Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility Criteria
2.2. Data Extraction
2.3. Data Synthesis and Analysis
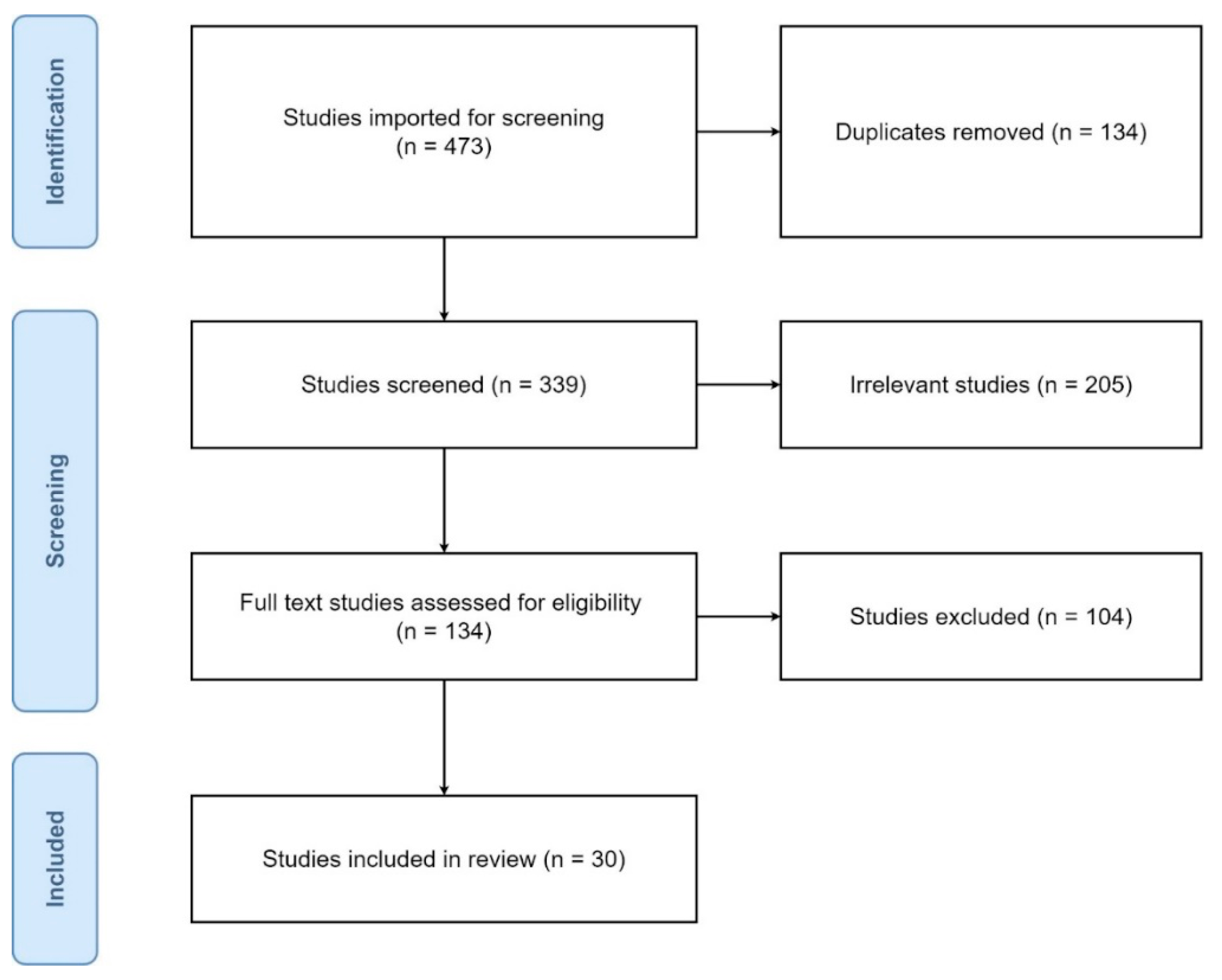
3. Results
3.1. Study Selection and Characteristics
3.2. Limitations
3.3. Certainty of Evidence
4. Discussion
4.1. Whole Skin Changes
4.2. Ophthalmic Changes
4.3. Nasopharyngeal Changes
4.4. Oral Changes
4.5. Mood and Neurological Changes
4.6. Musculoskeletal Changes
4.7. Gastrointestinal Changes
4.8. Infections
4.9. Others
4.10. Laboratory Abnormalities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5282379, Isotretinoin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isotretinoin (accessed on 28 February 2022).
- Pile, H.D.; Sadiq, N.M. Isotretinoin; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525949/ (accessed on 28 February 2022).
- Bagatin, E.; Costa, C.S.; da Rocha, M.A.D.; Picosse, F.R.; Kamamoto, C.S.L.; Pirmez, R.; Ianhez, M.; Miot, H.A. Consensus on the use of oral isotretinoin in dermatology—Brazilian Society of Dermatology. An. Bras. Dermatol. 2020, 95, 19–38. [Google Scholar] [CrossRef]
- Kochhar, D.M.; Penner, J.D. Developmental effects of isotretinoin and 4-oxo-isotretinoin: The role of metabolism in teratogenicity. Teratology 1987, 36, 67–75. [Google Scholar] [CrossRef]
- Nau, H. Teratogenicity of isotretinoin revisited: Species variation and the role of all-trans-retinoic acid. J. Am. Acad. Dermatol. 2001, 45, S183–S187. [Google Scholar] [CrossRef]
- Teo, W.L. The “Maskne” microbiome—Pathophysiology and therapeutics. Int. J. Dermatol. 2021, 60, 799–809. [Google Scholar] [CrossRef]
- Özkesici Kurt, B. The course of acne in healthcare workers during the COVID-19 pandemic and evaluation of possible risk factors. J. Cosmet. Dermatol. 2021, 20, 3730–3738. [Google Scholar] [CrossRef]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid. Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Stein Gold, L.; Segal, J.; Zaenglein, A.L. An Open-label, Phase IV Study Evaluating Lidose-isotretinoin Adminis-tered without Food in Patients with Severe Recalcitrant Nodular Acne: Low Relapse Rates Observed Over the 104-week Post-treatment Period. J. Clin. Aesthetic Dermatol. 2019, 12, 13–18. [Google Scholar]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00975143?term=isotretinoin&rslt=With&cond=Acne+Vulgaris&draw=2&rank=2&view=results (accessed on 28 February 2022).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01474590?term=isotretinoin&rslt=With&cond=Acne+Vulgaris&draw=2&rank=4&view=results (accessed on 28 February 2022).
- Li, Y.; Zhu, J.; Zhang, Y.; Liu, X.; Ye, J. Isotretinoin plus 420 nm intense pulsed light versus isotretinoin alone for the treatment of acne vulgaris: A randomized, controlled study of efficacy, safety, and patient satisfaction in Chinese subjects. Lasers Med. Sci. 2021, 36, 657–665. [Google Scholar] [CrossRef]
- Xia, J.; Hu, G.; Hu, D.; Geng, S.; Zeng, W. Concomitant Use of 1,550-nm Nonablative Fractional Laser with Low-Dose Isotretinoin for the Treatment of Acne Vulgaris in Asian Patients: A Randomized Split-Face Controlled Study. Dermatol. Surg. 2018, 44, 1201–1208. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Farag, A.; Hegazy, R.; Mongy, M.; Shalaby, S.; Kamel, M.M. Combined low-dose isotretinoin and pulsed dye laser versus standard-dose isotretinoin in the treatment of inflammatory acne. Lasers Surg. Med. 2021, 53, 603–609. [Google Scholar] [CrossRef]
- Rademaker, M.; Wishart, J.M.; Birchall, N.M. Isotretinoin 5 mg daily for low-grade adult acne vulgaris—A placebo-controlled, randomized double-blind study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 747–754. [Google Scholar] [CrossRef]
- Ahmad, H.M. Analysis of clinical efficacy, side effects, and laboratory changes among patients with acne vulgaris receiving single versus twice daily dose of oral isotretinoin. Dermatol. Ther. 2015, 28, 151–157. [Google Scholar] [CrossRef]
- Tan, J.; Humphrey, S.; Vender, R.; Barankin, B.; Gooderham, M.; Kerrouche, N.; Audibert, F.; Lynde, C. A treatment for severe nodular acne: A randomized investigator-blinded, controlled, noninferiority trial comparing fixed-dose adapalene/benzoyl peroxide plus doxycycline vs. oral isotretinoin. Br. J. Dermatol. 2014, 171, 1508–1516. [Google Scholar] [CrossRef]
- Oprica, C.; Emtestam, L.; Hagströmer, L.; Nord, C.E. Clinical and microbiological comparisons of isotretinoin vs. tetracycline in acne vulgaris. Acta Derm. Venereol. 2007, 87, 246–254. [Google Scholar] [CrossRef]
- Agarwal, U.; Bhola, K.; Besarwal, R. Oral isotretinoin in different dose regimens for acne vulgaris: A randomized comparative trial. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 688–694. [Google Scholar] [CrossRef]
- Gorpelioglu, C.; Özol, D.; Sarifakioglu, E. Influence of isotretinoin on nasal mucociliary clearance and lung function in patients with acne vulgaris. Int. J. Dermatol. 2010, 49, 87–90. [Google Scholar] [CrossRef]
- Akman, A.; Durusoy, C.; Senturk, M.; Koc, C.K.; Soyturk, D.; Alpsoy, E. Treatment of acne with intermittent and conventional isotretinoin: A randomized, controlled multicenter study. Arch. Dermatol. Res. 2007, 299, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Yoo, K.H.; Park, K.Y.; Han, T.Y.; Li, K.; Seo, S.J.; Hong, C.K. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: A randomized, controlled comparative study. Br. J. Dermatol. 2011, 164, 1369–1375. [Google Scholar] [CrossRef]
- Kus, S.; Gün, D.; Demirçay, Z.; Sur, H. Vitamin E does not reduce the side-effects of isotretinoin in the treatment of acne vulgaris. Int. J. Dermatol. 2005, 44, 248–251. [Google Scholar] [CrossRef]
- Kaymak, Y.; Ilter, N. The effectiveness of intermittent isotretinoin treatment in mild or moderate acne. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1256–1260. [Google Scholar] [CrossRef]
- DiGiovanna, J.J.; Langman, C.B.; Tschen, E.H.; Jones, T.; Menter, A.; Lowe, N.J.; Eichenfield, L.; Hebert, A.A.; Pariser, D.; Savin, R.P.; et al. Effect of a single course of isotretinoin therapy on bone mineral density in adolescent patients with severe, recalcitrant, nodular acne. J. Am. Acad. Dermatol. 2004, 51, 709–717. [Google Scholar] [CrossRef]
- Strauss, J.S.; Gottlieb, A.B.; Jones, T.; Koo, J.Y.; Leyden, J.J.; Lucky, A.; Pappas, A.A.; McLane, J.; Leach, E.E. Concomitant administration of vitamin E does not change the side effects of isotretinoin as used in acne vulgaris: A randomized trial. J. Am. Acad. Dermatol. 2000, 43, 777–784. [Google Scholar] [CrossRef]
- Hermes, B.; Praetel, C.; Henz, B.M. Medium dose isotretinoin for the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 117–121. [Google Scholar] [CrossRef]
- Lin, J.-L.; Shih, I.-H.; Yu, C.-C. Hemodialysis-related nodulocystic acne treated with isotretinoin. Nephron 1999, 81, 146–150. [Google Scholar] [CrossRef]
- Strauss, J.S.; Leyden, J.J.; Lucky, A.W.; Lookingbill, D.P.; Drake, L.A.; Hanifin, J.M.; Lowe, N.J.; Jones, T.M.; Stewart, D.M.; Jarratt, M.T.; et al. Safety of a new micronized formulation of isotretinoin in patients with severe recalcitrant nodular acne: A randomized trial comparing micronized isotretinoin with standard isotretinoin. J. Am. Acad. Dermatol. 2001, 45, 196–207. [Google Scholar] [CrossRef]
- Strauss, J.S.; Rapini, R.P.; Shalita, A.R.; Konecky, E.; Pochi, P.E.; Comite, H.; Exner, J.H. Isotretinoin therapy for acne: Results of a multicenter dose-response study. J. Am. Acad. Dermatol. 1984, 10, 490–496. [Google Scholar] [CrossRef]
- Lester, R.S.; Schachter, G.D.; Light, M.J. Isotretinoin and tetracycline in the management of severe nodulocystic acne. Int. J. Dermatol. 1985, 24, 252–257. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Socha-Szott, A.; Thomsen, R.J.; Pochi, P.E.; Shalita, A.R.; Strauss, J.S. Comparative effect of isotretinoin and etretinate on acne and sebaceous gland secretion. J. Am. Acad. Dermatol. 1982, 6, 760–765. [Google Scholar] [CrossRef]
- Jones, D.H.; King, K.; Miller, A.J.; Cunliffe, W.J. A dose-response study of I3-cis-retinoic acid in acne vulgaris. Br. J. Dermatol. 1983, 108, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, R.C.; Stamey, C.R.; Burkhart, C.N.; Lugo-Somolinos, A.; Morrell, D.S. High-Dose Isotretinoin Treatment and the Rate of Retrial, Relapse, and Adverse Effects in Patients with Acne Vulgaris. JAMA Dermatol. 2013, 149, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirnezami, M.; Rahimi, H. Is Oral Omega-3 Effective in Reducing Mucocutaneous Side Effects of Isotretinoin in Patients with Acne Vulgaris? Dermatol. Res. Pract. 2018, 2018, 6974045. [Google Scholar] [CrossRef] [PubMed]
- Kanigsberg, N.; DesGroseilliers, J.P. Use of 13-cis-retinoic acid in cystic acne. Can. Med. Assoc. J. 1983, 129, 224–228. [Google Scholar]
- Farrell, L.N.; Strauss, J.S.; Stranieri, A.M. The treatment of severe cystic acne with 13-cis-retinoic acid: Evaluation of sebum production and the clinical response in a multiple-dose trial. J. Am. Acad. Dermatol. 1980, 3, 602–611. [Google Scholar] [CrossRef]
- van der Meeren, H.L.; van der Schroeff, J.G.; Stijnen, T.; van Duren, J.A.; van der Dries, H.A.; van Voorst Vader, P.C. Dose-response relationship in isotretinoin therapy for conglobate acne. Dermatologica 1983, 167, 299–303. [Google Scholar] [CrossRef]
- Gencebay, G.; Aşkın, Ö.; Serdaroğlu, S. Evaluation of the changes in sebum, moisturization and elasticity in acne vulgaris patients receiving systemic isotretinoin treatment. Cutan. Ocul. Toxicol. 2021, 40, 140–144. [Google Scholar] [CrossRef]
- Bagatin, E.; Costa, C.S. The use of isotretinoin for acne—An update on optimal dosing, surveillance, and adverse effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Islamoğlu, Z.G.K.; Altınyazar, H.C. Effects of isotretinoin on the hair cycle. J. Cosmet. Dermatol. 2019, 18, 647–651. [Google Scholar] [CrossRef]
- Figueiras, D.D.A.; Ramos, T.B.; Marinho, A.K.D.O.F.; Bezerra, M.S.M.; Cauas, R.C. Paronychia and granulation tissue formation during treatment with isotretinoin. An. Bras. Dermatol. 2016, 91, 223–225. [Google Scholar] [CrossRef] [Green Version]
- Fouladgar, N.; Khabazkhoob, M.; Hanifnia, A.R.; Yekta, A.A.; Mirzajani, A. Evaluation of the effects of isotretinoin for treatment of acne on corneal sensitivity. J. Curr. Ophthalmol. 2018, 30, 326–329. [Google Scholar] [CrossRef]
- Karalezli, A.; Borazan, M.; Altinors, D.D.; Dursun, R.; Kiyici, H.; Akova, Y.A. Conjunctival impression cytology, ocular surface, and tear-film changes in patients treated with systemic isotretinoin. Cornea 2009, 28, 46–50. [Google Scholar] [CrossRef]
- Demirok, G.; Topalak, Y.; Gündüz, Ö.; Yildirim, D.; Kocamaz, M.F.; Şengün, A. The long-term effect of oral isotretinoin therapy on macula ganglion cell complex thickness. Cutan. Ocul. Toxicol. 2017, 36, 259–262. [Google Scholar] [CrossRef]
- Ucak, H.; Aykut, V.; Ozturk, S.; Cicek, D.; Erden, I.; Demir, B. Effect of Oral Isotretinoin Treatment on Retinal Nerve Fiber Layer Thickness. J. Cutan. Med. Surg. 2014, 18, 236–242. [Google Scholar] [CrossRef]
- Gediz, B.S.; Eroglu, F.C.; Aydogan, M.; Aydugan, M.T.; Hekimsoy, H.K. Choroidal vascular changes in acne patients under isotretinoin treatment. Cutan. Ocul. Toxicol. 2021, 40, 125–129. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Hafsi, W. Cheilitis; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470592/ (accessed on 28 February 2022).
- Kemeriz, F.; Kayabaşı, S.; Cemil, B.C.; Hızlı, Ö. Evaluation of oral isotretinoin effects on hearing system in patients with acne vulgaris: Reversible or not? Dermatol. Ther. 2021, 34, e14640. [Google Scholar] [CrossRef]
- McLane, J. Analysis of common side effects of isotretinoin. J. Am. Acad. Dermatol. 2001, 45, S188–S194. [Google Scholar] [CrossRef]
- Ormerod, A.D.; Thind, C.K.; Rice, S.A.; Reid, I.C.; Williams, J.H.G.; McCaffery, P.J.A. Influence of isotretinoin on hippocampal-based learning in human subjects. Psychopharmacology 2012, 221, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Javanbakht, A.M.A.; Pour, H.M.; Tarrahi, M.J. Effects of oral isotretinoin on serum folic acid levels. J. Drugs Dermatol. 2012, 11, 23–24. [Google Scholar]
- Polat, M.; Lenk, N.; Öztas, P.; Ilhan, M.N.; Artuz, F.; Alli, N.; Bingöl, S. Plasma homocysteine level is elevated in patients on isotretinoin therapy for cystic acne: A prospective controlled study. J. Dermatol. Treat. 2008, 19, 229–232. [Google Scholar] [CrossRef]
- Karadag, A.S.; Tutal, E.; Ertugrul, D.T.; Akin, K.O. Effect of isotretinoin treatment on plasma holotranscobalamin, vitamin B12, folic acid, and homocysteine levels: Non-controlled study. Int. J. Dermatol. 2011, 50, 1564–1569. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Karikas, G.A.; Georgala, S.; Michas, T.; Tsakiris, S. Elevated plasma homocysteine levels in patients on isotretinoin therapy for cystic acne. Int. J. Dermatol. 2001, 40, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ergun, T.; Seckin, D.; Ozaydin, N.; Bakar, Ö.; Comert, A.; Atsu, N.; Demircay, Z.; Yoney, H.; Zaimoglu, S. Isotretinoin has no negative effect on attention, executive function and mood. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Nevoralová, Z.; Dvořáková, D. Mood changes, depression and suicide risk during isotretinoin treatment: A prospective study. Int. J. Dermatol. 2013, 52, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, Y.; Taner, E.; Taner, Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int. J. Dermatol. 2009, 48, 41–46. [Google Scholar] [CrossRef]
- Bozdağ, K.E.; Gülseren, S.; Güven, F.; Çam, B. Evaluation of depressive symptoms in acne patients treated with isotretinoin. J. Dermatol. Treat. 2009, 20, 293–296. [Google Scholar] [CrossRef]
- Rehn, L.; Meririnne, E.; Isometsä, E.; Henriksson, M.; Höök-Nikanne, J. Depressive symptoms and suicidal ideation during isotretinoin treatment: A 12-week follow-up study of male Finnish military conscripts. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1294–1297. [Google Scholar] [CrossRef]
- Ferahbas, A.; Turan, M.T.; Esel, E.; Utas, S.; Kutlugun, C.; Kilic, C.G. A pilot study evaluating anxiety and depressive scores in acne patients treated with isotretinoin. J. Dermatol. Treat. 2004, 15, 153–157. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Peck, G.L.; Squillace, K.M.; Gantt, G.G. Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J. Am. Acad. Dermatol. 1987, 17, 25–32. [Google Scholar] [CrossRef]
- Ng, C.H.; Tam, M.M.; Celi, E.; Tate, B.; Schweitzer, I. Prospective study of depressive symptoms and quality of life in acne vulgaris patients treated with isotretinoin compared to antibiotic and topical therapy. Australas. J. Dermatol. 2002, 43, 262–268. [Google Scholar] [CrossRef]
- Chia, C.Y.; Lane, W.; Chibnall, J.; Allen, A.; Siegfried, E. Isotretinoin therapy and mood changes in adolescents with moderate to severe acne: A cohort study. Arch. Dermatol. 2005, 141, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadag, A.S.; Ertugrul, D.T.; Takcı, Z.; Bilgili, S.G.; Namuslu, M.; Ata, N.; Şekeroǧlu, R. The effect of isotretinoin on retinol-binding protein 4, leptin, adiponectin and insulin resistance in acne vulgaris patients. Dermatology 2015, 230, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Cemil, B.C.; Ayvaz, H.H.; Ozturk, G.; Ergin, C.; Akıs, H.K.; Gonul, M.; Arzuhal, E. Effects of isotretinoin on body mass index, serum adiponectin, leptin, and ghrelin levels in acne vulgaris patients. Postepy Dermatol. Allergol. 2016, 33, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaosmanoğlu, N.; Mülkoğlu, C. Analysis of musculoskeletal side effects of oral Isotretinoin treatment: A cross-sectional study. BMC Musculoskelet. Disord. 2020, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Hoover, K.B.; Miller, C.G.; Galante, N.C.; Langman, C.B. A double-blind, randomized, Phase III, multicenter study in 358 pediatric subjects receiving isotretinoin therapy demonstrates no effect on pediatric bone mineral density. Osteoporos. Int. 2015, 26, 2441–2447. [Google Scholar] [CrossRef]
- Leachman, S.A.; Insogna, K.L.; Katz, L.; Ellison, A.; Milstone, L.M. Bone densities in patients receiving isotretinoin for cystic acne. Arch. Dermatol. 1999, 135, 961–965. [Google Scholar] [CrossRef] [Green Version]
- Kindmark, A.; Rollman, O.; Mallmin, H.; Petrén-Mallmin, M.; Ljunghall, S.; Melhus, H. Oral isotretinoin therapy in severe acne induces transient suppression of biochemical markers of bone turnover and calcium homeostasis. Acta Derm. Venereol. 1998, 78, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Greene, J.P. An adolescent with abdominal pain taking isotretinoin for severe acne. South. Med. J. 2006, 99, 992–994. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jamal, M.M.; Nguyen, E.T.; Bechtold, M.L.; Nguyen, D.L. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 210–216. [Google Scholar] [CrossRef]
- Etminan, M.; Bird, S.T.; Delaney, J.A.; Bressler, B.; Brophy, J.M. Isotretinoin and risk for inflammatory bowel disease: A nested case-control study and meta-analysis of published and unpublished data. JAMA Dermatol. 2013, 149, 216–220. [Google Scholar] [CrossRef]
- Hazin, R.; Ibrahimi, O.A.; Hazin, M.I.; Kimyai-Asadi, A. Stevens-Johnson syndrome: Pathogenesis, diagnosis, and management. Ann. Med. 2008, 40, 129–138. [Google Scholar] [CrossRef]
- Abali, R.; Yuksel, M.A.; Aktas, C.; Celik, C.; Guzel, S.; Erfan, G.; Sahin, O. Decreased ovarian reserve in female Sprague–Dawley rats induced by isotretinoin (retinoic acid) exposure. Reprod. Biomed. Online 2013, 27, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktürk, A.S.; Abalı, R.; Yüksel, M.A.; Güzel, E.; Güzel, S.; Kıran, R. The effects of isotretinoin on the ovarian reserve of females with acne. Gynecol. Endocrinol. 2014, 30, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Cinar, L.; Açmaz, G.; Aksoy, U.; Aydin, T.; Vurdem, U.E.; Oz, L.; Karadag, O.I.; Kartal, D. The effect of isotretinoin on ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with acne. Gynecol. Obstet. Investig. 2015, 79, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Özturk, S.; Özturk, T.; Uçak, H.; Erden, I.; Demir, B.; Kayalı, A.; Cicek, D. Evaluation of ovarian reserve and function in female patients treated with oral isotretinoin for severe acne: An exploratory study. Cutan. Ocul. Toxicol. 2015, 34, 21–24. [Google Scholar] [CrossRef]
- Çinar, L.; Kartal, D.; Ergin, C.; Aksoy, H.; Karadag, M.A.; Aydin, T.; Cinar, E.; Borlu, M. The effect of systemic isotretinoin on male fertility. Cutan. Ocul. Toxicol. 2016, 35, 296–299. [Google Scholar] [CrossRef]
| Name | Other Names | Molecular Formula | Structure |
|---|---|---|---|
| Isotretinoin (ISO) | 13-cis-Retinoic Acid, Accutane, Ro 4-3780, Roaccutane | C20H28O2 | 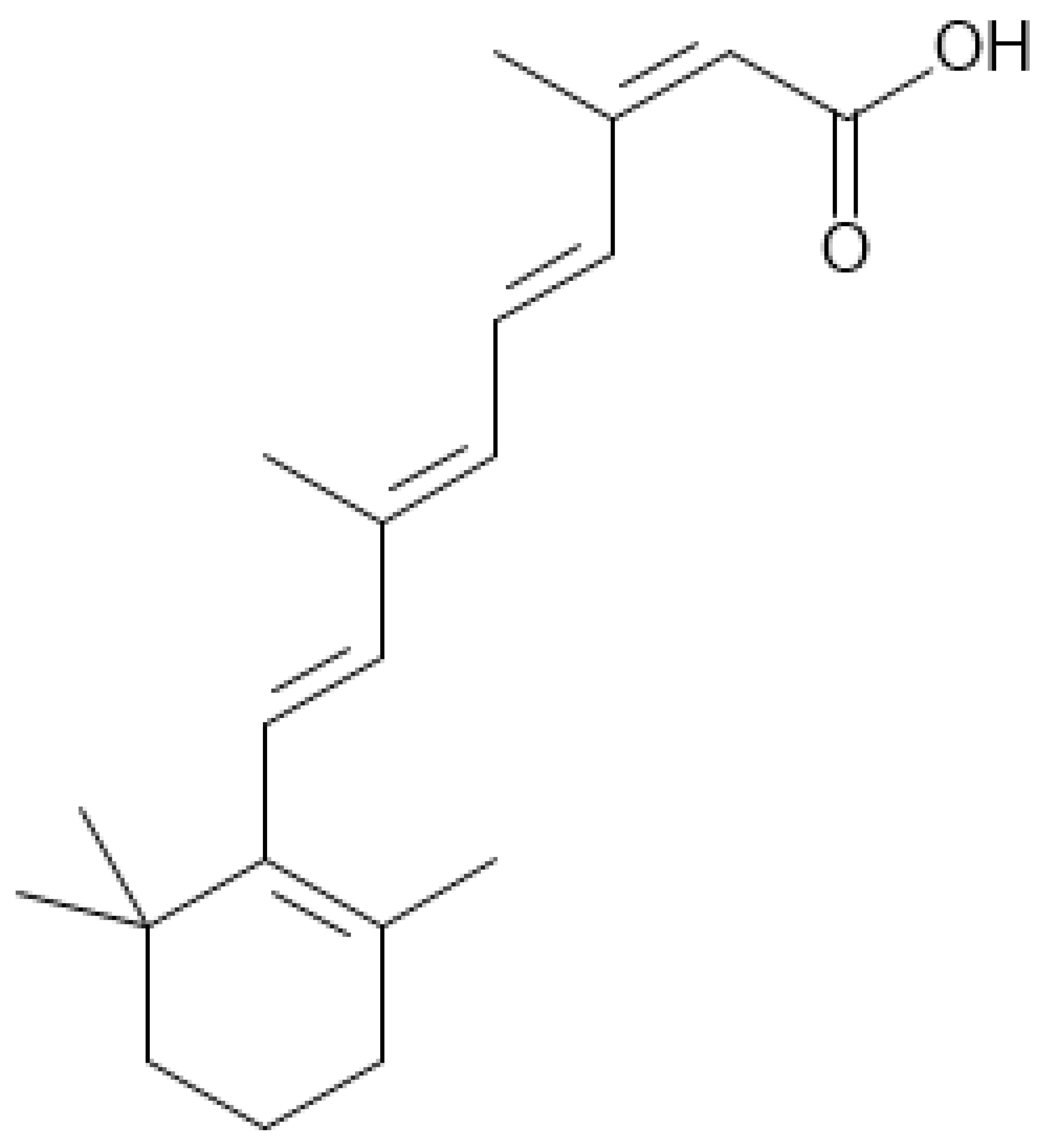 |
| Name/Author and Year of Publication | Population | Study Design | Drug Intervention and Scheme |
|---|---|---|---|
| Del Rosso et al., 2019 [11] | 166 | open-label CT | Lidose-ISO 0.5 mg/kg/day (4 weeks); 1.0 mg/kg/day (16 weeks); no treatment (104 weeks) |
| NCT00975143 [12] | 925 | RCT | ISO or CIP-ISO 0.5 mg/kg/day (2 weeks); 1.0 mg/kg/day (16 weeks) |
| Tan et al., 2014 [13] | 133 | RCT | ISO 0.5 mg/kg/day (4 weeks); 1.0 mg/kg/day (4 months) + vehicle gel |
| Li et al., 2020 [14] | 23 | RCT | ISO 0.5–0.75 mg/kg/day (8 weeks) + topical agents (adapalene 0.1% gel, fusidic acid 2% cream) |
| Xia et al., 2018 [15] | 18 | RCT | ISO 0.15–0.4 mg/kg/day (3 months) + 3 sessions of non-ablative fractional laser (NAFL) |
| Ibrahim et al., 2020 [16] | 46 | RCT | Group A: isotretinoin 0.25 mg/kg/day + 5 sessions of pulsed dry laser (PDL) (over 6 months); Group B: isotretinoin 0.5 mg/kg/day (over 6 months) |
| Rademaker et al., 2014 [17] | 60 | RCT | ISO 0.5 mg/kg/day (32 weeks) |
| Ahmad et al., 2015 [18] | 58 | RCT | ISO 0.5–1.0 mg/kg/day (3 months) |
| NCT01474590Tan [19] | 133 | RCT | ISO 0.5 mg/kg/day (4 weeks); ISO 1 mg/kg/day (16 weeks) + vehicle gel |
| Oprica et al., 2007 [20] | 52 | RCT | ISO 1.0 mg/kg/day (24 weeks) |
| Agarwal et al., 2011 [21] | 120 | RCT | Group A: ISO 1.0 mg/kg/day; Group B: ISO 1.0 mg/kg/alternate day; Group C: ISO 1.0 mg/kg/week/4 weeks (16 weeks); Group D: ISO 20 mg/every alternate day (16 weeks); for all groups oral azithromycin 500 mg/day/3 days a week (3 weeks) + topical 1% clindamycin phosphate/2 × day |
| Gorpelioglu et al., 2010 [22] | 40 | open-label CT | ISO 0.5–1.0 mg/kg/day (at least 3 months) |
| Akman et al., 2007 [23] | 66 | RCT | Group A: ISO 0.5 mg/kg/day/first 10 days of each month (6 months); Group B: ISO 0.5 mg/kg/day (1 month); ISO 0.5 mg/kg/day/the first 10 days of each month (5 months); Group C: ISO 0.5 mg/kg/day (6 months) |
| Lee et al., 2011 [24] | 60 | RCT | Group A: ISO 0.5–0.7 mg/kg/day; Group B: ISO 0.25–0.4 mg/kg/day; Group C: ISO 0.5–0.7 mg/kg/day for 1 week of every 4 weeks (24 weeks) |
| Kus et.al., 2005 [25] | 60 | RCT | Group A: ISO 1.0 mg/kg/day; Group B: ISO 1.0 mg/kg/day + vit. E 800 IU/day (16 weeks) |
| Kaymak et al., 2006 [26] | 41 | open-label CT | ISO 0.5–0.75 mg/kg/day, for 1 week every 4 weeks (6 months) |
| DiGiovanna et al., 2004 [27] | 217 | RCT | ISO 1.0 mg/kg/day (16–20 weeks) |
| Strauss et al., 2000 [28] | 140 | RCT | Group A: ISO 1.0 mg/kg/day; Group B: ISO 1.0 mg/kg/day + 800 IU/day vit. E (20 weeks) |
| Hermes et al., 1998 [29] | 94 | open-label CT | ISO ~0.43 mg/kg/day (~8.3 months) |
| Lin et al., 1999 [30] | 18 | RCT | ISO 10 mg/day (3 months) |
| Strauss et al., 2001 [31] | 300 | RCT | ISO 1.0 mg/kg/day (20 weeks) |
| Strauss et al., 1984 [32] | 150 | RCT | Group A: ISO 0.1 mg/kg/day; Group B: ISO 0.5 mg/kg/day; Group C: ISO 1.0 mg/kg/day (20 weeks) |
| Lester et al., 1985 [33] | 15 | RCT | ISO 0.5–2.0 mg/kg/day (16 weeks) |
| Goldstein et al., 1982 [34] | 28 | RCT | ISO 1.0 mg/kg/day (16 weeks) |
| Jones et al., 1983 [35] | 76 | RCT | ISO 0.1 mg/kg/day; Group B: ISO 0.5 mg/kg/day; Group C: ISO 1.0 mg/kg/day (16 weeks) |
| Blasiak et al., 2013 [36] | 116 | RCT | Group A: ISO mean cumulative dose < 220 mg/kg (5.8 months); Group B: ISO mean cumulative dose > 220 mg/kg (6.5 months) |
| Mirnezami et al., 2018 [37] | 118 | RCT | Group A: ISO 0.5 mg/kg/day; Group B: ISO 0.5 mg/kg/day + oral omega-3 1.0 g/day (16 weeks) |
| Kanigsberg et al., 1983 [38] | 33 | open-label CT | ISO 0.5–1.0 mg/kg/day (16 weeks) |
| Farrell et al., 1980 [39] | 14 | RCT | ISO 0.1–1.0 mg/kg/day (12 weeks) |
| Meeren et al., 1983 [40] | 58 | RCT | Group A: ISO 0.5 mg/kg/day; Group B: ISO 1.0 mg/kg/day (24 weeks) |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Dry skin | 3228 (45 IAs) | 1584 (49.07%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| Skin fragility | 235 (7 IAs) | 65 (27.66%) | ⨁⨁⨁◯ moderate due to indirectness |
| Erythemal changes | 2350 (33 IAs) | 653 (27.79%) | ⨁⨁⨁◯ moderate due to indirectness |
| Sunburn | 141 (3 IAs) | 17 (12.06%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Decreased sweating | 15 (1 IA) | 1 (6.67%) | ⨁◯◯◯ very low due to imprecision |
| Dryness of other mucosal tissues | 238 (6 IAs) | 70 (29.41%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| Hair changes | 837 (20 IAs) | 149 (17.90%) | ⨁⨁⨁◯ moderate due to indirectness |
| Hand changes | 692 (18 IAs) | 171 (24.71%) | ⨁⨁⨁◯ moderate due to indirectness |
| Skin atrophy | 58 (2 IAs) | 2 (3.45%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Flare of acne | 96 (4 IAs) | 46 (47.92%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Dry or irritated eyes | 2244 (27 IAs) | 603 (26.87%) | ⨁⨁⨁◯ moderate due to indirectness |
| Eye pain | 169 (4 IAs) | 29 (17.16%) | ⨁⨁⨁◯ moderate due to indirectness |
| Vision changes | 1313 (10 IAs) | 72 (5.48%) | ⨁⨁⨁◯ moderate due to indirectness |
| Conjunctival changes | 545 (11 IAs) | 125 (22.49%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Dry nose | 860 (18 IAs) | 346 (40.23%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| Nasopharyngitis | 1543 (8 IAs) | 205 (13.29%) | ⨁⨁⨁◯ moderate due to indirectness |
| UPTRI | 1058 (2 IAs) | 52 (4.91%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Epistaxis | 2250 (30 IAs) | 536 (23.82%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| Cough | 58 (1 IAs) | 2 (3.45%) | ⨁◯◯◯ very low due to imprecision |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Dry lips | 2309 (30 IAs) | 1344 (58.21%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| Cheilitis | 2528 (27 IAs) | 1049 (41.50%) | ⨁⨁⨁◯ moderate due to indirectness |
| Dry and sore mouth | 726 (21 IAs) | 275 (37.88%) | ⨁⨁⨁◯ moderate due to indirectness |
| Excessive thirst | 393 (10 IAs) | 138 (35.11%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Hearing changes | 116 (2 IAs) | 2 (1.72%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Headache | 1967 (17 IAs) | 207 (10.52%) | ⨁⨁⨁◯ moderate due to indirectness |
| Mood changes | 521 (11 IAs) | 53 (10.17%) | ⨁⨁⨁◯ moderate due to indirectness |
| Decreased appetite | 141 (3 IAs) | 23 (16.31%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Increased appetite | 156 (4 IAs) | 6 (3.85%) | ⨁⨁⨁◯ moderate due to indirectness |
| Fatigue and tiredness | 543 (16 IAs) | 132 (24.31%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Back pain | 1500 (4 IAs) | 294 (19.60%) | ⨁⨁⨁◯ moderate due to indirectness |
| Arthralgia | 1716 (15 IAs) | 297 (17.31%) | ⨁⨁⨁⨁ high due to dose-response gradient, however indirectness present |
| MS discomfort | 1717 (20 IAs) | 216 (12.58%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| GI disorders | 257 (5 IAs) | 25 (9.73%) | ⨁⨁⨁◯ moderate due to indirectness |
| Abdominal pain | 555 (9 IAs) | 51 (9.19%) | ⨁⨁⨁◯ moderate due to indirectness |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Infections | 29 (1 IA) | 11 (37.93%) | ⨁◯◯◯ very low due to imprecision |
| Herpes simplex | 15 (1 IA) | 0 (0.00%) | ⨁◯◯◯ very low due to imprecision |
| Fever | 141 (3 IAs) | 8 (5.67%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Stevens-Johnson syndrome | 58 (1 IA) | 2 (3.45%) | ⨁◯◯◯ very low due to imprecision |
| Morbilliform eruption | 133 (3 IAs) | 7 (5.26%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Pyogenic granuloma | 1058 (2 IAs) | 2 (0.19%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Irregular cycle | 15 (1 IA) | 0 (0.00%) | ⨁◯◯◯ very low due to imprecision |
| Outcomes | Number of Participants (IAs) | Prevalence (%) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Liver function tests abn | 2018 (40 IAs) | 99 (4.91%) | ⨁⨁⨁◯ moderate due to indirectness |
| AST ↑ | 1768 (38 IAs) | 77 (4.36%) | ⨁⨁⨁◯ moderate due to indirectness |
| ALT ↑ | 1692 (35 IAs) | 50 (2.96%) | ⨁⨁⨁◯ moderate due to indirectness |
| GGT ↑ | 579 (6 IAs) | 24 (4.15%) | ⨁⨁⨁◯ moderate due to indirectness |
| ALP ↑ | 1034 (17 IAs) | 8 (0.77%) | ⨁⨁⨁◯ moderate due to indirectness |
| Total protein ↑ | 321 (7 IAs) | 16 (4.98%) | ⨁⨁⨁◯ moderate due to indirectness |
| Albumin ↑ | 188 (5 IAs) | 0 (0.00%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| LDH ↑ | 621 (8 IAs) | 58 (9.34%) | ⨁⨁⨁◯ moderate due to indirectness |
| Total bilirubin ↑ | 278 (9 IAs) | 2 (0.72%) | ⨁⨁⨁◯ moderate due to indirectness |
| Direct bilirubin ↑ | 90 (4 IAs) | 1 (1.11%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Lipid test abn | 2010 (39 IAs) | 290 (14.43%) | ⨁⨁⨁◯ moderate due to indirectness |
| Total cholesterol ↑ | 1927 (37 IAs) | 142 (7.37%) | ⨁⨁⨁◯ moderate due to indirectness |
| HDL ↓ | 508 (13 IAs) | 83 (16.34%) | ⨁⨁⨁◯ moderate due to indirectness |
| LDL ↑ | 367 (10 IAs) | 87 (23.71%) | ⨁⨁⨁◯ moderate due to indirectness |
| VLDL ↑ | 213 (4 IAs) | 59 (27.70%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Triglycerides ↑ | 2000 (39 IAs) | 206 (10.30%) | ⨁⨁⨁◯ moderate due to indirectness |
| Blood count abn | 1062 (17 IAs) | 21 (1.98%) | ⨁⨁⨁◯ moderate due to indirectness |
| RBC abn | 1054 (16 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Reticulocytes abn | 155 (4 IAs) | 0 (0.00%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Hb abn | 1054 (16 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| HCT abn | 1054 (16 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| WBC abn | 1054 (16 IAs) | 18 (1.71%) | ⨁⨁⨁◯ moderate due to indirectness |
| Neutrophiles abn | 593 (7 IAs) | 3 (0.51%) | ⨁⨁⨁◯ moderate due to indirectness |
| Eosinophiles abn | 593 (7 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Basophiles abn | 593 (7 IAs) | 3 (0.51%) | ⨁⨁⨁◯ moderate due to indirectness |
| Monocytes abn | 593 (7 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Lymphocytes abn | 593 (7 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Platelets abn | 1062 (17 IAs) | 11 (1.04%) | ⨁⨁⨁◯ moderate due to indirectness |
| ERS ↑ | 14 (1 IAs) | 7 (50.00%) | ⨁◯◯◯ very low due to imprecision |
| Urine test abn | 296 (9 IAs) | 16 (5.41%) | ⨁⨁⨁◯ moderate due to indirectness |
| Specific gravity ↑ | 141 (3 IAs) | 16 (11.35%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| WBC ↑ | 141 (3 IAs) | 11 (7.80%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| Kidney function test abn | 1286 (9 IAs) | 55 (3.66%) | ⨁⨁⨁◯ moderate due to indirectness |
| Blood urea nitrogen ↑ | 321 (7 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Uric acid ↑ | 293 (6 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| CK ↑ | 1463 (9 IAs) | 55 (3.76%) | ⨁⨁⨁◯ moderate due to indirectness |
| Glucose abn | 361 (8 IAs) | 0 (0.00%) | ⨁⨁⨁◯ moderate due to indirectness |
| Calcium-phosphate abn | 231 (2 IAs) | 0 (0.00%) | ⨁◯◯◯ very low due to imprecision |
| Ca abn | 231 (2 IAs) | 0 (0.00%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| P abn | 231 (2 IAs) | 0 (0.00%) | ⨁⨁◯◯ low due to indirectness and imprecision |
| 25(OH)D abn | 217 (1 IAs) | 0 (0.00%) | ⨁◯◯◯ very low due to imprecision |
| Semen test abn | 28 (1 IAs) | 0 (0.00%) | ⨁◯◯◯ very low due to imprecision |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapała, J.; Lewandowska, J.; Placek, W.; Owczarczyk-Saczonek, A. Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6463. https://doi.org/10.3390/ijerph19116463
Kapała J, Lewandowska J, Placek W, Owczarczyk-Saczonek A. Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(11):6463. https://doi.org/10.3390/ijerph19116463
Chicago/Turabian StyleKapała, Jan, Julia Lewandowska, Waldemar Placek, and Agnieszka Owczarczyk-Saczonek. 2022. "Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 11: 6463. https://doi.org/10.3390/ijerph19116463







