Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Microalgae and Culture Conditions
2.1.2. Reagents and Solvents
2.2. Methods
2.2.1. Chlorella vulgaris Biomass Characterization
2.2.2. Immobilization of Chlorella vulgaris Cells in Alginate Beads
2.2.3. Chlorella vulgaris Concentration Measurement
2.2.4. Determination of FLX Concentration
2.2.5. Determination of Nutrients (Total Phosphorus and Total Nitrogen) Concentration
2.2.6. Biosorption Studies
Kinetic Experiments
Equilibrium Experiments
Data and Statistical Analysis
2.2.7. Removal of FLX and Nutrients from Real Treated Municipal Wastewater
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Biomass Characterization
3.1.1. FT-IR Results
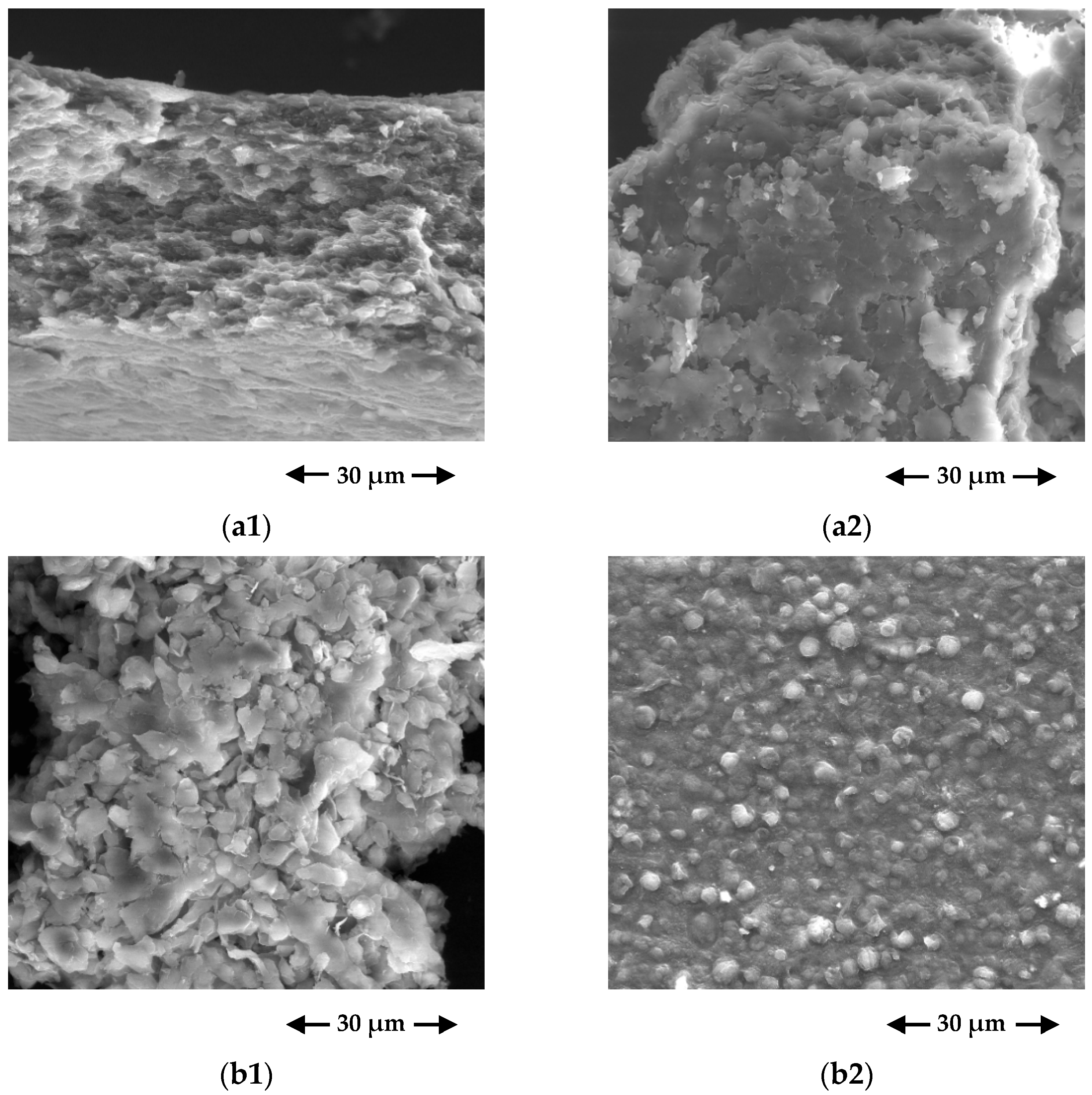
3.1.2. SEM/EDS Results
3.1.3. pHPZC and pH Effect Results
3.2. Kinetic and Equilibrium Studies
3.2.1. Kinetic Results
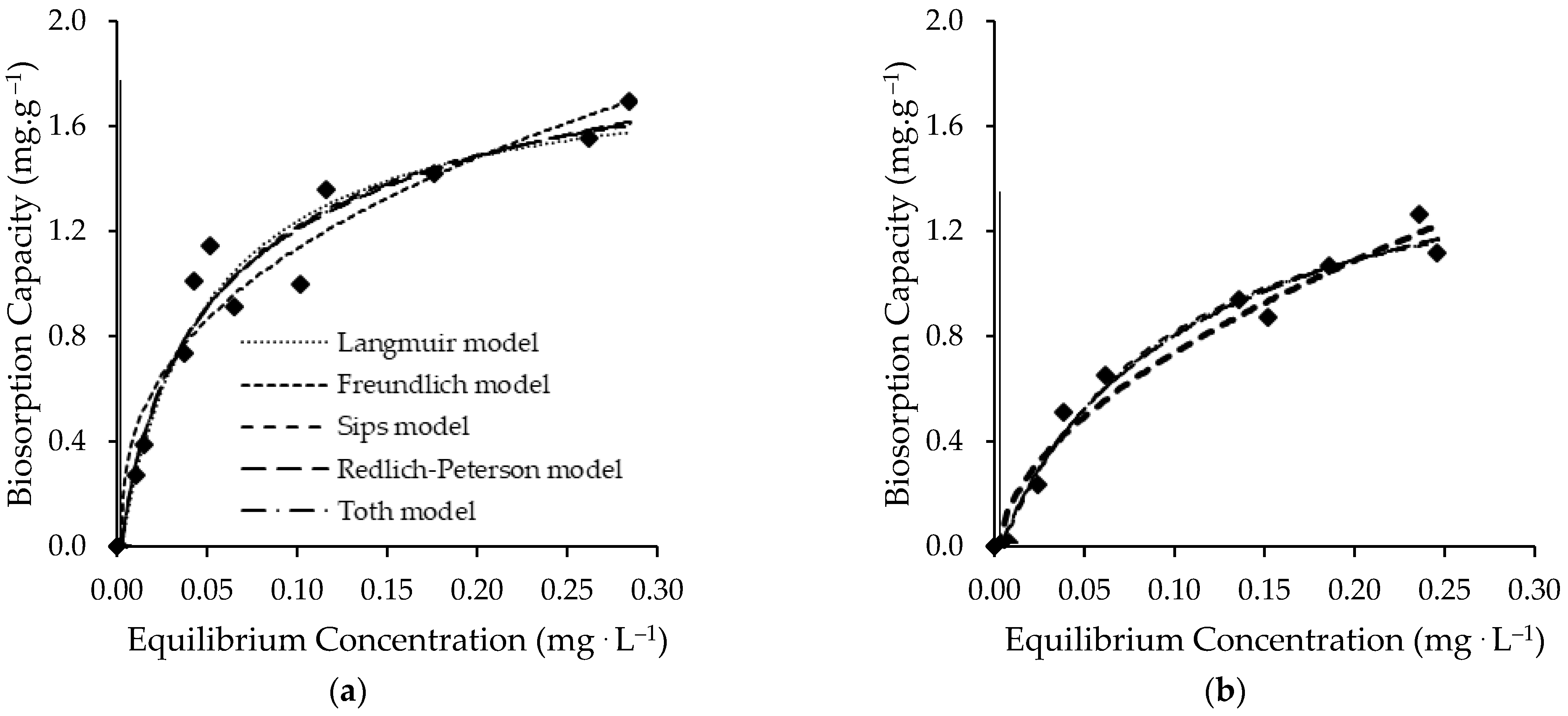
3.2.2. Equilibrium Results
3.3. Removal of FLX and Nutrients from Real Treated Municipal Wastewaters
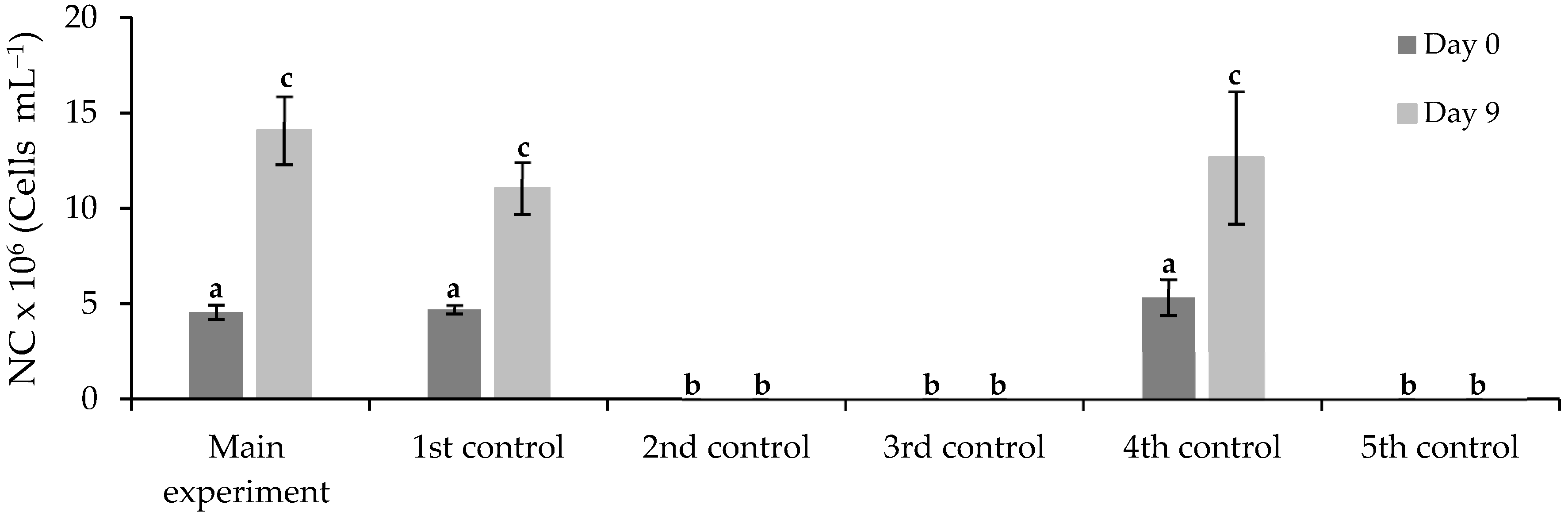
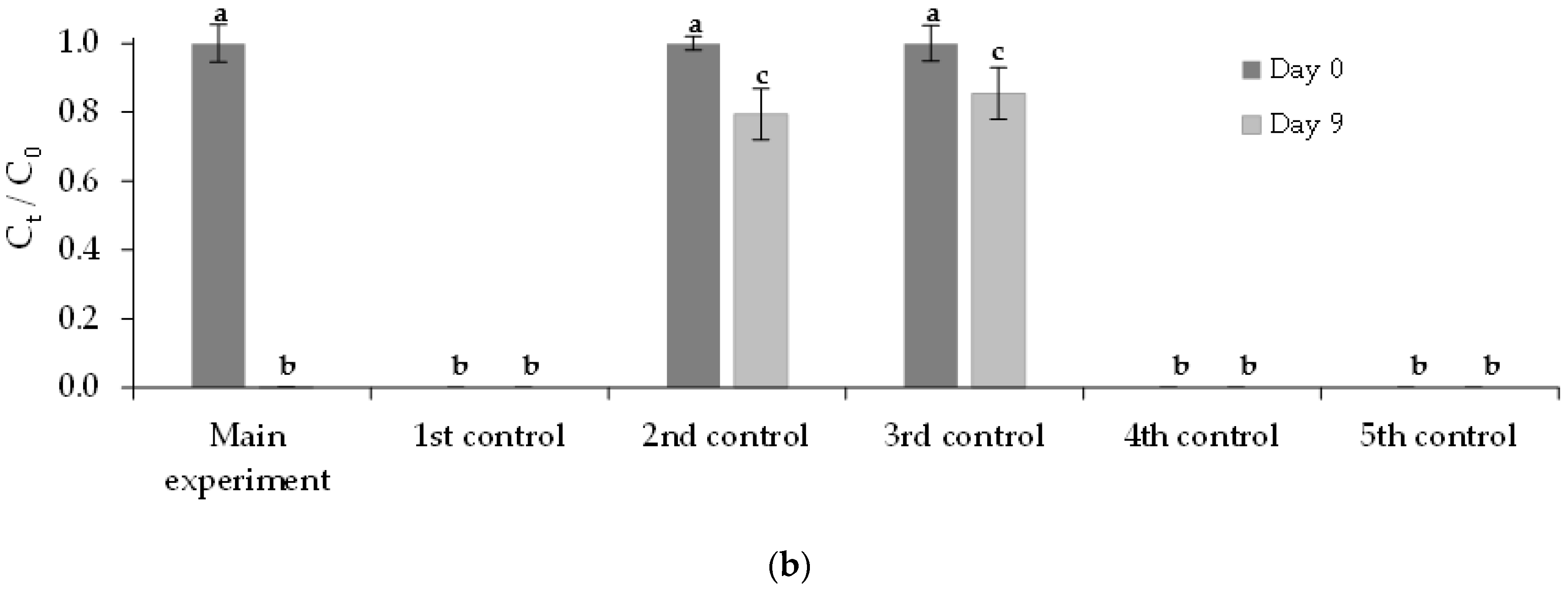
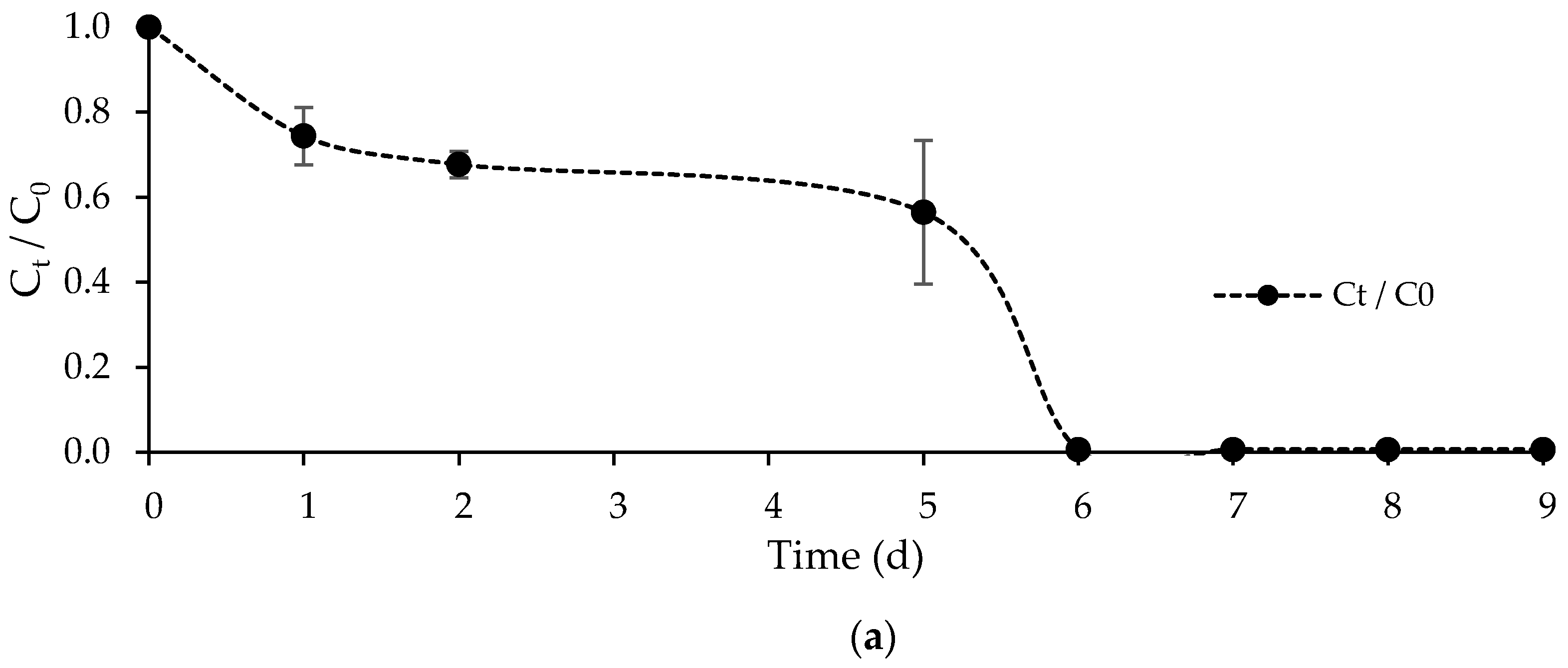
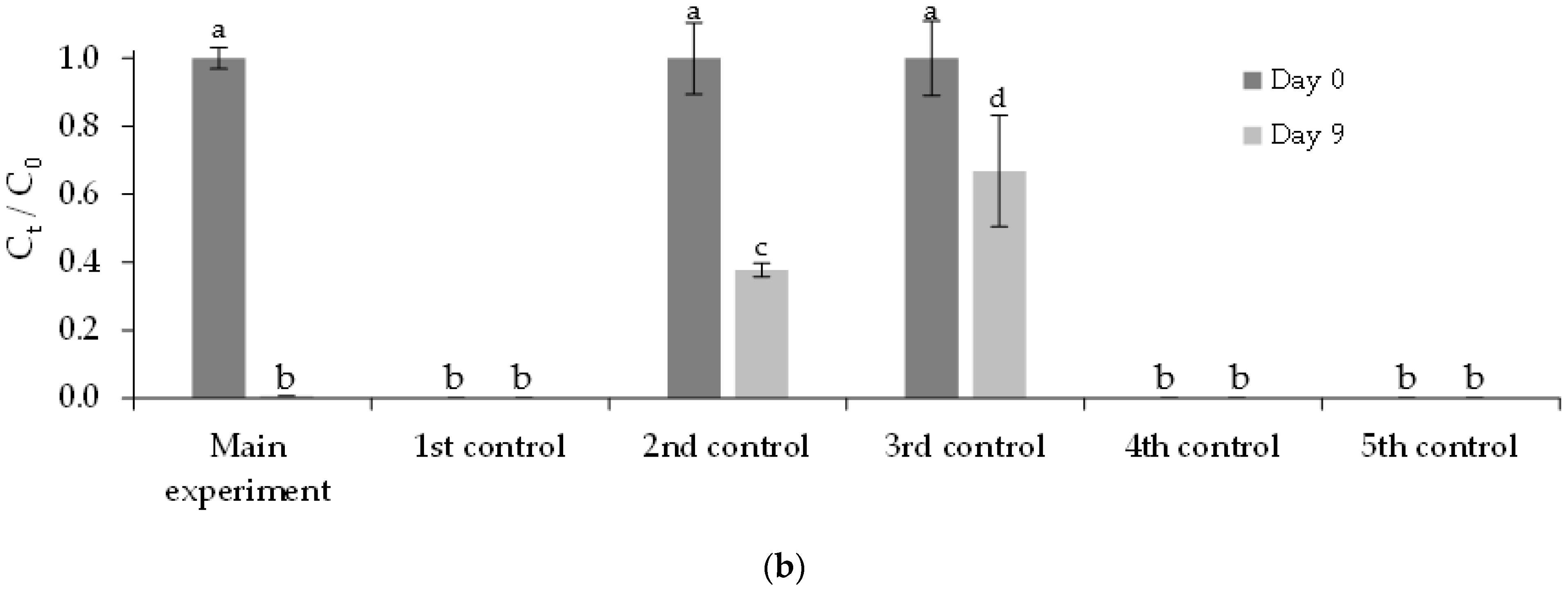
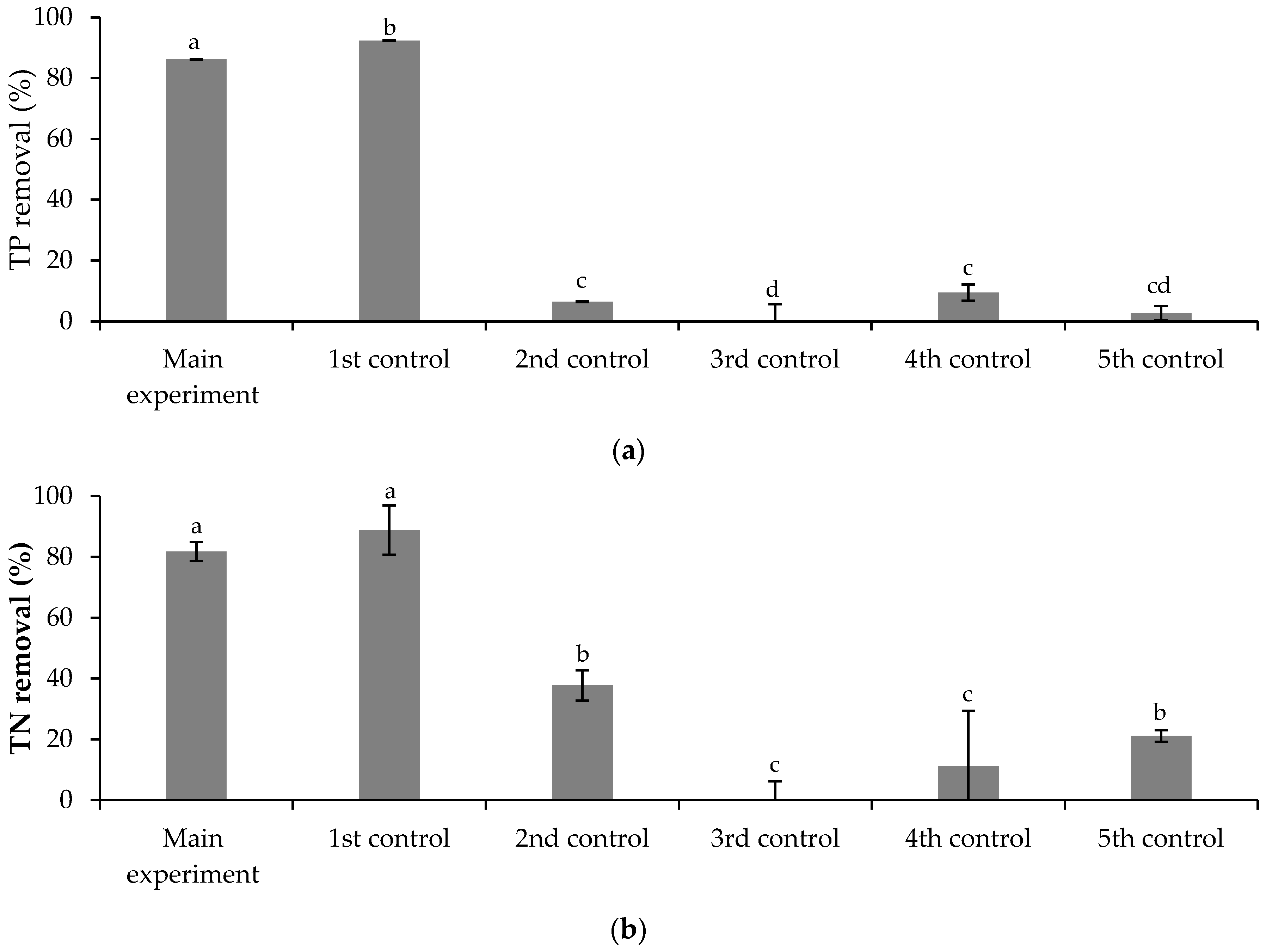
3.3.1. Free Chlorella vulgaris Assays Results
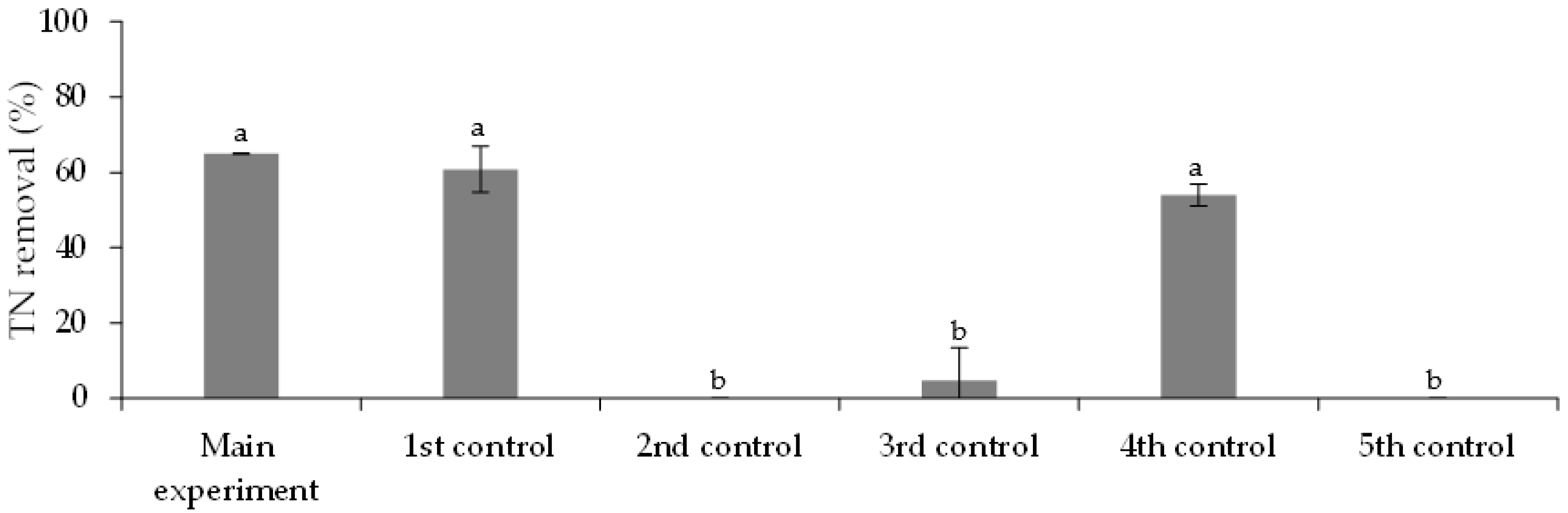
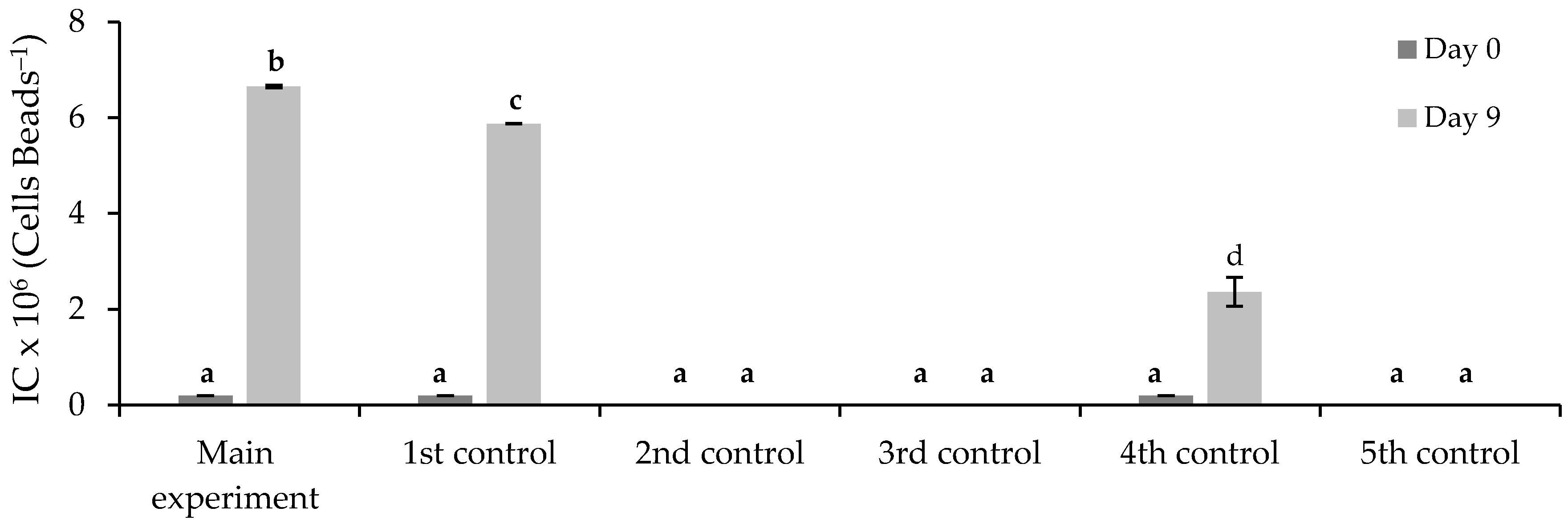
3.3.2. Immobilized Chlorella vulgaris Assays Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolpin, D.W.; Furlong, E.; Meyer, M.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Fong, P.P.; Molnar, N. Norfluoxetine Induces Spawning and Parturition in Estuarine and Freshwater Bivalves. Bull. Environ. Contam. Toxicol. 2008, 81, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Bernadt, M. Drug treatment of depression. In Seminars in General Adult Psychiatry, 2nd ed.; Stein, G., Wilkinson, G., Eds.; Royal College of Psychiatrists: London, UK, 2007; ISBN 9781904671442. [Google Scholar]
- Saussereau, E.; Lacroix, C.; Guerbet, M.; Cellier, D.; Spiroux, J.; Goullé, J.-P. Determination of Levels of Current Drugs in Hospital and Urban Wastewater. Bull. Environ. Contam. Toxicol. 2013, 91, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Lino, C.; Meisel, L.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef]
- Christensen, A.M.; Faaborg-Andersen, S.; Ingerslev, F.; Baun, A. Mixture and single-substance toxicity of selective serotonin reuptake inhibitors toward algae and crustaceans. Environ. Toxicol. Chem. 2007, 26, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Nentwig, G. Effects of Pharmaceuticals on Aquatic Invertebrates. Part II: The Antidepressant Drug Fluoxetine. Arch. Environ. Contam. Toxicol. 2007, 52, 163–170. [Google Scholar] [CrossRef]
- McDonald, M.D.; Gonzalez, A.; Sloman, K.A. Higher levels of aggression are observed in socially dominant toadfish treated with the selective serotonin reuptake inhibitor, fluoxetine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 107–112. [Google Scholar] [CrossRef]
- Kohlert, J.G.; Mangan, B.P.; Kodra, C.; Drako, L.; Long, E.; Simpson, H. Decreased Aggressive and Locomotor Behaviors in Betta Splendens after Exposure to Fluoxetine. Psychol. Rep. 2012, 110, 51–62. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Lado, W.E.; Zamora, J.M.; Duarte-Guterman, P.; Langlois, V.S.; Metcalfe, C.D.; Chang, J.P.; Moon, T.W.; Trudeau, V.L. Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus. Aquat. Toxicol. 2010, 100, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Gaworecki, K.M.; Klaine, S.J. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008, 88, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Beulig, A.; Fowler, J. Fish on prozac: Effect of serotonin reuptake inhibitors on cognition in goldfish. Behav. Neurosci. 2008, 122, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Foran, C.M.; Weston, J.; Slattery, M.; Brooks, B.W.; Huggett, D.B. Reproductive assessment of Japanese medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch. Environ. Contam. Toxicol. 2004, 46, 511–517. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.; Zhang, T.; Valero, J. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Mehariya, S.; Karthikeyan, P.O. (Eds.) Algal Biorefineries and The Circular Bioeconomy, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2022; Volume 2, pp. 183–250. ISBN 978-1-032-04893-2. [Google Scholar]
- Silva, A.; Stawiński, W.; Romacho, J.; Santos, L.H.; Figueiredo, S.A.; Freitas, O.M.; Delerue-Matos, C. Adsorption of Fluoxetine and Venlafaxine onto the Marine Seaweed Bifurcaria bifurcata. Environ. Eng. Sci. 2019, 36, 573–582. [Google Scholar] [CrossRef]
- Silva, A.; Coimbra, R.N.; Escapa, C.; Figueiredo, S.A.; Freitas, O.M.; Otero, M. Green Microalgae Scenedesmus Obliquus Utilization for the Adsorptive Removal of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) from Water Samples. Int. J. Environ. Res. Public Health 2020, 17, 3707. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Barbosa, M.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- González, L.E.; Cañizares, R.O.; Baena, S. Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.E.; de Coimbra, R.N.; Bermejo, S.P.; Pérez, A.I.G.; Cabero, M.O. Comparative Assessment of Pharmaceutical Removal from Wastewater by the Microalgae Chlorella sorokiniana, Chlorella vulgaris and Scenedesmus obliquus. In Biological Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2. In Guideline on Defined Approaches for Skin Sensitisation (OECD GL No. 497); OECD Publishing: Paris, France, 2011; p. 25. [Google Scholar]
- ChemAxon. Marvin Software. Available online: https://chemaxon.com (accessed on 10 January 2019).
- AMAP Chemicals. Fluoxetine. Available online: https://chemicals.amap.no/chemicals/fluoxetine (accessed on 10 January 2019).
- Monteiro, S.; Martins, J.M.S.S.; Magalhães, F.; Carvalho, L. Low Density Wood-Based Particleboards Bonded with Foamable Sour Cassava Starch: Preliminary Studies. Polymers 2016, 8, 354. [Google Scholar] [CrossRef]
- Lau, P.S.; Tam, N.F.-Y.; Wong, Y.S. Wastewater Nutrients (N and P) Removal by Carrageenan and Alginate Immobilized Chlorella Vulgaris. Environ. Technol. 1997, 18, 945–951. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/short-term-chronic-freshwater-wet-manual_2002.pdf (accessed on 10 January 2019).
- Wastewater Connect. Available online: http://wastewaterconnect.co.za/wastewater-info-hub-entry?id=12 (accessed on 6 March 2020).
- Rachlin, J.W.; Grosso, A. The effects of pH on the growth of Chlorella vulgaris and its interactions with cadmium toxicity. Arch. Environ. Contam. Toxicol. 1991, 20, 505–508. [Google Scholar] [CrossRef]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2003; ISBN 9780070418783. [Google Scholar]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Lagergren, S. About theory of so-called adsorption of soluble substances. Kungliga Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Tóth, J. State Equation of the Solid-gas Interface Layers. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–328. [Google Scholar]
- Duygu, D.Y.; Udoh, A.U.; Ozer, T.B.; Akbulut, A.; Erkaya, I.A.; Yildiz, K.; Guler, D. Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar] [CrossRef]
- Ponnuswamy, I.; Soundararajan, M.; Syed, S. Isolation and Characterization of Green Microalgae for Carbon Sequestration, Waste Water Treatment and Bio-fuel Production. Int. J. Bio-Sci. Bio-Technol. 2013, 5, 17–26. [Google Scholar]
- Grace, C.E.E.; Lakshmi, P.K.; Meenakshi, S.; Vaidyanathan, S.; Srisudha, S.; Mary, M.B. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117382. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and Drying Methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Risley, D.S.; Bopp, R.J. Fluoxetine. In Profiles of Drug Substances, Excipients and Related Methodology; Florey, K., Ed.; Academic Press: London, UK, 1991; Volume 19, ISBN 01226008194. [Google Scholar]
- Ayranci, E.; Hoda, N.; Bayram, E. Adsorption of benzoic acid onto high specific area activated carbon cloth. J. Colloid Interface Sci. 2005, 284, 83–88. [Google Scholar] [CrossRef]
- Schneider, J.D. pH Effects on the Sorption of Fluoxetine and Sulfamethoxazole by Three Powdered Activated Carbons, in Civil and Environmental Engineering. Master’s Thesis, Utah State University, Logan, UT, USA, May 2018. [Google Scholar]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; De Gruyter: Berlin, Germany, 2012; ISBN 9783110240221. [Google Scholar]
- Xing, B.; Pignatello, J.J. SORPTION|Organic Chemicals. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, MS, USA, 2005; pp. 537–548. ISBN 9780124095489. [Google Scholar]
- Cavalcante, J.d.A. Análise Experimental da Cinética de Secagem da Alga Marinha Sargassum sp. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2003. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Pomiès, M.; Choubert, J.-M.; Wisniewski, C.; Coquery, M. Modelling of micropollutant removal in biological wastewater treatments: A review. Sci. Total Environ. 2013, 443, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, M.; Chaibakhsh, N.; Naeemi, A.S. Optimized treatment of wastewater containing cytotoxic drugs by living and dead biomass of the freshwater microalga, Chlorella vulgaris. Ecol. Eng. 2018, 111, 85–93. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Mera, R.; Abalde, J. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J. Hazard. Mater. 2016, 320, 315–325. [Google Scholar] [CrossRef]
- Dvoretsky, D.S.; Akulinin, E.; Dvoretsky, S.; Temnov, M.; Androsova, A. Defining optimal conditions for Chlorella vulgaris microalgae biomass cell walls disruption in the process of biofuel production. In Proceedings of the 16th International Multidisciplinry Scientific GeoConference SGEM 2016, Albena, Bulgaria, 30 June–6 July 2016; pp. 261–267. [Google Scholar]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; de Carvalho, M.M.M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-based biosorbents as cost-effective alternatives to commercial adsorbents for the retention of fluoxetine from water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef] [Green Version]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; de Carvalho, M.M.M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2018, 648, 582–600. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Limits to Growth. In Wastewater Treatment with Algae, 1st ed.; Tam, N.F.Y., Wong, Y.S., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; Volume 7, pp. 203–226. ISBN 9783662108635. [Google Scholar]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Phytoplankton stoichiometry. Ecol. Res. 2008, 23, 479–485. [Google Scholar] [CrossRef]
- Yoo, I.-K.; Seong, G.H.; Chang, H.N.; Park, J.K. Encapsulation of Lactobacillus casei cells in liquid-core alginate capsules for lactic acid production. Enzym. Microb. Technol. 1996, 19, 428–433. [Google Scholar] [CrossRef]
- Roger, S.; Talbot, D.; Bee, A. Preparation and effect of Ca2+ on water solubility, particle release and swelling properties of magnetic alginate films. J. Magn. Magn. Mater. 2006, 305, 221–227. [Google Scholar] [CrossRef]
- Cortez, S.; Nicolau, A.; Flickinger, M.C.; Mota, M. Biocoatings: A new challenge for environmental biotechnology. Biochem. Eng. J. 2017, 121, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Sforza, E.; Calvaruso, C.; La Rocca, N.; Bertucco, A. Luxury uptake of phosphorus in Nannochloropsis salina: Effect of P concentration and light on P uptake in batch and continuous cultures. Biochem. Eng. J. 2018, 134, 69–79. [Google Scholar] [CrossRef]





| Assay | Purposes |
|---|---|
| Main experiment |
|
| 1st control |
|
| 2nd control |
|
| 3rd control |
|
| 4th control |
|
| 5th control |
|
| Assay | Municipal Wastewater (% v/v) | Chlorella vulgaris Culture (% v/v) | FLX Solution (% v/v) | Medium OECD 1 (% v/v) | |
|---|---|---|---|---|---|
| Free Cells | Immobilized Cells | ||||
| Main experiment | 72 | 20 | 0 (390 ± 1 beads) | 8 | 0 |
| 1st control | 72 | 20 | 0 (390 ± 1 beads) | 0 | 8 |
| 2nd control | 72 | 0 | 0 | 8 | 20 |
| 3rd control | 0 | 0 | 0 | 8 | 92 |
| 4th control | 0 | 20 | 0 (390 ± 1 beads) | 0 | 80 |
| 5th control | 72 | 0 | 0 | 0 | 28 |
| Chlorella vulgaris Sample | Element Mass Fraction (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | O | Na | Mg | Si | P | S | K | Ca | ||
| Living | Before FLX biosorption | 55.1 | ---- | 34.3 | 0.8 | 0.7 | 3.3 | 2.1 | 1.2 | 1.6 | 1.0 |
| After FLX biosorption | 47.8 | 8.4 | 39.0 | ---- | 0.5 | 0.6 | 1.0 | 0.8 | 1.2 | 0.7 | |
| Lyophilized | Before FLX biosorption | 56.4 | ---- | 38.2 | 0.5 | 0.8 | ---- | 1.7 | 1.0 | 0.5 | 0.9 |
| After FLX biosorption | 54.3 | ---- | 40.1 | 0.4 | 0.6 | 1.2 | 1.1 | 1.4 | ---- | 1.1 | |
| Model | Parameters 1 | Living C. vulgaris Biomass | Non-Living C. vulgaris Biomass |
|---|---|---|---|
| Elovich | |||
| α (mg·g−1·min−1) | 4 ± 3 | 7 × 109 ± 9 × 1010 | |
| β (g·mg−1) | 8 ± 1 | 12 ± 6 | |
| SSE | 0.262 | 0.440 | |
| 0.099 | 0.177 | ||
| 0.827 | 0.913 | ||
| 0.0097 | 0.0314 | ||
| AIC | −130 | −52 | |
| BIC | −126 | −49 | |
| Pseudo-first-order | |||
| (mg·g−1) | 0.96 ± 0.02 | 2.33 ± 0.04 | |
| k1 (min−1) | 0.20 ± 0.02 | 0.7 ± 0.1 | |
| SSE | 0.217 | 0.317 | |
| 0.090 | 0.150 | ||
| 0.857 | 0.938 | ||
| 0.0080 | 0.0226 | ||
| AIC | −136 | −57 | |
| BIC | −132 | −54 | |
| Pseudo-second order | Best-fit | Best-fit | |
| (mg·g−1) | 1.03 ± 0.03 | 2.39 ± 0.05 | |
| k2 (g·mg−1·min−1) | 0.3 ± 0.1 | 0.7 ± 0.2 | |
| SSE | 0.173 | 0.276 | |
| 0.080 | 0.140 | ||
| 0.886 | 0.946 | ||
| 0.0064 | 0.0197 | ||
| AIC | −142 | −59 | |
| BIC | −138 | −57 |
| Model | Parameters 1 | Living C. vulgaris Biomass | Non-Living C. vulgaris Biomass |
|---|---|---|---|
| Freundlich | |||
| 2.7 ± 0.3 | 1.8 ± 0.2 | ||
| ((mg·g−1)·(L·mg−1)1:nF) | 2.8 ± 0.3 | 2.8 ± 0.4 | |
| SSE | 0.228 | 0.066 | |
| 0.151 | 0.091 | ||
| 0.923 | 0.961 | ||
| 0.0228 | 0.0083 | ||
| AIC | −42 | −44 | |
| BIC | −10 | −43 | |
| Langmuir | Best-fit | Best-fit | |
| (mg·g−1) | 1.9 ± 0.1 | 1.6 ± 0.2 | |
| (L·mg−1) | 20 ± 5 | 11 ± 3 | |
| SSE | 0.175 | 0.043 | |
| 0.132 | 0.073 | ||
| 0.941 | 0.975 | ||
| 0.0175 | 0.0054 | ||
| AIC | −45 | −48 | |
| BIC | −43 | −48 | |
| Langmuir−Freundlich | |||
| (Sips) | (mg·g−1) | 2.1 ± 0.5 | 1.5 ± 0.4 |
| 0.8 ± 0.3 | 1.1 ± 0.3 | ||
| (L·mg−1) | 15 ± 10 | 13 ± 8 | |
| SSE | 0.170 | 0.043 | |
| 0.137 | 0.078 | ||
| 0.936 | 0.971 | ||
| 0.0189 | 0.0061 | ||
| AIC | −43 | −47 | |
| BIC | −41 | −45 | |
| Redlich−Peterson | |||
| (L·g−1) | 53 ± 30 | 20 ± 10 | |
| (L·mg−1)βRP | 24 ± 11 | 11 ± 3 | |
| 0.9 ± 0.2 | 0.9 ± 0.4 | ||
| SSE | 0.162 | 0.043 | |
| 0.134 | 0.078 | ||
| 0.939 | 0.971 | ||
| 0.0181 | 0.0061 | ||
| AIC | −44 | −47 | |
| BIC | −42 | −45 | |
| Tóth | |||
| (mg·g−1) | 2 ± 1 | 2 ± 1 | |
| 0.7 ± 0.5 | 0.9 ± 0.7 | ||
| (L·mg−1) | 0.1 ± 0.1 | 0.1 ± 0.2 | |
| SSE | 0.167 | 0.043 | |
| 0.136 | 0.078 | ||
| 0.938 | 0.971 | ||
| 0.0185 | 0.0061 | ||
| AIC | −43 | −47 | |
| BIC | −41 | −45 |
| Sorbent | Experimental Conditions | (mg·g−1) | References | |
|---|---|---|---|---|
| Time (min) | Temperature (°C) | |||
| Commercial activated carbon | <360 | 25 | 96.2 | [61] |
| Papermill sludge-based non-activated carbon | <360 | 25 | 120.4 | [61] |
| Papermill sludge-based activated carbon with ZnCl2 | <360 | 25 | 28.4 | [61] |
| Papermill sludge-based activated carbon with NaOH | <360 | 25 | 136.6 | [61] |
| Eucalyptus biochar | 15 | ~20 | 6.41 | [62] |
| Hollow trees biochar | 15 | ~20 | 3.04 | [62] |
| Vine biochar | 15 | ~20 | 2.80 | [62] |
| Synthetic zeolite 13× | >600 | 25 | 32.11 | [63] |
| Synthetic zeolite 4A | >1200 | 25 | 21.86 | [63] |
| Spent coffee grounds | 180–600 | 25 | 14.31 | [63] |
| Pine bark | 180–600 | 25 | 6.53 | [63] |
| Cork waste | 180–600 | 25 | 4.74 | [63] |
| Non-living Bifurcaria bifurcata biomass | 180 | ~20 | 6.81 | [19] |
| Living Chlorella vulgaris biomass | 120 | ~20 | 1.9 | This study |
| Non-living Chlorella vulgaris biomass | 120 | ~20 | 1.6 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.D.M.; Fernandes, D.F.; Figueiredo, S.A.; Freitas, O.M.; Delerue-Matos, C. Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation. Int. J. Environ. Res. Public Health 2022, 19, 6081. https://doi.org/10.3390/ijerph19106081
Silva ADM, Fernandes DF, Figueiredo SA, Freitas OM, Delerue-Matos C. Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation. International Journal of Environmental Research and Public Health. 2022; 19(10):6081. https://doi.org/10.3390/ijerph19106081
Chicago/Turabian StyleSilva, Andreia D. M., Diana F. Fernandes, Sónia A. Figueiredo, Olga M. Freitas, and Cristina Delerue-Matos. 2022. "Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation" International Journal of Environmental Research and Public Health 19, no. 10: 6081. https://doi.org/10.3390/ijerph19106081
APA StyleSilva, A. D. M., Fernandes, D. F., Figueiredo, S. A., Freitas, O. M., & Delerue-Matos, C. (2022). Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation. International Journal of Environmental Research and Public Health, 19(10), 6081. https://doi.org/10.3390/ijerph19106081











