The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review
Abstract
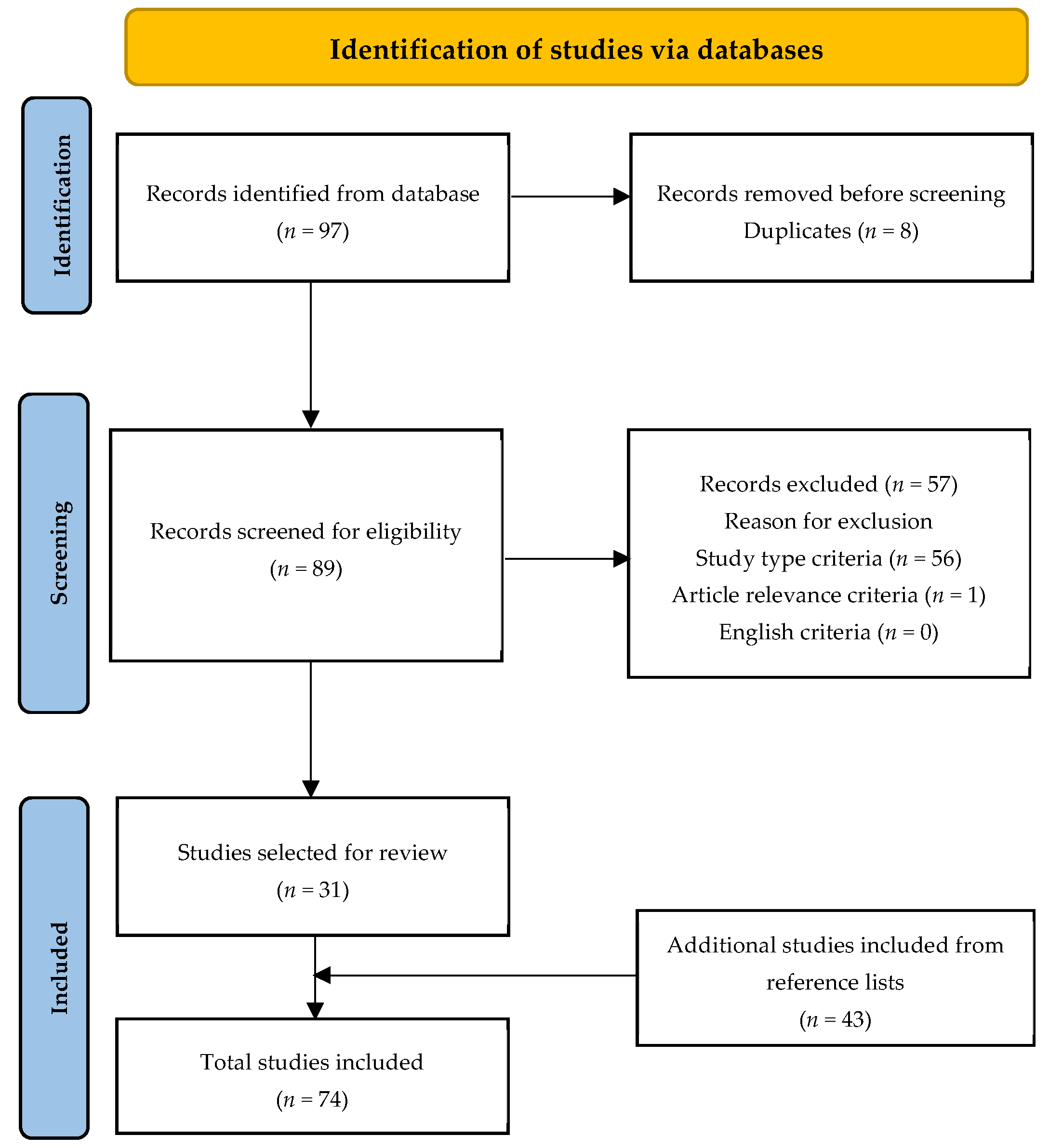
:1. Introduction
2. Materials and Methods
3. NAC Considerations
4. NAC in Trichotillomania
5. NAC in Excoriation Disorder
6. NAC in Onychophagia and Onychotillomania
7. Discussion
8. Limitations and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sani, G.; Gualtieri, I.; Paolini, M.; Bonanni, L.; Spinazzola, E.; Maggiora, M.; Pinzone, V.; Brugnoli, R.; Angeletti, G.; Girardi, P.; et al. Drug Treatment of Trichotillomania (Hair-Pulling Disorder), Excoriation (Skin-picking) Disorder, and Nail-biting (Onychophagia). Curr. Neuropharmacol. 2019, 17, 775–786. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 2013. Available online: http://dsm.psychiatryonline.org/book.aspx?bookid=556 (accessed on 8 March 2022).
- Nwankwo, C.O.; Jafferany, M. N-Acetylcysteine in psychodermatological disorders. Dermatol. Ther. 2019, 32, e13073. [Google Scholar] [CrossRef] [PubMed]
- Penzel, F. Body-Focused Repetitive Disorder. Hair Pulling, Skin Picking and Biting: Body-Focused Repetitive Disorders. What Exactly Are BFRBs? Available online: https://www.aamft.org/Consumer_Updates/Body_Focused_Repetitive_Disorders.aspx (accessed on 8 March 2022).
- Sampaio, D.G.; Grant, J.E. Body-focused repetitive behaviors and the dermatology patient. Clin. Dermatol. 2018, 36, 723–727. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Keuthen, N.J.; Lochner, C.; Stein, D.J. Skin picking disorder. Am. J. Psychiatry 2012, 169, 1143–1149. [Google Scholar] [CrossRef]
- Pacan, P.; Grzesiak, M.; Reich, A.; Kantorska-Janiec, M.; Szepietowski, J. Onychophagia and Onychotillomania: Prevalence, Clinical Picture and Comorbidities. Acta Derm. Venereol. 2014, 94, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.H.; Yücel, M.; Yun, J.; Yoon, Y.B.; Cho, K.I.K.; Parkes, L.; Kim, S.N.; Kwon, J.S. Altered functional network architecture in orbitofronto-striato-thalamic circuit of unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2017, 38, 109–119. [Google Scholar] [CrossRef]
- Hou, J.-M.; Zhao, M.; Zhang, W.; Song, L.-H.; Wu, W.-J.; Wang, J.; Zhou, D.-Q.; Xie, B.; He, M.; Guo, J.-W.; et al. Resting-state functional connectivity abnormalities in patients with obsessive–compulsive disorder and their healthy first-degree relatives. J. Psychiatry Neurosci. 2014, 39, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Hanna, G.L.; Rosenberg, D.R.; Arnold, P.D. The role of glutamate signaling in the pathogenesis and treatment of obsessive–compulsive disorder. Pharmacol. Biochem. Behav. 2012, 100, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K. Astroglia and Obsessive Compulsive Disorder. Adv Neurobiol. 2021, 26, 139–149. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef]
- Minarini, A.; Ferrari, S.; Galletti, M.; Giambalvo, N.; Perrone, D.; Rioli, G.; Galeazzi, G.M. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.P.; Moreira, R. Oral N-acetylcysteine in the treatment of obsessive-compulsive disorder: A systematic review of the clinical evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.L.; Patel, V.; Debord, L.C.; Rosen, T. A review of N-acetylcysteine in the treatment of grooming disorders. Int. J. Dermatol. 2019, 58, 502–510. [Google Scholar] [CrossRef]
- Janeczek, M.; Moy, L.; Riopelle, A.; Vetter, O.; Reserva, J.; Tung, R.; Swan, J. The Potential Uses of N-acetylcysteine in Dermatology: A Review. J. Clin. Aesthetic Dermatol. 2019, 12, 20–26. [Google Scholar]
- Bhattacharyya, S.; Khanna, S.; Chakrabarty, K.; Mahadevan, A.; Christopher, R.; Shankar, S.K. Anti-Brain Autoantibodies and Altered Excitatory Neurotransmitters in Obsessive–Compulsive Disorder. Neuropsychopharmacology 2009, 34, 2489–2496. [Google Scholar] [CrossRef]
- Yücel, M.; Wood, S.; Wellard, M.; Harrison, B.J.; Fornito, A.; Pujol, J.; Velakoulis, D.; Pantelis, C. Anterior Cingulate Glutamate–Glutamine Levels Predict Symptom Severity in Women with Obsessive–Compulsive Disorder. Aust. N. Z. J. Psychiatry 2008, 42, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, K.; Bhattacharyya, S.; Christopher, R.; Khanna, S. Glutamatergic Dysfunction in OCD. Neuropsychopharmacology 2005, 30, 1735–1740. [Google Scholar] [CrossRef] [Green Version]
- Hadi, F.; Kashefinejad, S.; Kamalzadeh, L.; Hoobehfekr, S.; Shalbafan, M. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2021, 22, 69. [Google Scholar] [CrossRef]
- Baker, D.A.; Madayag, A.; Kristiansen, L.; Meador-Woodruff, J.H.; Haroutunian, V.; Raju, I. Contribution of Cystine–Glutamate Antiporters to the Psychotomimetic Effects of Phencyclidine. Neuropsychopharmacology 2008, 33, 1760–1772. [Google Scholar] [CrossRef] [Green Version]
- Behl, A.; Swami, G.; Sircar, S.S.; Bhatia, M.S.; Banerjee, B.D. Relationship of Possible Stress-Related Biochemical Markers to Oxidative/Antioxidative Status in Obsessive-Compulsive Disorder. Neuropsychobiology 2010, 61, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ersan, S.; Bakir, S.; Ersan, E.E.; Dogan, O. Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive–compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, E.; Cetinkaya, S.; Ersan, S.; Kucukosman, S.; Ersan, E.E. Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive–compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Amin, S.S.; Mohtashim, M. N-acetylcysteine in dermatology. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Van Ameringen, M.; Mancini, C.; Patterson, B.; Bennett, M.; Oakman, J. A randomized, double-blind, placebo-controlled trial of olanzapine in the treatment of trichotillomania. J. Clin. Psychiatry 2010, 71, 1336–1343. [Google Scholar] [CrossRef]
- Leppink, E.W.; Redden, S.A.; Grant, J.E. A double-blind, placebo-controlled study of inositol in trichotillomania. Int. Clin. Psychopharmacol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Schreiber, L.R.; Kim, S.W. The opiate antagonist, naltrexone, in the treatment of trichotillomania: Results of a double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 2014, 34, 134–138. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Kim, S.W. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: A double-blind, placebo-controlled study. Arch. Gen. Psychiatry 2009, 66, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Bloch, M.H.; Panza, K.E.; Grant, J.E.; Pittenger, C.; Leckman, J.F. N-Acetylcysteine in the Treatment of Pediatric Trichotillomania: A Randomized, Double-Blind, Placebo-Controlled Add-On Trial. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Schumer, M.C.; Panza, K.E.; Mulqueen, J.M.; Jakubovski, E.; Bloch, M.H. Long-term outcome in pediatric trichotillomania. Depress. Anxiety 2015, 32, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, S.; Hong, X.; Lu, S.; Tang, S.; Shen, Y.; Feng, M.; Guo, P.; Fang, Y. A case of trichotillomania with binge eating disorder: Combined with N-acetylcysteine synergistic therapy. Ann. Gen. Psychiatry 2021, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Keuthen, N.; Greenberg, E. Assessment and treatment of trichotillomania (hair pulling disorder) and excoriation (skin picking) disorder. Clin. Dermatol. 2018, 36, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, F.; Keleş, S. Repetitive Behaviors Treated with N-Acetylcysteine: Case Series. Clin. Neuropharmacol. 2019, 42, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.; Andrade, T.C.; Brito, F.F.; Silva, G.V.; Cavalcante, M.L.; Martelli, A.C. Trichotillomania: A case report with clinical and dermatoscopic differential diagnosis with alopecia areata. An. Bras. Dermatol. 2017, 92, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Barroso, L.A.L.; Sternberg, F.; de Fraia e Souza, M.N.I.; Nunes, G.J.D.B. Trichotillomania: A good response to treatment with N-acetylcysteine. Bras. Dermatol. 2017, 92, 537–539. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, D.; Seçkin, D. N-Acetylcysteine in the treatment of trichotillomania: Remarkable results in two patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Bhagwandas, K. N-acetylcysteine in trichotillomania: A panacea for compulsive skin disorders? Br. J. Dermatol. 2014, 171, 1253–1255. [Google Scholar] [CrossRef]
- Rodrigues-Barata, A.R.; Tosti, A.; Rodríguez-Pichardo, A.; Camacho-Martínez, F. N-acetylcysteine in the Treatment of Trichotillomania. Int. J. Trichology 2012, 4, 176–178. [Google Scholar] [CrossRef] [Green Version]
- Odlaug, B.L.; Grant, J.E. N-Acetyl Cysteine in the Treatment of Grooming Disorders. J. Clin. Psychopharmacol. 2007, 27, 227–229. [Google Scholar] [CrossRef]
- Nemeh, M.N.; Hogeling, M. Pediatric skin picking disorder: A review of management. Pediatr. Dermatol. 2022. [Google Scholar] [CrossRef]
- Schuck, K.; Keijsers, G.P.; Rinck, M. The effects of brief cognitive-behaviour therapy for pathological skin picking: A randomized comparison to wait-list control. Behav. Res. Ther. 2011, 49, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.J.; Woods, D.W.; Twohig, M.P. Habit reversal as a treatment for chronic skin picking: A pilot investigation. Behav. Modif. 2006, 30, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Lochner, C.; Roos, A.; Stein, D. Excoriation (skin-picking) disorder: A systematic review of treatment options. Neuropsychiatr. Dis. Treat. 2017, 13, 1867–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, J.E.; Chamberlain, S.R.; Redden, S.A.; Leppink, E.W.; Odlaug, B.L.; Kim, S.W. N-Acetylcysteine in the Treatment of Excoriation Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Hwang, A.S.; Campbell, E.H.; Sartori-Valinotti, J.C. Evidence of N-acetylcysteine efficacy for skin picking disorder: A retrospective cohort study. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Miller, J.L.; Angulo, M. An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am. J. Med. Genet. Part A 2014, 164, 421–424. [Google Scholar] [CrossRef]
- Wieting, J.; Deest, M.; Bleich, S.; Frieling, H.; Eberlein, C. N-Acetylcysteine provides limited efficacy as treatment option for skin picking in Prader–Willi syndrome. Am. J. Med. Genet. Part A 2022, 188, 828–835. [Google Scholar] [CrossRef]
- Ozcan, D. N-acetylcysteine for managing neurotic excoriation: Encouraging results in two patients. An. Bras. Dermatol. 2021, 96, 390–391. [Google Scholar] [CrossRef]
- Silva-Netto, R.; Jesus, G.; Nogueira, M.; Tavares, H. N-acetylcysteine in the treatment of skin-picking disorder. Braz. J. Psychiatry 2014, 36, 101. [Google Scholar] [CrossRef] [Green Version]
- Percinel, I.; Yazici, K.U. Glutamatergic dysfunction in skin-picking disorder: Treatment of a pediatric patient with N-acetylcysteine. J. Clin. Psychopharmacol. 2014, 34, 772–774. [Google Scholar] [CrossRef]
- Velazquez, L.; Ward-Chene, L.; Loosigian, S.R. Fluoxetine in the treatment of self-mutilating behavior. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.L.; Lenane, M.C.; Swedo, S.E.; Rettew, D.C.; Rapoport, J.L. A Double-blind Comparison of Clomipramine and Desipramine Treatment of Severe Onychophagia (Nail Biting). Arch. Gen. Psychiatry 1991, 48, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wadden, P.; Pawliuk, G. Cessation of nail-biting and bupropion. Can. J. Psychiatry 1999, 44, 709–710. [Google Scholar] [PubMed]
- Sharma, V.; Sommerdyk, C. Lithium Treatment of Chronic Nail Biting. Prim. Care Companion CNS Disord. 2014, 16, 27432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.K.; Lipner, S.R. Update on Diagnosis and Management of Onychophagia and Onychotillomania. Int. J. Environ. Res. Public Health 2022, 19, 3392. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Derakhshan, N.; Berk, M. N-acetylcysteine versus placebo for treating nail biting, a double blind randomized placebo controlled clinical trial. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2013, 12, 223–228. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Derakhshan, N. N-acetylcysteine for treatment of autism, a case report. J. Res. Med. Sci. 2012, 17, 985–987. [Google Scholar]
- Berk, M.; Jeavons, S.; Dean, O.M.; Dodd, S.; Moss, K.; Gama, C.S.; Malhi, G.S. Nail-Biting Stuff? The Effect of N-acetyl Cysteine on Nail-Biting. CNS Spectr. 2009, 14, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Magid, M.; Mennella, C.; Kuhn, H.; Stamu-O’Brien, C.; Kroumpouzos, G. Onychophagia and onychotillomania can be effectively managed. J. Am. Acad. Dermatol. 2017, 77, e143–e144. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.L.C.; Diniz, J.B.; Requena, G.; Joaquim, M.A.; Pittenger, C.; Bloch, M.H.; Miguel, E.C.; Shavitt, R.G. Randomized, Double-Blind, Placebo-Controlled Trial of N-Acetylcysteine Augmentation for Treatment-Resistant Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2017, 78, e766–e773. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.; Castle, D.; Bousman, C.; Oliver, G.; Cribb, L.; Blair-West, S.; Brakoulias, V.; Camfield, D.; Ee, C.; et al. N-acetyl cysteine (NAC) augmentation in the treatment of obsessive-compulsive disorder: A phase III, 20-week, double-blind, randomized, placebo-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 117, 110550. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Oliver, G.; Camfield, D.A.; Dean, O.M.; Dowling, N.; Smith, D.J.; Murphy, J.; Menon, R.; Berk, M.; Blair-West, S.; et al. N-Acetyl Cysteine (NAC) in the Treatment of Obsessive-Compulsive Disorder: A 16-Week, Double-Blind, Randomised, Placebo-Controlled Study. CNS Drugs 2015, 29, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, R.C.J.; Berk, M.; Kalivas, P.W.; Back, S.E.; Kanaan, R.A. Correction to: The Potential of N Acetyl L Cysteine (NAC) in the Treatment of Psychiatric Disorders. CNS Drugs 2022, 36, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. N-Acetylcysteine ethyl ester (NACET): A novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem. Pharmacol. 2012, 84, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.E.; Jenike, E.A.; Hezel, D.M.; Stack, D.E.; Dodman, N.H.; Shuster, L.; Jenike, M.A. A Single-Blinded Case-Control Study of Memantine in Severe Obsessive-Compulsive Disorder. J. Clin. Psychopharmacol. 2010, 30, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, W.M.; Benedict, M.M.; Doerfer, J.; Perrin, M.; Panek, L.; Cleveland, W.L.; Javitt, D.C. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J. Psychiatr. Res. 2009, 43, 664–670. [Google Scholar] [CrossRef]
- Coric, V.; Milanovic, S.; Wasylink, S.; Patel, P.; Malison, R.; Krystal, J.H. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with obsessive-compulsive disorder and major depressive disorder. Psychopharmacology 2003, 167, 219–220. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Gao, T.; Du, J.; Tian, S.; Liu, W. A meta-analysis of the effects of non-invasive brain stimulation on obsessive-compulsive disorder. Psychiatry Res. 2022, 312, 114530. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, M.; Padberg, F. A perfect match: Noninvasive brain stimulation and psychotherapy. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, S27–S33. [Google Scholar] [CrossRef] [PubMed]
| Summary of NAC Treatment Studies in Trichotillomania | |||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Patients | Age (Year) | Comorbidities | NAC Dose | Other Concurrent Medications | Outcomes |
| Grant, Odlaug, and Kim (2009) [30] | RDBPCT | Adult (n = 50) | 18–65 | Depression, anxiety, OCD, PTSD, SPD, bulimia | 1200–2400 mg/day | SSRIs, SNRIs, stimulants, psychotherapy | The NAC group showed higher efficacy (F1,47 = 32.152, p < 0.001) compared to the placebo group based on MGH-HPS. The NAC group also showed improvement in hair pulling severity (F1,47 = 18.245, p < 0.001) and resistance and control (F1,47 = 37.067, p < 0.001) compared to placebo. |
| Bloch et al. (2013) [31] | RDBPCT | Pediatric (n = 39) | 8–17 | ADHD, depression, anxiety, OCD, tic disorder, SPD | 600–2400 mg/day | SSRIs, antipsychotics, atomoxetine, psychotherapy | No significant difference between NAC and placebo group based on MGH-HPS (p = 0.55). Moderate decrease in hair pulling noted in both groups (p = 0.002). |
| Zhao et al. (2021) [33] | Case report | Adult (n = 1) | 25, F | BED, anxiety, depression | 600–1800 mg/day | Fluvoxamine 150 mg/day, bupropion 300 mg/day | After 2 weeks, stable mood and reduced hair pulling behavior reported. At 14 weeks, patient reported no hair pulling or binge eating episodes, and improved anxiety and depression. |
| Jones, Keuthen, and Greenberg (2018) [34] | Case report | Adult (n = 1) | 18, F | OCD, depression, anxiety, SPD | 2700 mg/day | Fluoxetine 40 mg/day, psychotherapy | After 16 weeks, patient had significant reduction in hair pulling, skin picking, depression, anxiety, and OCD symptoms. Full remission was not reached. |
| Kilic and Keles (2018) [35] | Case report | Adult (n = 1) | 18, F | Depression, anxiety | 1200 mg/day | Fluoxetine 40 mg/day | After 3 weeks, patient showed decreased hair pulling urges and behavior. All depression and anxiety symptoms ceased. At 6-month follow-up, no hair pulling was noted. |
| Pino et al. (2017) [36] | Case report | Pediatric (n = 1) | 12, F | Not specified | 2400 mg/day | Doxepin 10 mg/day, fluoxetine 20 mg/day, pimozide 2 mg/day | After 6 months, patient had improved hair density and dermoscopy findings. |
| Barroso et al. (2017) [37] | Case report | Pediatric (n = 1) | 11, M | Asthma, atopic dermatitis | 1200–1800 mg/day | None | After 3 months, patient showed improvement at 1200 mg/day. Remission with complete hair regrowth was achieved at 1800 mg/day for 3 months. |
| Ozcan and Seckin (2016) [38] | Case report | Adult and pediatric (n = 2) | 30, F; 14, F | Not specified; ADHD | 1200 mg/day | None; methylphenidate | Case 1: After 2 months, hair pulling decreased with complete remission within 4 months. No recurrence of hair pulling was noted at 7-month follow-up. Case 2: After 2 weeks, significant improvement of hair pulling noted with complete hair regrowth after 6 months. No recurrence of hair pulling noted at 8-month follow-up. |
| Taylor and Bhagwandas (2014) [39] | Case report | Adult (n = 1) | 58, F | Unexplained weight loss | 1200 mg/day | None | After 4 weeks, patient showed noticeable regrowth of hair, which further improved at 10 weeks. Progress continued and maintained at 32 weeks. |
| Rodrigues-Barata et al. (2012) [40] | Case report | Adult (n = 2) | 23, F; 19, F | Alopecia; Not specified | 1200 mg/day | None | Case 1: Within 2 months, hair regrowth was observed; Case 2: Complete regrowth was observed after 3 months of treatment. |
| Odlaug and Grant (2007) [41] | Case report | Adult (n = 2) | 28, M; 40, F | ADHD, nail biting; Not specified | 600–1800 mg/day; 600–2400 mg/day | None | Case 1: Dose was increased from 600 to 1800 mg/day over several weeks. Complete cessation of hair pulling after 1 week on 1800 mg/day. Case 2: Dose was increased from 600 to 2400 mg/day. Complete cessation of urges and hair pulling after 2 weeks on 2400 mg/day. |
| Summary of NAC Treatment Studies in Excoriation Disorder. | |||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Patients | Age (Year) | Comorbidities | NAC Dose | Other Concurrent Medications | Outcomes |
| Grant et al. (2016) [46] | RDBPCT | Adult (n = 66) | 18–65 | Depression, anxiety, TTM, nail biting | 1200–3000 mg/day | Psychotropic medications | After 12 weeks, NAC treatment group showed significant improvement in skin picking severity compared to placebo based on NE-YBOCS and CGI-Serverity scale (p = 0.048, p = 0.03). |
| Hwang, Campbell, and Sartori-Valinotti (2021) [47] | Retrospective cohort study | Adult (n = 28) | Mean: 57.2 | Not specified | 1200–2400 mg/day | Doxepin, duloxetine | After 12 weeks, 61.5% of patients reported a positive response to NAC. |
| Miller and Angulo (2013) [48] | Open-label pilot study | Adult and pediatric (n = 35) | 5–39 | Prader—Willi syndrome | 450–1200 mg/day | Valproic acid, quetiapine, risperidone, spironolactone, growth hormone, metformin, levothyroxine, modafinil | After 12 weeks, 100% of patients reported reduced skin picking behavior and 71% achieved complete resolution. |
| Wieting et al. (2021) [49] | Retrospective cohort study | Adult (n = 14) | 17–53 | Prader—Willi syndrome | 1800–2400 mg/day | Risperidone, pipamperone, aripiprazole, sertraline, milnacipran | After 12 weeks, 6 patients reported no changes in symptoms and 2 had worsened symptoms based on CGI-Improvement scale. |
| Ozcan (2021) [50] | Case report | Adult (n = 2) | 75, F; 36, F | Not specified | 1200 mg/day | None | Case 1: After 2 weeks, patient reported decreased skin picking. Treatment was continued for 3 months and no relapse at 6-month follow-up. Case 2: After 6 weeks, complete cessation skin picking reported and no relapse at 3-month follow-up. |
| Kilic and Keles (2019) [35] | Case report | Adult (n = 1) | 42, F | Depression | 1200 mg/day | Venlafaxine 225 mg/day | After 10 days, patient reported decreased skin picking symptoms. Complete cessation of skin picking achieved at 3 months. |
| Silva-Netto et al. (2014) [51] | Case report | Adult (n = 3) | 45, F; 40, F; 31, F | TTM, depression; Bipolar disorder; Depression, pathological jealousy, internet addiction | 1200–1800 mg/day; 1200 mg/day; 1200 mg/day | Venlafaxine 75 mg/day; lithium 600 mg/day, quetiapine 50 mg/day; fluoxetine 20 mg/day | Case 1: Skin picking resolved completely. Case 2: Complete resolution of skin picking achieved and maintained for 10 months. Case 3: Substantial improvement of skin picking. |
| Percinel and Yazici (2014) [52] | Case report | Pediatric (n = 1) | 12, F | Not specified | 600–1800 mg/day | None | 4 weeks after a dose increase from 600 to 1200 mg/day, skin picking urges and behavior decreased dramatically and complete remission achieved after 4 weeks on 1800 mg/day. |
| Odlaug and Grant (2007) [41] | Case report | Adult (n = 1) | 52, F | Bulimia nervosa, compulsive buying | 600–1800 mg/day | None | Patient reported 50% decrease of picking urges and behaviors within 1 week after dose increase from 600 to 1200 mg/day. Patient had no skin picking behavior after a dose increase to 1800 mg/day. |
| Summary of NAC Treatment Studies in Onychophagia | |||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Patients | Age (Year) | Comorbidities | NAC Dose | Other Concurrent Medications | Outcomes |
| Ghanizadeh et al. (2013) [58] | RDBPCT | Pediatric (n = 42) | 6–18 | OCD, depression, anxiety, ADHD, tic disorder, SPD | 200–800 mg/day | SSRIs, SNRIs, stimulants, antipsychotics, TCAs | Significantly increased nail length in treatment group compared to placebo after 1 month (p < 0.04). No significant difference noted after 2 months. |
| Kilic and Keles (2019) [35] | Case report | Adult (n = 1) | 24, M | Not specified | 1200–1800 mg/day | None | After 3 weeks on 1800 mg/day, patient lost urge to bite his nails. Efficacy was maintained after 6 weeks. |
| Ghanizadeh and Derakhshan (2012) [59] | Case report | Pediatric (n = 1) | 8, M | Autism | 800 mg/day | Risperidone 2 mg/day and thioridazine 10 mg/day | After 1 month of NAC, patient’s parents reported reduced nail biting and autism symptoms. |
| Berk et al. (2009) [60] | Case report | Adult (n = 3) | 46, F; 44, F; 46, M | Bipolar disorder; Depression, anxiety, bipolar disorder; Depression, bipolar disorder | 2000 mg/day; 2000 mg/day; Not specified | Lithium 900 mg/day, quetiapine 300 mg/day; Mirtazapine 15 mg; None | Case 1: After 2 weeks, patient completely stopped nail biting and results were maintained at 7-month follow-up; Case 2: After 4 months, patient completed stopped nail biting and results were maintained at 2-month follow-up; Case 3: After 28 weeks, patient reported reduction in nail biting. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.K.; Lipner, S.R. The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 6370. https://doi.org/10.3390/ijerph19116370
Lee DK, Lipner SR. The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review. International Journal of Environmental Research and Public Health. 2022; 19(11):6370. https://doi.org/10.3390/ijerph19116370
Chicago/Turabian StyleLee, Debra K., and Shari R. Lipner. 2022. "The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review" International Journal of Environmental Research and Public Health 19, no. 11: 6370. https://doi.org/10.3390/ijerph19116370
APA StyleLee, D. K., & Lipner, S. R. (2022). The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review. International Journal of Environmental Research and Public Health, 19(11), 6370. https://doi.org/10.3390/ijerph19116370






