To Be Active or to Stop? A Cross-Sectional Retrospective Study Exploring Provider Advice and Patient Fears Surrounding Physical Activity in Pregnancies Complicated by Fetal Growth Restriction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
- On average, how many days per week do you engage in moderate-to-vigorous physical activity (such as a brisk walk)?
- On average, how many minutes do you engage in physical activity at this level?
2.3. Data Analysis
3. Results
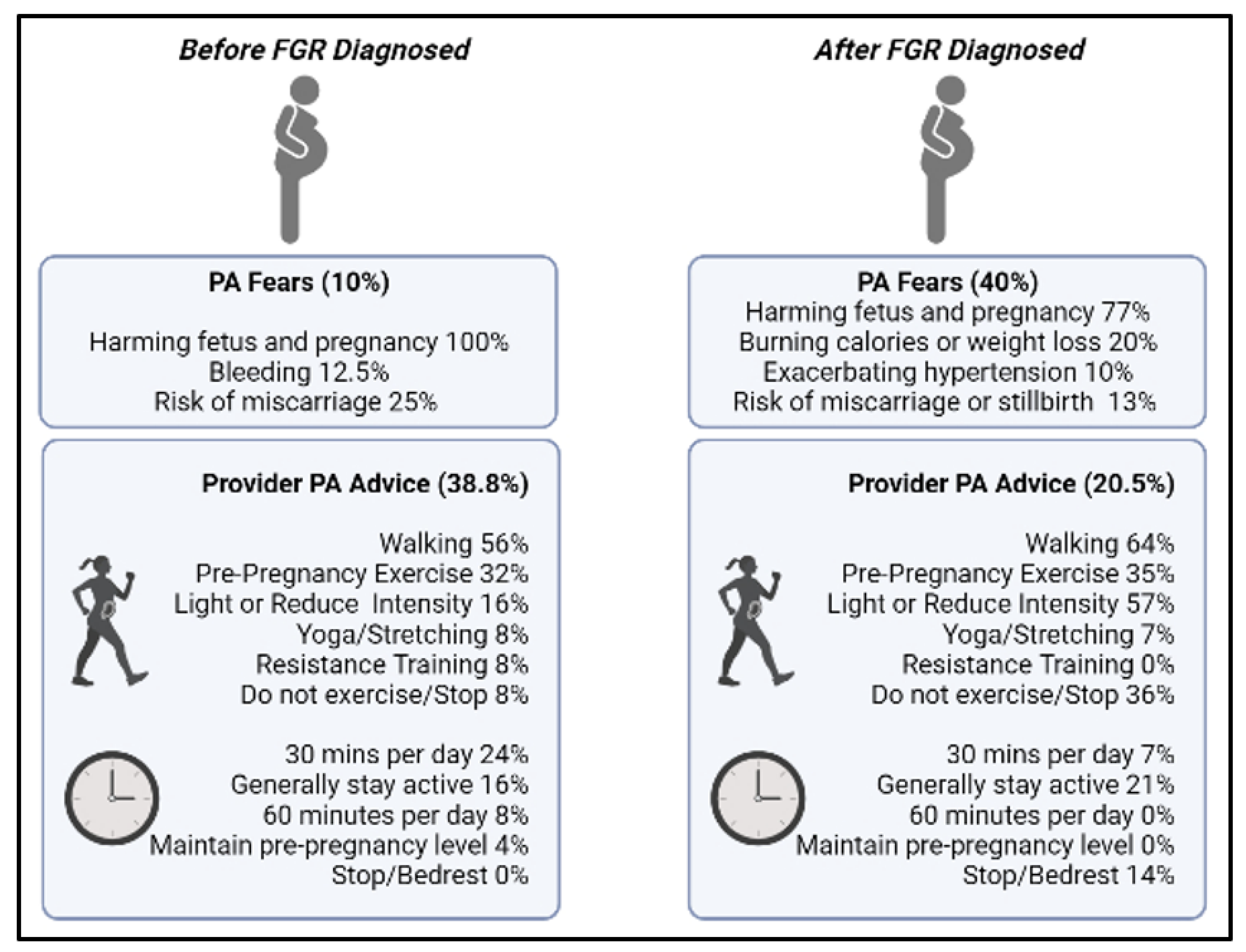
3.1. Provider Conversations Regarding Physical Activity during Pregnancy
3.2. Physical Activity Levels
3.3. Content Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albu, A.; Anca, A.; Horhoianu, V.; Horhoianu, I. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25408721 (accessed on 1 April 2022).
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134: Fetal growth restriction. Obstet. Gynecol. 2013, 121, 1122–1133. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Alberry, M.; Soothill, P. Management of fetal growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F62–F67. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction. Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Pineles, B.L.; Crimmins, S.; Turan, O. Timing of Delivery in Pregnancies Complicated by Suspected Fetal Growth Restriction without Doppler Abnormalities. Am. J. Perinatol. 2020, 37, 647–651. [Google Scholar] [CrossRef]
- Skow, R.J.; King, E.C.; Steinback, C.D.; Davenport, M.H. The influence of prenatal exercise and pre-eclampsia on maternal vascular function. Clin. Sci. 2017, 131, 2223–2240. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; De Plata, A.C.A.; Escudero, M.M.; Echeverry, I.; Ortega, J.G.; Salazar, B.; Rey, J.J.; Hormiga, C.; Lopez-Jaramillo, P. Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. J. Obstet. Gynaecol. Res. 2011, 37, 1601–1608. [Google Scholar] [CrossRef]
- de Oliveria Melo, A.S.; Silva, J.L.; Tavares, J.S.; Barros, V.O.; Leite, D.F.; Amorim, M.M. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012, 120, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Moyer, C.; Reoyo, O.R.; May, L. The Influence of Prenatal Exercise on Offspring Health: A Review. Clin. Med. Insights Women’s Health 2016, 9, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Meah, V.L.; Davies, G.A.; Davenport, M.H. Why can’t I exercise during pregnancy? Time to revisit medical ‘absolute’ and ‘relative’ contraindications: Systematic review of evidence of harm and a call to action. Br. J. Sports Med. 2020, 54, 1395–1404. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [Google Scholar] [CrossRef] [Green Version]
- AAbrar, A.; Fairbrother, N.; Smith, A.P.; Skoll, A.; Albert, A. Anxiety among women experiencing medically complicated pregnancy: A systematic review and meta-analysis. Birth 2019, 47, 13–20. [Google Scholar] [CrossRef]
- Fairbrother, N.; Young, A.; Zhang, A.; Janssen, P.; Antony, M.M. The prevalence and incidence of perinatal anxiety disorders among women experiencing a medically complicated pregnancy. Arch. Women’s Ment. Health 2017, 20, 311–319. [Google Scholar] [CrossRef]
- Winkel, S.; Einsle, F.; Pieper, L.; Höfler, M.; Wittchen, H.-U.; Martini, J. Associations of anxiety disorders, depressive disorders and body weight with hypertension during pregnancy. Arch. Women’s Ment. Health 2015, 18, 473–483. [Google Scholar] [CrossRef]
- Brown, H.K.; Wilton, A.S.; Ray, J.G.; Dennis, C.-L.; Guttmann, A.; Vigod, S.N. Chronic physical conditions and risk for perinatal mental illness: A population-based retrospective cohort study. PLoS Med. 2019, 16, e1002864. [Google Scholar] [CrossRef]
- Hennington, B.S.; Alexander, B.T. Linking intrauterine growth restriction and blood pressure: Insight into the human origins of cardiovascular disease. Circulation 2013, 128, 2179–2180. [Google Scholar] [CrossRef] [Green Version]
- Yawn, B.P.; Suman, V.J.; Jacobsen, S. Maternal Recall of Distant Pregnancy Events. J. Clin. Epidemiol. 1998, 51, 399–405. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Declaración de la Iniciativa STROBE (Strengthening the Reporting of Observational studies in Epidemiology): Directrices para la comunicación de estudios observacionales. Gac. Sanit. 2008, 22, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Golightly, Y.M.; Allen, K.D.; Ambrose, K.R.; Stiller, J.L.; Evenson, K.R.; Voisin, C.; Hootman, J.M.; Callahan, L.F. Physical Activity as a Vital Sign: A Systematic Review. Prev. Chronic Dis. 2017, 14, E123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borodulin, K.; Mäki-Opas, T.; Leino-Arjas, P.; Tammelin, T.H.; Heliövaara, M.; Martelin, T.; Kestilä, L.; Prättälä, R. Leisure time physical activity in a 22-year follow-up among Finnish adults. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guba, E.; Lincoln, Y. Competing paradigms in qualitative research. In Handbook of Qualitative Research, 1st ed.; SAGE Publications: Thousand Oaks, CA, USA, 1994; pp. 105–117. [Google Scholar]
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef]
- Powers, B.; Knapp, T. Dictionary of Nursing Theory and Research, 3rd ed.; Springer Publishing Company: New York, NY, USA, 2006. [Google Scholar]
- Okafor, U.; Goon, D. Physical Activity Advice and Counselling by Healthcare Providers: A Scoping Review. Healthcare 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., 3rd; Kim, H.; Burciu, B.; Schmidt, S.; Petry, K.; Lopez, B. Continuing regular exercise during pregnancy: Effect of exercise volume on fetoplacental growth. Am. J. Obstet. Gynecol. 2002, 186, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Meah, V.L.; Davies, G.A.; Davenport, M.H.; Siassakos, D. Modify, don’t stop! Time to reconsider the ‘relative’ and ‘absolute’ contraindications to physical activity in pregnancy: An opinion piece. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 331–335. [Google Scholar] [CrossRef]
- Bauer, I.; Hartkopf, J.; Kullmann, S.; Schleger, F.; Hallschmid, M.; Pauluschke-Fröhlich, J.; Fritsche, A.; Preissl, H. Spotlight on the fetus: How physical activity during pregnancy influences fetal health: A narrative review. BMJ Open Sport Exerc. Med. 2020, 6, e000658. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.C.; Verma, R.P. Fetal Growth Restriction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562268/ (accessed on 14 August 2021).
- Osuchukwu, O.O.; Reed, D.J. Small for Gestational Age. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563247/ (accessed on 23 January 2022).
- Borodulin, K.M.; Evenson, K.R.; Wen, F.; Herring, A.H.; Benson, A.M. Physical Activity Patterns during Pregnancy. Med. Sci. Sports Exerc. 2008, 40, 1901–1908. [Google Scholar] [CrossRef] [Green Version]
- Clapp, J.F. Long-term outcome after exercising throughout pregnancy: Fitness and cardiovascular risk. Am. J. Obstet. Gynecol. 2008, 199, 489.e1–489.e6. [Google Scholar] [CrossRef] [Green Version]
- Pathirathna, M.L.; Sekijima, K.; Sadakata, M.; Fujiwara, N.; Muramatsu, Y.; Wimalasiri, K.M.S. Effects of Physical Activity During Pregnancy on Neonatal Birth Weight. Sci. Rep. 2019, 9, 6000. [Google Scholar] [CrossRef]
- Watkins, V.Y.; Zhao, P.; Frolova, A.I.; Carter, E.B.; Kelly, J.C.; Odibo, A.O.; England, S.K.; Raghuraman, N. The impact of physical activity during pregnancy on fetal growth. Am. J. Obstet. Gynecol. 2022, 226, S376. [Google Scholar] [CrossRef]
- George, A.; Luz, R.F.; De Tychey, C.; Thilly, N.; Spitz, E. Anxiety symptoms and coping strategies in the perinatal period. BMC Pregnancy Childbirth 2013, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Beyuo, T.; Oppong, S.A.; Owusu, A.; Moyer, C.A.; Lawrence, E. Exploring self-blame and the perceived causes of preeclampsia in urban Ghana. Int. J. Gynecol. Obstet. 2021, 152, 280–281. [Google Scholar] [CrossRef]
- Sheen, K.; Spiby, H.; Slade, P. The experience and impact of traumatic perinatal event experiences in midwives: A qualitative investigation. Int. J. Nurs. Stud. 2016, 53, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, M.C. ‘To make it through each day still pregnant’: Pregnancy bed rest and the disciplining of the maternal body. J. Gend. Stud. 2011, 20, 209–221. [Google Scholar] [CrossRef]
- Forray, A.; Mayes, L.C.; Magriples, U.; Epperson, C.N. Prevalence of post-traumatic stress disorder in pregnant women with prior pregnancy complications. J. Matern. Neonatal Med. 2009, 22, 522–527. [Google Scholar] [CrossRef]
- Ben-Ami, I.; Maymon, R.; Svirsky, R.; Cuckle, H.; Jauniaux, E. Down Syndrome Screening in Assisted Conception Twins. Obstet. Gynecol. Surv. 2013, 68, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Tinius, R.; Nagpal, T.S.; Edens, K.; Duchette, C.; Blankenship, M. Exploring Beliefs about Exercise among Pregnant Women in Rural Communities. J. Midwifery Women’s Health 2020, 65, 538–545. [Google Scholar] [CrossRef]
- Whitaker, K.M.; Wilcox, S.; Liu, J.; Blair, S.N.; Pate, R.R. Pregnant women’s perceptions of weight gain, physical activity, and nutrition using Theory of Planned Behavior constructs. J. Behav. Med. 2016, 39, 41–54. [Google Scholar] [CrossRef]
- Haakstad, L.A.H.; Vistad, I.; Sagedal, L.R.; Lohne-Seiler, H.; Torstveit, M.K. How does a lifestyle intervention during pregnancy influence perceived barriers to leisure-time physical activity? The Norwegian fit for delivery study, a randomized controlled trial. BMC Pregnancy Childbirth 2018, 18, 127. [Google Scholar] [CrossRef] [Green Version]

| Characteristic (N = 78) | Mean ± SD or N (%) |
|---|---|
| Age (years) | 31.5 ± 5.0 (range 18–41) |
| Pre-pregnancy weight (lbs) | 157.9 ± 48.7 |
| Pre-pregnancy height (inches) | 64.0 ± 4.7 |
| Pre-pregnancy body mass index (kg/m2) | 26.8 ± 7.7 |
| Time of FGR diagnosis (gestation age in weeks) | 25.5 ± 5.9 |
| Parity | |
| Nulliparous | 38 (48.7) |
| Multiparous | 40 (51.2) |
| Race | |
| White | 54 (69.2) |
| Black | 3 (3.8) |
| Asian | 3 (3.8) |
| Missing | 18 (23.0) |
| Ethnicity | |
| Hispanic, Latino, Spanish origin | 2 (2.6) |
| Non-Hispanic | 59 (75.6) |
| Missing | 17 (21.8) |
| Educational Attainment | |
| No school | 1 (1.3) |
| High school diploma | 7 (9.0) |
| GED | 2 (2.6) |
| Some college credit | 16 (20.5) |
| Associate’s degree | 4 (5.1) |
| Bachelor’s degree | 17 (21.8) |
| Master’s degree | 12 (15.4) |
| Professional degree | 1 (1.3) |
| Doctorate degree | 2 (2.6) |
| Missing | 16 (20.5) |
| Gestation Age at Delivery (weeks) | 35.1 ± 4.8 |
| Infant Birthweight (g) | 1834 ± 752 |
| Infant Percentile at Birth (%) | |
| <3% | 48 (61.5) |
| 3–10% | 15 (19.2) |
| >10% | 9 (11.5) |
| UTD | 6 (7.7) |
| Pregnancy Complication * | N (%) |
| High blood pressure | 25 (32.1) |
| Oligohydraminos | 20 (25.6) |
| Anxiety/stress/depression | 18 (23.1) |
| Group B Strep | 11 (14.1) |
| Gestational diabetes | 10 (12.8) |
| Preterm labor | 2 (2.6) |
| Hyperemesis gravidarium | 1(1.3) |
| Fatigue | 1 (1.3) |
| Pain—low back, ankles | 1 (1.3) |
| Shingles | 1 (1.3) |
| Fetal Complication# | N (%) |
| Reverse fetal Doppler | 8 (10.3) |
| Absent fetal Doppler | 14 (17.9) |
| Abnormal BPP | 12 (15.4) |
| Abnormal NST | 11 (14.1) |
| Physical Activity Level (min/Week) | Estimated Fetal Weight (%) (Mean: 13.6 ± 27.7%) | Birthweight (Mean: 4.0 ± 1.3 lbs) |
|---|---|---|
| 1st Trimester | R = −0.148, p = 0.232 | R = 0.145, p = 0.252 |
| 2nd Trimester | R = −0.056, p = 0.651 | R = 0.266, p = 0.023 * |
| 3rd Trimester | R = −0.093, p = 0.470 | R = 0.179, p = 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinius, R.A.; Maples, J.M.; Schafer, M.A.; Paudel, A.; Fortner, K.B.; Zite, N.B.; Nagpal, T.S. To Be Active or to Stop? A Cross-Sectional Retrospective Study Exploring Provider Advice and Patient Fears Surrounding Physical Activity in Pregnancies Complicated by Fetal Growth Restriction. Int. J. Environ. Res. Public Health 2022, 19, 6076. https://doi.org/10.3390/ijerph19106076
Tinius RA, Maples JM, Schafer MA, Paudel A, Fortner KB, Zite NB, Nagpal TS. To Be Active or to Stop? A Cross-Sectional Retrospective Study Exploring Provider Advice and Patient Fears Surrounding Physical Activity in Pregnancies Complicated by Fetal Growth Restriction. International Journal of Environmental Research and Public Health. 2022; 19(10):6076. https://doi.org/10.3390/ijerph19106076
Chicago/Turabian StyleTinius, Rachel A., Jill M. Maples, Mark A. Schafer, Alissa Paudel, Kimberly B. Fortner, Nikki B. Zite, and Taniya S. Nagpal. 2022. "To Be Active or to Stop? A Cross-Sectional Retrospective Study Exploring Provider Advice and Patient Fears Surrounding Physical Activity in Pregnancies Complicated by Fetal Growth Restriction" International Journal of Environmental Research and Public Health 19, no. 10: 6076. https://doi.org/10.3390/ijerph19106076
APA StyleTinius, R. A., Maples, J. M., Schafer, M. A., Paudel, A., Fortner, K. B., Zite, N. B., & Nagpal, T. S. (2022). To Be Active or to Stop? A Cross-Sectional Retrospective Study Exploring Provider Advice and Patient Fears Surrounding Physical Activity in Pregnancies Complicated by Fetal Growth Restriction. International Journal of Environmental Research and Public Health, 19(10), 6076. https://doi.org/10.3390/ijerph19106076







