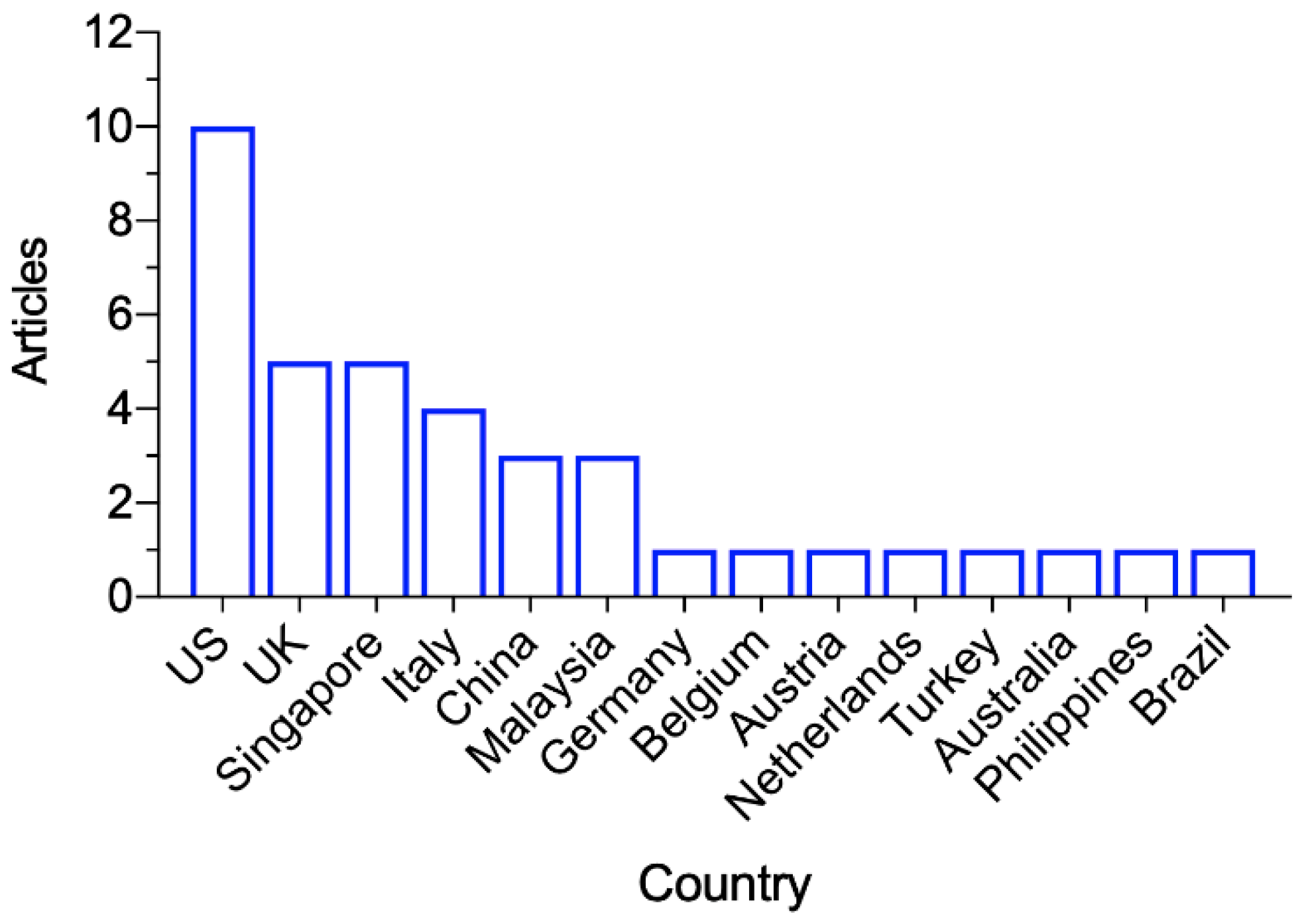
3.2. Study Characteristics
3.3. Risk-of-Bias Assessment
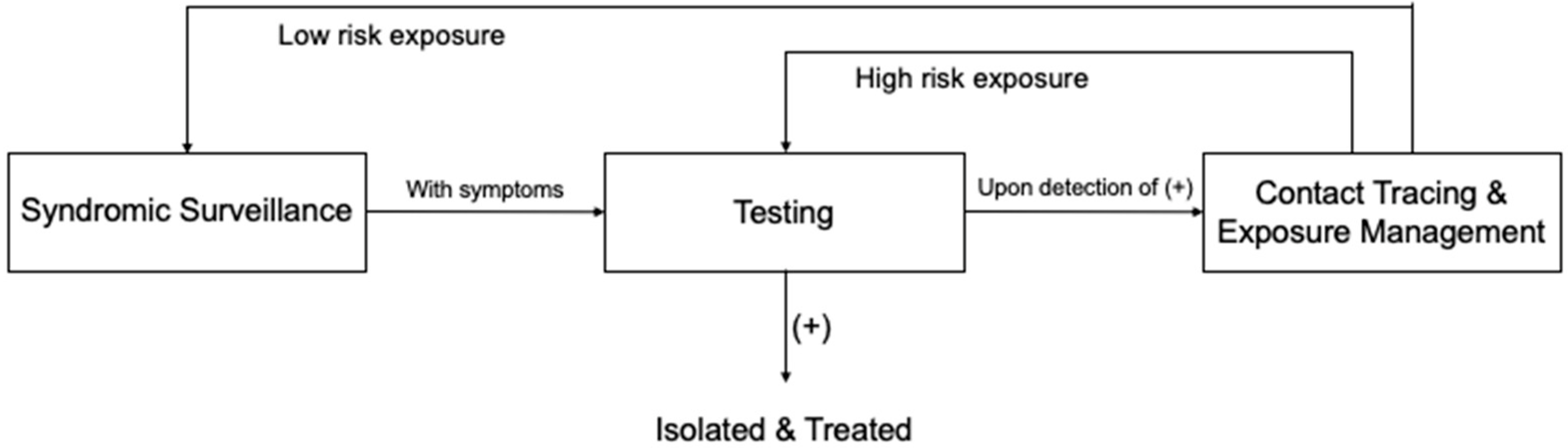
3.4. A Whole-Process Workflow Framework
3.4.1. Syndromic Surveillance
3.4.2. Testing
3.4.3. Contact Tracing and Exposure Management
4. Discussion
4.1. Future Directions
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Chen, Z.; Azman, A.S.; Deng, X.; Sun, R.; Zhao, Z.; Zheng, N.; Chen, X.; Lu, W.; Zhuang, T. Serological evidence of human infection with SARS-CoV-2: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e598–e609. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int (accessed on 1 May 2022).
- Treibel, T.A.; Manisty, C.; Burton, M.; McKnight, A.; Lambourne, J.; Augusto, J.B.; Couto-Parada, X.; Cutino-Moguel, T.; Noursadeghi, M.; Moon, J.C. COVID-19: PCR screening of asymptomatic healthcare workers at London hospital. Lancet 2020, 395, 1608–1610. [Google Scholar] [CrossRef]
- Aghaizu, A.; Elam, G.; Ncube, F.; Thomson, G.; Szilágyi, E.; Eckmanns, T.; Poulakou, G.; Catchpole, M. Preventing the next ‘SARS’-European healthcare workers’ attitudes towards monitoring their health for the surveillance of newly emerging infections: Qualitative study. BMC Public Health 2011, 11, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielicki, J.A.; Duval, X.; Gobat, N.; Goossens, H.; Koopmans, M.; Tacconelli, E.; van der Werf, S. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect. Dis. 2020, 20, E261–E267. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, M.H.; Shen, C.W.; Hsieh, M.H.; Wu, L.K.; Chen, L.C.; Cheng, T.J.; Chen, L.S.; Tsai, J.R.; Hsiao, S.H. Secure Health Care Workers’ Health and Safety Methodically during COVID-19 Epidemic in Taiwan. Asia-Pac. J. Public Health 2020, 32, 485–488. [Google Scholar] [CrossRef]
- Venkatachalam, I.; Conceicao, E.P.; Aung, M.K.; How, M.K.B.; Wee, L.E.; Sim, J.X.Y.; Tan, B.H.; Ling, M.L. Healthcare workers as a sentinel surveillance population in the early phase of the COVID-19 pandemic. Singap. Med. J. 2021, 1, 21. [Google Scholar] [CrossRef]
- WHO. Prevention, Identification and Management of Health Worker Infection in the Context of COVID-19. 2020. Available online: https://www.who.int/publications/i/item/10665-336265 (accessed on 22 February 2022).
- WHO. Surveillance Protocol for SARS-CoV-2 Infection among Health Workers. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-HCW_Surveillance_Protocol-2020.1 (accessed on 22 February 2022).
- CDC. Case Investigation and Contact Tracing: Part of a Multipronged Approach to Fight the COVID-19 Pandemic. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/principles-contact-tracing.html (accessed on 22 February 2022).
- Chong, D.W.Q.; Jayaraj, V.J.; Rampal, S.; Said, M.A.; Farid, N.D.N.; Zaki, R.A.; Hairi, N.N.; Hoe, V.C.W.; Isahak, M.; Ponnampalavanar, S.; et al. Establishment of a hospital-based health care workers surveillance programme to keep them safe during the COVID-19 pandemic. J. Glob. Health 2020, 10, 0203100. [Google Scholar] [CrossRef]
- McMichael, T.M.; Currie, D.W.; Clark, S.; Pogosjans, S.; Kay, M.; Schwartz, N.G.; Lewis, J.; Baer, A.; Kawakami, V.; Lukoff, M.D.; et al. Epidemiology of COVID-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020, 382, 2005–2011. [Google Scholar] [CrossRef]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: A cross-sectional study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [Green Version]
- Lüers, J.-C.; Klußmann, J.P.; Guntinas-Lichius, O. The COVID-19 pandemic and otolaryngology: What it comes down to? Laryngorhinootologie 2020, 99, 287–291. [Google Scholar]
- Black, J.R.M.; Bailey, C.; Przewrocka, J.; Dijkstra, K.K.; Swanton, C. COVID-19: The case for health-care worker screening to prevent hospital transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef]
- Boustead, K.; McDowall, K.; Baker, K.F.; Pareja-Cebrian, L.; Gibson, L.; Cunningham, M.; Murphy, E. Establishing a healthcare worker screening programme for COVID-19. Occup. Med. 2020, 70, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Vedala, K.; Swaim, S.; Welch, S.; Calendar, A.; Kakkera, K.; Khasawneh, K.; Kamoga, R. Identifying asymptomatic healthcare workers with COVID-19 in a community hospital: An institution’s experience. J. Community Hosp. Int. 2020, 10, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Buchtele, N.; Rabitsch, W.; Knaus, H.A.; Wohlfarth, P. Containment of a traceable COVID-19 outbreak among healthcare workers at a hematopoietic stem cell transplantation unit. Bone Marrow Transpl. 2020, 55, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wang, J.; Zong, L.; Zhang, J.; Sun, H.; Walline, J.H.; Sun, P.; Xu, S.; Li, Y.; Wang, C.; Liu, J. Identifying the effects of an upgraded ‘fever clinic’on COVID-19 control and the workload of emergency department: Retrospective study in a tertiary hospital in China. BMJ Open 2020, 10, e039177. [Google Scholar] [CrossRef]
- Zhang, H.P.; Dimitrov, D.; Simpson, L.; Plaks, N.; Singh, B.; Penney, S.; Charles, J.; Sheehan, R.; Flammini, S.; Murphy, S.; et al. A Web-Based, Mobile-Responsive Application to Screen Health Care Workers for COVID-19 Symptoms: Rapid Design, Deployment, and Usage. JMIR Form. Res. 2020, 4, e19533. [Google Scholar] [CrossRef]
- Wee, L.E.; Sim, X.Y.J.; Conceicao, E.P.; Aung, M.K.; Goh, J.Q.; Yeo, D.W.T.; Gan, W.H.; Chua, Y.Y.; Wijaya, L.; Tan, T.T.; et al. Containment of COVID-19 cases among healthcare workers: The role of surveillance, early detection, and outbreak management. Infect. Control Hosp. Epidemiol. 2020, 41, 765–771. [Google Scholar] [CrossRef]
- Garzaro, G.; Clari, M.; Ciocan, C.; Grillo, E.; Mansour, I.; Godono, A.; Borgna, L.G.; Sciannameo, V.; Costa, G.; Raciti, I.M.; et al. COVID-19 infection and diffusion among the healthcare workforce in a large university-hospital in northwest Italy. Med. Lav. 2020, 111, 184–194. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuan, J.T.; Tay, M.Z.; Lim, D.W.; Htun, H.L.; Kyaw, W.M.; Lee, L.T.; Ang, B.; Chow, A. Dancing with COVID-19 after the Hammer is Lifted: Enhancing Healthcare Worker Surveillance. J. Infect. 2020, 81, E13–E15. [Google Scholar] [CrossRef]
- Coppeta, L.; Somma, G.; Ippoliti, L.; Ferrari, C.; D’Alessandro, I.; Pietroiusti, A.; Aurilio, M.T. Contact Screening for Healthcare Workers Exposed to Patients with COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 9082. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Wong, S.C.; Tong, D.W.; Chuang, V.W.; Chen, J.H.; Lee, L.L.; To, K.K.; Hung, I.F.; Ho, P.L.; Yeung, D.T.; et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.; Hittel, N.; Nienhaus, A. Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers-A Cost Model. Int. J. Environ. Res. Public Health 2021, 18, 10767. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Herigon, J.C.; Uptegraft, C.; Samuel, B.; Brown, D.L.; Bickel, J.; Hron, J.D. Use of clinical data to augment healthcare worker contact tracing during the COVID-19 pandemic. J. Am. Med. Inform. Assoc. 2021, 29, 142–148. [Google Scholar] [CrossRef]
- Cordioli, M.; Mirandola, M.; Gios, L.; Gaspari, S.; Carelli, M.; Lotti, V.; Sandri, A.; Vicentini, C.; Gibellini, D.; Carrara, E.; et al. COVID-19 seroprevalence amongst healthcare workers: Potential biases in estimating infection prevalence. Epidemiol. Infect. 2022, 150, E48. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.; Price, D.A.; Murphy, E.; van der Loeff, I.S.; Baker, K.F.; Lendrem, D.; Lendrem, C.; Schmid, M.L.; Pareja-Cebrian, L.; Welch, A.; et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020, 395, E77–E78. [Google Scholar] [CrossRef]
- Rivett, L.; Sridhar, S.; Sparkes, D.; Routledge, M.; Jones, N.K.; Forrest, S.; Young, J.; Pereira-Dias, J.; Hamilton, W.L.; Ferris, M.; et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife 2020, 9, e58728. [Google Scholar] [CrossRef]
- Khalil, A.; Hill, R.; Ladhani, S.; Pattisson, K.; O’Brien, P. COVID-19 screening of health-care workers in a London maternity hospital. Lancet Infect. Dis. 2021, 21, 23–24. [Google Scholar] [CrossRef]
- Flynn, E.F.; Kuhn, E.; Shaik, M.; Tarr, E.; Scattolini, N.; Ballantine, A. Drive-Through COVID-19 Testing during the 2020 Pandemic: A Safe, Efficient, and Scalable Model for Pediatric Patients and Health Care Workers. Acad. Pediatr. 2020, 20, 753–755. [Google Scholar] [CrossRef]
- Yombi, J.C.; De Greef, J.; Marsin, A.S.; Simon, A.; Rodriguez-Villalobos, H.; Penaloza, A.; Belkhir, L. Symptom-based screening for COVID-19 in healthcare workers: The importance of fever. J. Hosp. Infect. 2020, 105, 428–429. [Google Scholar] [CrossRef]
- Blain, H.; Rolland, Y.; Tuaillon, E.; Giacosa, N.; Albrand, M.; Jaussent, A.; Benetos, A.; Miot, S.; Bousquet, J. Efficacy of a Test-Retest Strategy in Residents and Health Care Personnel of a Nursing Home Facing a COVID-19 Outbreak. J. Am. Med. Dir. Assoc. 2020, 21, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.M.G.; Lazaro, J.; Sayo, A.R.; Han, S.M.; Ukawa, T.; Suzuki, S.; Takaya, S.; Telan, E.; Solante, R.; Ariyoshi, K.; et al. COVID-19 Screening for Healthcare Workers in a Tertiary Infectious Diseases Referral Hospital in Manila, the Philippines. Am. J. Trop. Med. Hyg. 2020, 103, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Kacmaz, A.B.; Sumbul, B.; Bolukcu, S.; Okay, G.; Durdu, B.; Akkoyunlu, Y.; Meric Koc, M. Utility of Rapid Antibody Test for Screening COVID-19 among Healthcare Professionals. Bezmialem Sci. 2020, 8, 22–26. [Google Scholar] [CrossRef]
- Tong, X.; Ning, M.Z.; Huang, R.; Jia, B.; Yan, X.M.; Xiong, Y.L.; Wu, W.H.; Liu, J.C.; Chen, Y.X.; Wu, C. Surveillance of SARS-CoV-2 infection among frontline health care workers in Wuhan during COVID-19 outbreak. Immun. Inflamm. Dis. 2020, 8, 840–843. [Google Scholar] [CrossRef]
- Racine-Brzostek, S.E.; Yang, H.S.; Chadburn, A.; Orlander, D.; An, A.J.L.; Campion, T.R.; Yee, J.; Chen, Z.M.; Loda, M.; Zhao, Z.; et al. COVID-19 Viral and Serology Testing in New York City Health Care Workers. Am. J. Clin. Pathol. 2020, 154, 592–595. [Google Scholar] [CrossRef]
- Del Castillo, G.; Castrofino, A.; Grosso, F.; Barone, A.; Crottogini, L.; Toso, C.; Pellegrinelli, L.; Pariani, E.; Castaldi, S.; Cereda, D. COVID-19 serological testing for Healthcare Workers in Lombardy, Italy. Eur. J. Public Health 2020, 30, V131. [Google Scholar] [CrossRef]
- Domeracki, S.; Clapp, R.N.; Taylor, K.; Lu, C.Y.M.; Lampiris, H.; Blanc, P.D. Cycle Threshold to Test Positivity in COVID-19 for Return to Work Clearance in Health Care Workers. J. Occup. Environ. Med. 2020, 62, 889–891. [Google Scholar] [CrossRef]
- Buising, K.L.; Williamson, D.; Cowie, B.C.; MacLachlan, J.; Orr, E.; MacIsaac, C.; Williams, E.; Bond, K.; Muhi, S.; McCarthy, J.; et al. A hospital-wide response to multiple outbreaks of COVID-19 in health care workers: Lessons learned from the field. Med. J. Aust. 2020, 214, 101–104.e1. [Google Scholar] [CrossRef]
- Mullins, K.E.; Merrill, V.; Ward, M.; King, B.; Rock, P.; Caswell, M.; Ahlman, M.; Harris, A.D.; Christenson, R. Validation of COVID-19 serologic tests and large scale screening of asymptomatic healthcare workers. Clin. Biochem. 2021, 90, 23–27. [Google Scholar] [CrossRef]
- Fernandes, F.S.; Toniass, S.D.C.; Leitune, J.C.B.; Brum, M.C.B.; Leotti, V.B.; Dantas, F.F.; Chaves, E.B.M.; Joveleviths, D. COVID-19 among healthcare workers in a Southern Brazilian Hospital and evaluation of a diagnostic strategy based on the RT-PCR test and retest for SARS-CoV-2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3365–3374. [Google Scholar] [CrossRef]
- Kolwijck, E.; Brouwers-Boers, M.; Broertjes, J.; van Heeswijk, K.; Runderkamp, N.; Meijer, A.; Hermans, M.H.A.; Leenders, A. Validation and implementation of the Panbio COVID-19 Ag rapid test for the diagnosis of SARS-CoV-2 infection in symptomatic hospital healthcare workers. Infect. Prev. Pract. 2021, 3, 100142. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.; Heskin, J.; Randell, P.; Mughal, N.; Moore, L.S.; Jones, R.; Davies, G.W.; Rayment, M. Real-world evaluation of COVID-19 lateral flow device (LFD) mass-testing in healthcare workers at a London hospital; a prospective cohort analysis. J. Infect. 2021, 83, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Azami, N.A.M.; Murad, N.A.A.; Nawi, A.M.; Salleh, S.A.; Periyasamy, P.; Kori, N.; Hasan, M.R.; Ahmad, N.; Sulong, A.; Othman, H.; et al. COVID-19 in Malaysia: Exposure assessment and prevention practices among healthcare workers at a teaching hospital. J. Infect. Dev. Ctries. 2021, 15, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.I.; Conceicao, E.P.; Aung, M.K.; Aung, M.O.; Yong, Y.; Venkatachalam, I.; Sim, J.X.Y. Rostered routine testing for healthcare workers and universal inpatient screening: The role of expanded hospital surveillance during an outbreak of coronavirus disease 2019 (COVID-19) in the surrounding community. Infect. Control Hosp. Epidemiol. 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Swift, M.D.; Challener, D.W.; Berbari, E.F.; Tommaso, C.P.; Christopherson, D.R.; Binnicker, M.J.; Breeher, L.E. Utility of Follow-up COVID-19 Antigen Tests after Acute SARS-CoV-2 Infection among Healthcare Personnel. Clin. Infect. Dis. 2022, ciac235. [Google Scholar] [CrossRef]
- Ho, H.J.; Zhang, Z.X.Z.; Huang, Z.L.; Aung, A.H.; Lim, W.Y.; Chow, A. Use of a Real-Time Locating System for Contact Tracing of Health Care Workers during the COVID-19 Pandemic at an Infectious Disease Center in Singapore: Validation Study. J. Med. Internet Res. 2020, 22, e19437. [Google Scholar] [CrossRef]
- Ho, H.J.; Lim, W.Y.; Ang, B.; Chow, A. Use of surveillance technology to enhance exposure management for healthcare workers during the COVID-19 pandemic. J. Hosp. Infect. 2021, 107, 101–102. [Google Scholar] [CrossRef]
- Wan, K.S.; Tok, P.S.K.; Yoga Ratnam, K.K.; Aziz, N.; Isahak, M.; Ahmad Zaki, R.; Nik Farid, N.D.; Hairi, N.N.; Rampal, S.; Ng, C.W.; et al. Implementation of a COVID-19 surveillance programme for healthcare workers in a teaching hospital in an upper-middle-income country. PLoS ONE 2021, 16, e0249394. [Google Scholar] [CrossRef]
- Monsalud, C.F.L.; Lind, M.F.G.; Hines, C.M.; Schora, D.; Grant, J.; McElvania, E.; Singh, K. Mitigating staff shortages: Risk of permitting healthcare workers to return to work after coronavirus disease 2019 (COVID-19) exposure. Infect. Control Hosp. Epidemiol. 2021, 1–2. [Google Scholar] [CrossRef]
- Anelli, F.; Leoni, G.; Monaco, R.; Nume, C.; Rossi, R.C.; Marinoni, G.; Spata, G.; De Giorgi, D.; Peccarisi, L.; Miani, A.; et al. Italian doctors call for protecting healthcare workers and boosting community surveillance during COVID-19 outbreak. BMJ 2020, 368, m1254. [Google Scholar] [CrossRef]
- Treibel, T.A.; Manisty, C.; Andiapen, M.; Pade, C.; Jensen, M.; Fontana, M.; Couto-Parada, X.; Cutino-Moguel, T.; Noursadeghi, M.; Moon, J.C. Asymptomatic healthcare worker screening during the COVID-19 pandemic Reply. Lancet 2020, 396, 1394–1395. [Google Scholar] [CrossRef]
- Chow, A.; Htun, H.L.; Kyaw, W.M.; Lee, L.T.; Ang, B. Asymptomatic healthcare worker screening during the COVID-19 pandemic. Lancet 2020, 396, 1393–1394. [Google Scholar] [CrossRef]
- Chan, M.C.; Cho, T.J.; Chang, F.Y.; Lin, J.C. Surveillance for coronavirus diseases 2019 (COVID-19) among health care workers at a medical center in Taiwan, March to August 2020. J. Formos. Med. Assoc. 2021, 120, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- CDC. Centers for Disease Control. Interim Guidance for Antigen Testing for SARS-CoV-2. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 22 April 2022).
- Ibrahim, L.F.; Cheng, D.R.; Babl, F.E.; Bryant, P.A.; Crawford, N.W.; Daley, A.J.; Lewena, S.; McNab, S.; Noakes, K.; Steer, A.C.; et al. COVID-19 in health-care workers: Testing and outcomes at a Victorian tertiary children’s hospital. J. Paediatr. Child Health 2020, 56, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Contact tracing in the European Union: Public health management of persons, including healthcare workers, who have had contact with COVID-19 cases—fourth update. Available online: https://www.ecdc.europa.eu/en/covid-19-contact-tracing-public-health-management (accessed on 24 February 2022).
- NHC. The Prevention and Control Plan for COVID-19 (8th Edition). 2021. Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202105/6f1e8ec6c4a540d99fafef52fc86d0f8.shtml (accessed on 24 February 2022).
- Chirico, F.; Magnavita, N. The Crucial Role of Occupational Health Surveillance for Health-care Workers during the COVID-19 Pandemic. Workplace Health Saf. 2020, 69, 5–6. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Botelho-Nevers, E.; Launay, O. Are the conditions met to make COVID-19 vaccination mandatory for healthcare professionals? Infect. Dis. Now 2021, 51, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Günther, D. Contact Classification in COVID-19 Tracing. arXiv preprint 2020, arXiv:2008.00431. [Google Scholar]
- Agbehadji, I.E.; Awuzie, B.O.; Ngowi, A.B.; Millham, R.C. Review of big data analytics, artificial intelligence and nature-inspired computing models towards accurate detection of COVID-19 pandemic cases and contact tracing. Int. J. Environ. Res. Public Health 2020, 17, 5330. [Google Scholar] [CrossRef]
- Comelli, A.; Consonni, D.; Lombardi, A.; Viero, G.; Oggioni, M.; Bono, P.; Renteria, S.C.U.; Ceriotti, F.; Mangioni, D.; Muscatello, A.; et al. Nasopharyngeal Testing among Healthcare Workers (HCWs) of a Large University Hospital in Milan, Italy during Two Epidemic Waves of COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 8748. [Google Scholar] [CrossRef]
- Seto, W.H.; Conly, J.M.; Pessoa-Silva, C.L.; Malik, M.; Eremin, S. Infection prevention and control measures for acute respiratory infections in healthcare settings: An update. East. Mediterr. Health J. 2013, 19 (Suppl. S1), S39–S47. [Google Scholar] [CrossRef]
- Anand, R.V.; Prabhu, J.; Kumar, P.J.; Manivannan, S.S.; Rajendran, S.; Kumar, K.R.; Susi, S.; Jothikumar, R. IoT role in prevention of COVID-19 and health care workforces behavioural intention in India-an empirical examination. Int. J. Pervasive Comput. 2020, 16, 331–340. [Google Scholar] [CrossRef]
- Kumar, S.; Raut, R.D.; Narkhede, B.E. A proposed collaborative framework by using artificial intelligence-internet of things (AI-IoT) in COVID-19 pandemic situation for healthcare workers. Int. J. Healthc. Manag. 2020, 13, 337–345. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| No. | Study Characteristics and Summary Report | |
|---|---|---|
| 1 | Author | Zhang et al. [21] |
| Month/Year | April 2020 | |
| Country | US | |
| Study Type | Observational study | |
| Measures | Syndromic Surveillance: A web-based mobile responsive HCW symptom screening application | |
| Results | Over a 7-day period, having quickly identified 0.36% symptomatic HCWs that otherwise could have come to work, increasing efficiency and effectiveness | |
| 2 | Author | Hunter et al. [30] |
| Month/Year | April 2020 | |
| Country | UK | |
| Study Type | Observational study | |
| Measures | Testing: Testing of staff with compatible symptoms and conveying results rapidly via email | |
| Results | In 3 weeks, enabled 1414 (out of 1654) HCWs to return more rapidly to service | |
| 3 | Author | Treibel et al. [3] |
| Month/Year | May 2020 | |
| Country | UK | |
| Study Type | Observational study | |
| Measures | Testing: Testing the asymptomatic HCWs especially during potential new waves of infection | |
| Results | Asymptomatic HCWs should be given easy access to testing, especially during new waves of infection | |
| 4 | Author | Wee et al. [22] |
| Month/Year | May 2020 | |
| Country | Singapore | |
| Study Type | Observational study | |
| Measures | Syndromic Surveillance: Ongoing syndromic surveillance and centralized reporting of fever and ARI symptoms Testing: Testing the symptomatic HCWs if symptoms not resolve after 5 days Contact Tracing & Exposure Management: (1) Contact tracing conducted upon detection of a confirmed case; (2) Exposure risk assessment based on duration of contact, type of activity, and PPE use during the contact; (3) To test the exposed HCWs developing symptoms; to quarantine HCWs having significant unprotected exposure; to active monitor symptoms of the HCWs with low risk of exposure; | |
| Results | Over a 16-week period, 14 cases of HCW infection and 4 clusters detected After measures taken, zero nosocomial transmission detected Early detection having reduced quarantine of HCWs | |
| 5 | Author | Garzaro et al. [23] |
| Month/Year | May 2020 | |
| Country | Italy | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: HCWs identified as low risk of exposure to self-monitor symptoms including cough, fever, dyspnoea, anosmia; Testing: Early testing enabling faster return-to-work thus alleviating staff shortages; Contact Tracing & Exposure Management: (1) Fast identification of contacts with the infected critical to lowering nosocomial transmission; (2) A structured risk-management for HCW exposure: (i) stratifying risks into high risk: presenting symptoms; moderate risk: exposure >15 min, or <2 m, without PPE; low risk: <15 min, or >2 m, with PPE; (ii) high risk HCWs to get tested and home quarantined; moderate risk HCWs to use surgical masks while awaiting the test results; low risk HCWs to self-monitor symptoms; | |
| Results | The monitoring measures having significantly reduced time between exposure, warning, and testing (p < 0.001) | |
| 6 | Author | Rivett et al. [31] |
| Month/Year | May 2020 | |
| Country | UK | |
| Study Type | Observational Study | |
| Measures | Testing: Comprehensive testing of both symptomatic & asymptomatic HCWs | |
| Results | Data suggesting the true asymptomatic carriage rate being 0.5% Comprehensive testing of HCWs with minimal/no symptoms critical for protecting HCWs and patients | |
| 7 | Author | Khalil et al. [32] |
| Month/Year (Accepted) | May 2020 | |
| Country | UK | |
| Study Type | Observational Study | |
| Measures | Testing: Universal testing of HCWs | |
| Results | 34% positive HCW cases being asymptomatic while 59% symptomatic HCWs tested negative, indicating crucial needs for routine testing of all HCWs to (1) identify asymptomatic infected HCWs in an early stage, and (2) mitigate staff shortages due to unnecessary quarantine | |
| 8 | Author | Flynn et al. [33] |
| Month/Year | May 2020 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Testing: A drive-through testing model | |
| Results | The drive-through testing model having increased test efficiency, avoided long lines, conserved PPE | |
| 9 | Author | Buchtele et al. [18] |
| Month/Year | May 2020 | |
| Country | Austria | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: Extensive contact tracing implemented among HCWs caring for immunocompromised patients, with all those having face-to-face contact with the confirmed case since the case’s onset of symptoms to get tested regardless of length of exposure | |
| Results | Extensive contact tracing and mass testing having prevented further spread of nosocomial transmission | |
| 10 | Author | Ho et al. [50] |
| Month/Year | May 2020 | |
| Country | Singapore | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: RTLS-based (real-time location systems) contact tracing demonstrated having better validity than traditional EMR-based (electronic medical record) methods; | |
| Results | An integration of RTLS and EMR providing the best performance for contact tracing with a sensitivity of 77.8% and a specificity of 73.4% | |
| 11 | Author | Yombi et al. [34] |
| Month/Year | May 2020 | |
| Country | Belgium | |
| Study Type | Observational Study | |
| Measures | Testing: Fever as a criterion for testing | |
| Results | Fever having a positive impact on the yield of PCR for SARS-CoV-2 (p < 0.001), using fever as a selection criterion resulting in more efficient screening | |
| 12 | Author | Blain et al. [35] |
| Month/Year | June 2020 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Testing: A test-retest strategy | |
| Results | 11% asymptomatic HCWs with negative PCR results developing antibodies later in time Repeated testing effective in identifying asymptomatic infected HCWs | |
| 13 | Author | Wang et al. [24] |
| Month/Year | July 2020 | |
| Country | Singapore | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: A comprehensive HCW sickness surveillance system: online reporting platform, medical screening, and testing for all the symptomatic HCWs Contact Tracing & Exposure Management: Exposure factors: serving in COVID-19 area/in non-COVID-19 area with known close contacts/in non-COVID-19 area with no known close contacts | |
| Results | Despite enhanced monitoring mechanism, no HCW was identified with infection, suggesting universal testing of HCWs not necessary for hospitals with adequate PPE protocol | |
| 14 | Author | Villanueva et al. [36] |
| Month/Year | July 2020 | |
| Country | Philippines | |
| Study Type | Observational Study | |
| Measures | Testing: Criteria for testing: close contact with or high-risk exposure to a COVID-19 case, presence of symptoms Contact Tracing & Exposure Management: Categorizing exposure into high/medium/low risks based on duration of contact, PPE use, whether an aerosol generating procedure | |
| Results | Early screening for HCW infection having reduced nosocomial transmission | |
| 15 | Author | Mehta et al. [17] |
| Month/Year | July 2020 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: Aggressive contact tracing enabling the identification & monitoring of asymptomatic and/or pre-symptomatic HCWs | |
| Results | Aggressive and effective contact tracing providing greater yield than mass testing of every individual | |
| 16 | Author | Kacmaz et al. [37] |
| Month/Year | August 2020 | |
| Country | Turkey | |
| Study Type | Observational Study | |
| Measures | Testing: rapid antibody testing | |
| Results | Reliability of antibody testing needing further validation but useful in COVID-19 screening among HCWs to evaluate IPC measures and prevent intra-hospital infection | |
| 17 | Author | Tong et al. [38] |
| Month/Year | August 2020 | |
| Country | China (Mainland) | |
| Study Type | Observational Study | |
| Measures | Testing: A combination of PCR testing, serological testing, and radiological assessment conducted among HCWs caring for COVID-19 patients in the early stage of the outbreak | |
| Results | With the measures taken, no nosocomial infection detected | |
| 18 | Author | Racine-Brzostek et al. [39] |
| Month/Year | September 2020 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Testing: PCR + antibody testing | |
| Results | 100% PCR positive HCWs tested positive for antibody testing High rates of seroprevalence suggesting the need for expanded PCR testing for HCWs | |
| 19 | Author | Del Castillo et al. [40] |
| Month/Year | September 2020 | |
| Country | Italy | |
| Study Type | Observational Study | |
| Measures | Testing: Serological testing followed by PCR testing if positive to IgG | |
| Results | Serological IgG testing combined with PCR testing found to be a valid screening intervention | |
| 20 | Author | Ho et al. [51] |
| Month/Year | September 2020 | |
| Country | Singapore | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: Utility of surveillance technologies such as RTLS and CCTV systems to enhance HCW exposure management | |
| Results | 30 min), enabling more effective contact tracing than traditional methods | |
| 21 | Author | Chong et al. [11] |
| Month/Year | October 2020 | |
| Country | Malaysia | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: HCWs with identifiable exposure risk under daily syndromic surveillance (self-assessment and self-reporting of symptoms through an online system) for 14 days since last exposure to an infection Testing: Targeted testing of close contacts Contact Tracing & Exposure Management: (1) Intensive contact tracing with identified close contacts having their exposure assessed and grouped into high/medium/low risk based on duration of exposure, presence of symptoms, PPE use, and whether an aerosol-generating procedure; (2) All close contacts to get tested and under daily symptom surveillance for 14 days; (3) HCWs with high risk exposure to be quarantined for 14 days; with medium risk 7 days; with low risk 2 days of sick leave | |
| Results | In a period of 5 months, 2401 risk assessments carried out among 1408 HCWs The surveillance program having limited nosocomial transmission, with a cumulative incidence of HCW infection of 0.3% | |
| 22 | Author | Chen et al. [6] |
| Month/Year | November 2020 | |
| Country | China (Taiwan) | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: Centralized reporting of fever and ARI symptoms Testing: Testing the symptomatic Contact Tracing & Exposure Management: HCW exposure history reporting system | |
| Results | With the measures taken, no HCW infection detected | |
| 23 | Author | Domeracki et al. [41] |
| Month/Year | November 2020 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Testing: PCR cycle threshold (Ct) data used for HCW return to work (RTW) decisions | |
| Results | Initial Ct data significantly correlated with the time period between first diagnosis and RTW clearance (r = −0.80, p < 0.01), supplementing the dichotomized positive-or-negative PCR results | |
| 24 | Author | Buising et al. [42] |
| Month/Year | November 2020 | |
| Country | Australia | |
| Study Type | Observational Study | |
| Measures | Testing: Frequent testing of HCWs and patients in wards with outbreaks and quick turnaround time for test results | |
| Results | Rapid and accessible testing enabling real-time outbreak management | |
| 25 | Author | Coppeta et al. [25] |
| Month/Year | December 2020 | |
| Country | Italy | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: Exposed HCWs placed under an active syndromic surveillance program Contact Tracing & Exposure Management: Evaluating (1) distance from the infected, (2) duration of exposure, (3) the kind of medical service provided during the exposure, and (4) use of PPE | |
| Results | Typical symptoms presented in 92% HCW positive cases, but in only 33.3% negative cases (p < 0.01), suggesting symptoms being the best predictors of positive PCR results Close contact (within 2 m for more than 15 min) not statistically connected to contagion Use of mask significantly related to contagion (p < 0.01) | |
| 26 | Author | Mullins et al. [43] |
| Month/Year | January 2021 | |
| Country | US | |
| Study Type | Experimental Study | |
| Measures | Testing: Parallel orthogonal testing of (1) Ortho Vitros Test, a commercial immunodiagnostic system, and (2) UMMC ELISA, a manually developed ELISA for total SARS-CoV-2 antibodies and full-length spike ectodomain protein | |
| Results | Positive predictive value: Ortho Vitros (82.2%), UMMC ELISA (100%) Negative predictive value: Ortho Vitros (100%), UMMC ELISA (99.9%) Parallel orthogonal testing of both demonstrated to improve the predictive value (+: 100%, −: 100%) | |
| 27 | Author | Cheng et al. [26] |
| Month/Year | March 2021 | |
| Country | China (Hong Kong) | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: electronic syndromic surveillance system activated since the 1st imported case Testing: (1) PCR testing for symptomatic HCWs and HCWs classified as close contacts; (2) Repeated testing according to clinical assessment Contact Tracing & Exposure Management: (1) infection control team leading epidemiological investigation; (2) classifying the infected into hospital-acquired, community-acquired, and undetermined | |
| Results | Infection rate of HCWs (0.46‰) significantly lower than that of general population (0.71‰) (p < 0.01) No nosocomial transmission detected among HCWs | |
| 28 | Author | Monsalud et al. [53] |
| Month/Year | March 2021 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: (1) high-risk exposure HCWs (having participated in aerosol-generating procedures without adequate PPE; ongoing exposure to infected household members) required to self-quarantine and PCR testing; (2) low-risk exposure HCWs (all the other exposed HCWs) placed under surveillance | |
| Results | 7.6% low-risk exposure HCWs identified as PCR-positive | |
| 29 | Author | Wan et al. [52] |
| Month/Year | March 2021 | |
| Country | Malaysia | |
| Study Type | Observational Study | |
| Measures | Contact Tracing & Exposure Management: (1) contact tracing initiated once a COVID-19 case identified, collating info on the movement of the case 48 h before the onset of symptoms/diagnosis, forming a list of contacts; (2) level of risk of the contacts assessed and classified into different groups; (3) detailing management algorithm for low/medium/high-risk HCWs | |
| Results | Risk-based assessment with high sensitivity (100%) and specificity (72%) Risk categories and symptoms significantly correlated with positive cases (p < 0.001) | |
| 30 | Author | Fernandes et al. [44] |
| Month/Year | April 2021 | |
| Country | Brazil | |
| Study Type | Observational Study | |
| Measures | Testing: PCR testing for the symptomatic HCWs and, if negative, a 2nd PCR test after the 5th day since symptom onset | |
| Results | The 2nd PCR testing having detected 4.9% of the positive cases | |
| 31 | Author | Kolwijck et al. [45] |
| Month/Year | April 2021 | |
| Country | The Netherlands | |
| Study Type | Observational Study | |
| Measures | Testing: Antigen test for symptomatic HCWs, and (1) if tested positive, considered COVID-19 infection; (2) if tested negative, followed by PCR testing | |
| Results | The antigen-based testing strategy proved to be effective and easy to implement, with 72.5% sensitivity and 97% negative predictive value | |
| 32 | Author | Lamb et al. [46] |
| Month/Year | July 2021 | |
| Country | UK | |
| Study Type | Observational Study | |
| Measures | Testing: Mass antigen testing for HCWs, followed by PCR testing if antigen tested positive | |
| Results | Antigen testing proven to be an effective screening tool, with a positive predictive value of 94.21% | |
| 33 | Author | Azami et al. [47] |
| Month/Year | July 2021 | |
| Country | Malaysia | |
| Study Type | Observational Study | |
| Measures | Testing: PCR + serological testing Contact Tracing & Exposure Management: (1) Online questionnaire; (2) Evaluating risk based on HCWs’ occupational exposure and adherence to IPC practices | |
| Results | With measures taken, nosocomial infection having reduced, with an HCW infection rate of 0.5% | |
| 34 | Author | Wee et al. [48] |
| Month/Year | August 2021 | |
| Country | Singapore | |
| Study Type | Observational Study | |
| Measures | Testing: Rostered routine testing for HCWs + mass screening of all inpatients | |
| Results | Enhancing early identification and contact tracing for HCW cases Significantly reducing the time infected inpatients spent in the general ward prior to isolation (p < 0.01) | |
| 35 | Author | Diel et al. [27] |
| Month/Year | October 2021 | |
| Country | Germany | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: Exposed HCWs required to self-observe COVID-19-related symptoms Testing: Antigen testing every other day for exposed HCWs + additional PCR testing if one becoming symptomatic | |
| Results | Monitoring exposed HCWs with the measures in this study greatly reducing costs by 87.0%, compared with sending the exposed HCWs into quarantine | |
| 36 | Author | Hong et al. [28] |
| Month/Year | October 2021 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: HCWs confirmed with exposure registered for twice-a-day symptom monitoring for 14 days via email Contact Tracing & Exposure Management: Using electronic health record clinical event data (EHR report), in addition to traditional interviews, staff records, radio-frequency identification data, wifi access logs, bluetooth data, and etc. to enhance contact screening | |
| Results | 22.2% exposures detected by EHR report, which would have been neglected based on traditional contact tracing methods | |
| 37 | Author | Cordioli et al. [29] |
| Month/Year | February 2022 | |
| Country | Italy | |
| Study Type | Observational Study | |
| Measures | Syndromic Surveillance: Monitoring COVID-19 pathognomonic signs and symptoms Testing: Serological + PCR testing | |
| Results | Using a 3-diagnostic criterion (PCR + serological testing + pathognomonic presentation) to assess infection prevalence: COVID-19 prevalence varied based on different criterion: serological (6.7%), PCR (8.1%), serological/PCR (10.0%), pathognomonic presentation (9.6%), at least one of the above-mentioned criteria (17.6%) The probability of positive serological result decreasing by 1.1% every 10 days from the infection Data suggesting serological testing informative on infection susceptibility but not best for predicting previous infection | |
| 38 | Author | Tande et al. [49] |
| Month/Year | March2022 | |
| Country | US | |
| Study Type | Observational Study | |
| Measures | Testing: Rapid antigen test for infected HCWs who meet the criteria to return to work, on the 5th day (or later) since symptom onset/diagnosis of COVID-19 | |
| Results | The rapid antigen test, helpful to guide return-to-work decisions, having reduced isolation time by 2 days/person | |
| Author and Year | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. [21] April 2020 | Low | Low | Low | Low | Moderate | Low | Low | Low |
| Hunter et al. [30] April 2020 | Moderate | Moderate | Low | Low | Moderate | Moderate | Low | Moderate |
| Treibel et al. [3] May 2020 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Wee et al. [22] May 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Garzaro et al. [23] May 2020 | Low | Low | Low | Low | Low | Low | Low | Low |
| Rivett et al. [31] May 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Khalil et al. [32] May 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Low |
| Flynn et al. [33] May 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Buchtele et al. [18] May 2020 | Moderate | Moderate | Low | Low | Low | Low | Low | Low |
| Ho et al. [50] May 2020 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Low |
| Yombi et al. [34] May 2020 | Moderate | Moderate | Low | Low | Moderate | Low | Moderate | Moderate |
| Blain et al. [35] June 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Wang et al. [24] July 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Villanueva et al. [36] July 2020 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Low |
| Mehta et al. [17] July 2020 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Kacmaz et al. [37] August 2020 | Moderate | Moderate | Low | Low | Low | Moderate | Low | Low |
| Tong et al. [38] August 2020 | Moderate | Moderate | Low | Low | Low | Moderate | Low | Low |
| Racine-Brzostek et al. [39] September 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Del Castillo et al. [40] September 2020 | Moderate | Low | Low | Low | Moderate | Low | Moderate | Low |
| Ho et al. [51] September 2020 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Low |
| Chong et al. [11] October 2020 | Moderate | Moderate | Low | Low | Low | Low | Low | Low |
| Chen et al. [6] November 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Domeracki et al. [41] November 2020 | Moderate | Moderate | Low | Low | Low | Low | Low | Low |
| Buising et al. [42] November 2020 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Coppeta et al. [25] December 2020 | Moderate | Low | Low | Low | Low | Low | Moderate | Low |
| Mullins et al. [43] January 2021 | Low | Low | Low | Low | Low | Low | Low | Low |
| Cheng et al. [26] March 2021 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Monsalud et al. [53] March 2021 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Low |
| Wan et al. [52] March 2021 | Moderate | Low | Low | Low | Moderate | Low | Low | Low |
| Fernandes et al. [44] April 2021 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Kolwijck et al. [45] April 2021 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Lamb et al. [46] July 2021 | Moderate | Low | Low | Low | Moderate | Low | Low | Low |
| Azami et al. [47] July 2021 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Low |
| Wee et al. [48] August 2021 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Low |
| Diel et al. [27] October 201 | Low | Low | Low | Low | Low | Low | Low | Low |
| Hong et al. [28] October 2021 | Moderate | Low | Low | Low | Low | Low | Moderate | Low |
| Cordioli et al. [29] February 2022 | Low | Low | Low | Low | Low | Low | Low | Low |
| Tende et al. [49] March 2022 | Moderate | Low | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Guo, X.; Chen, Z.; Chen, Y. An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis. Int. J. Environ. Res. Public Health 2022, 19, 5943. https://doi.org/10.3390/ijerph19105943
Mei Y, Guo X, Chen Z, Chen Y. An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis. International Journal of Environmental Research and Public Health. 2022; 19(10):5943. https://doi.org/10.3390/ijerph19105943
Chicago/Turabian StyleMei, Yueli, Xiuyun Guo, Zhihao Chen, and Yingzhi Chen. 2022. "An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis" International Journal of Environmental Research and Public Health 19, no. 10: 5943. https://doi.org/10.3390/ijerph19105943
APA StyleMei, Y., Guo, X., Chen, Z., & Chen, Y. (2022). An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis. International Journal of Environmental Research and Public Health, 19(10), 5943. https://doi.org/10.3390/ijerph19105943






