Food Waste Management Employing UV-Induced Black Soldier Flies: Metabolomic Analysis of Bioactive Components, Antioxidant Properties, and Antibacterial Potential
Abstract
:1. Introduction
2. Results
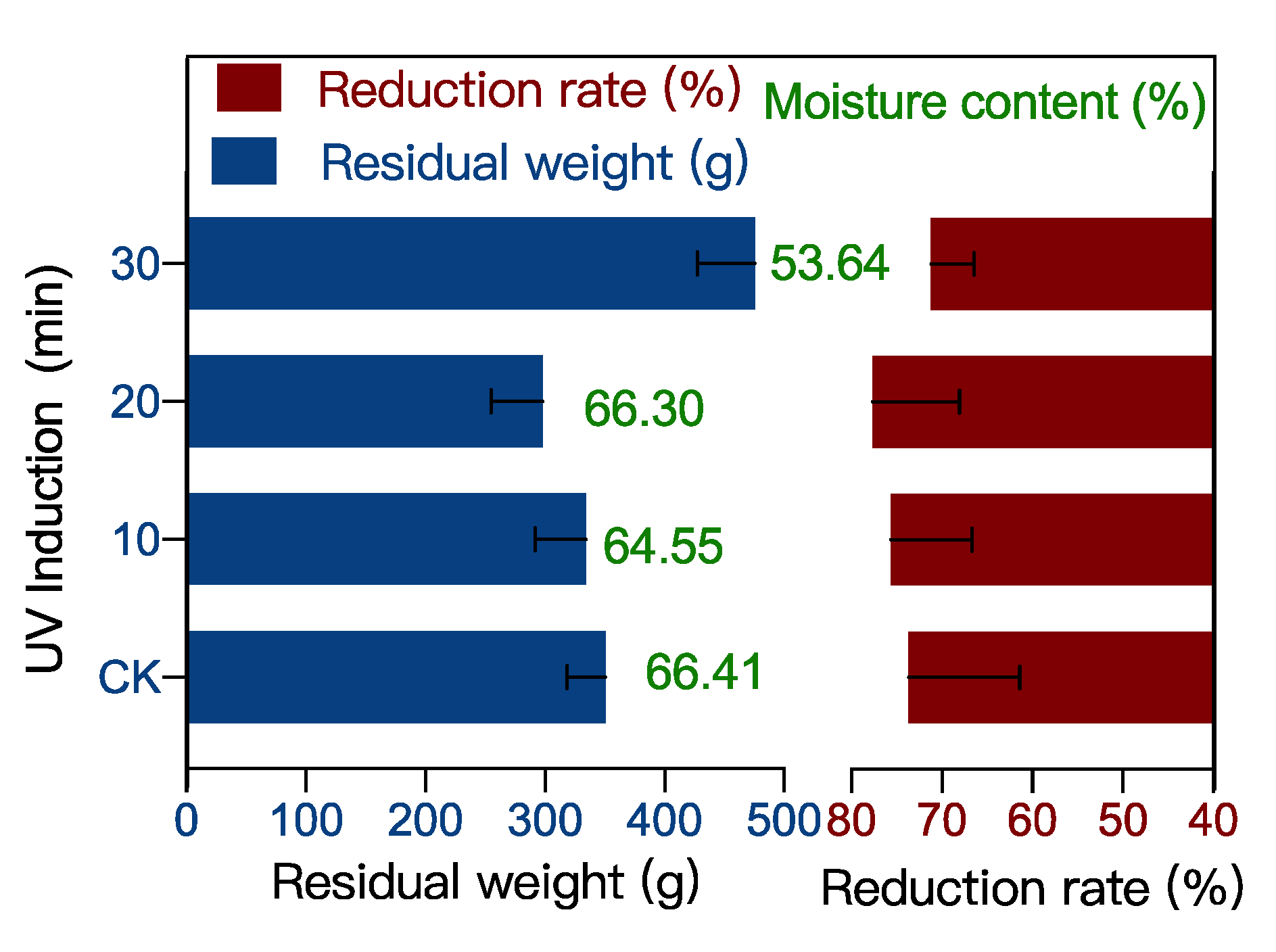
2.1. Food Waste Reduction Potential of UV-Induced BSF
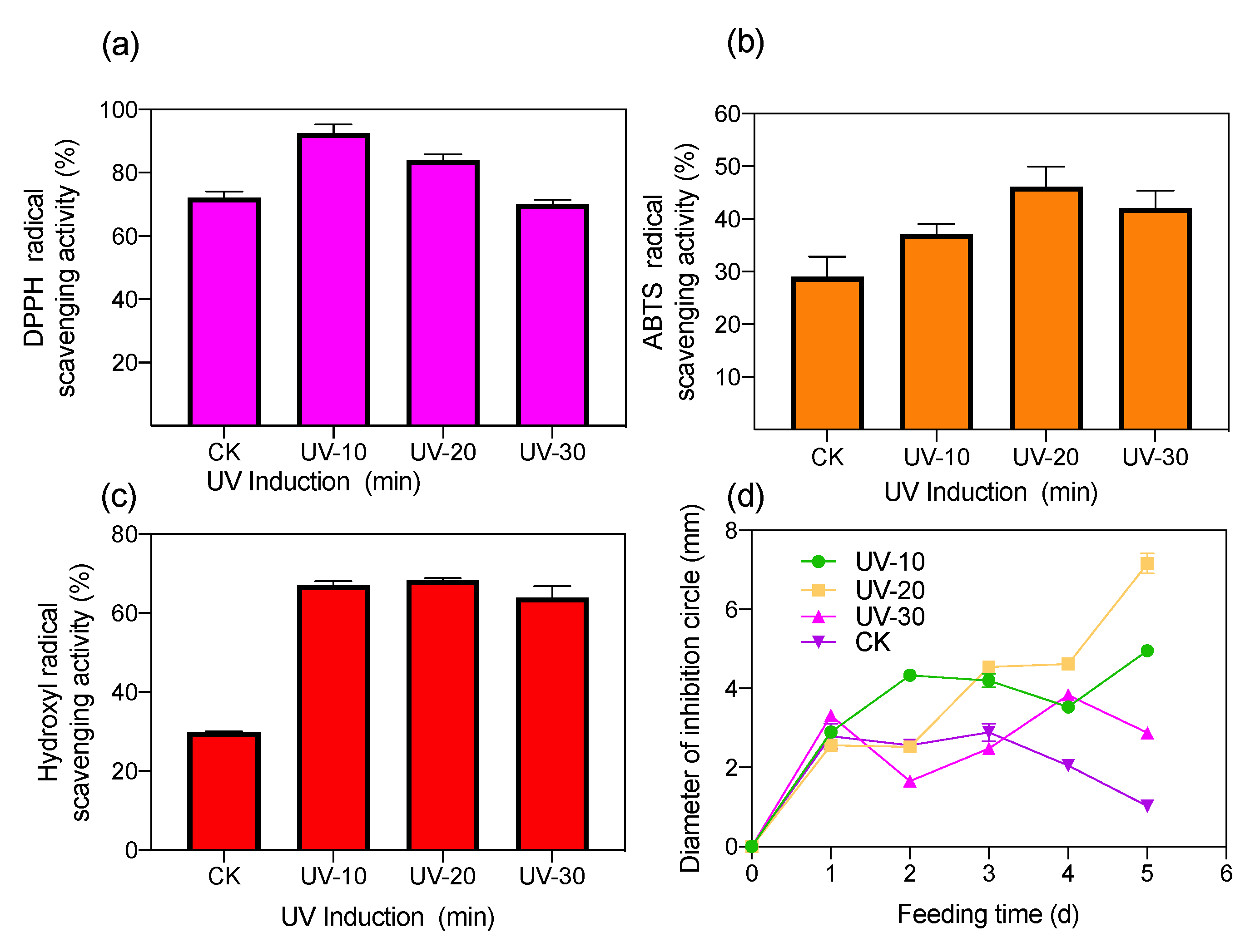
2.2. Antioxidant and Antimicrobial Activity of UV-Induced BSF Extract
2.3. Metabolic Profiling of UV-Induced BSF Extracts
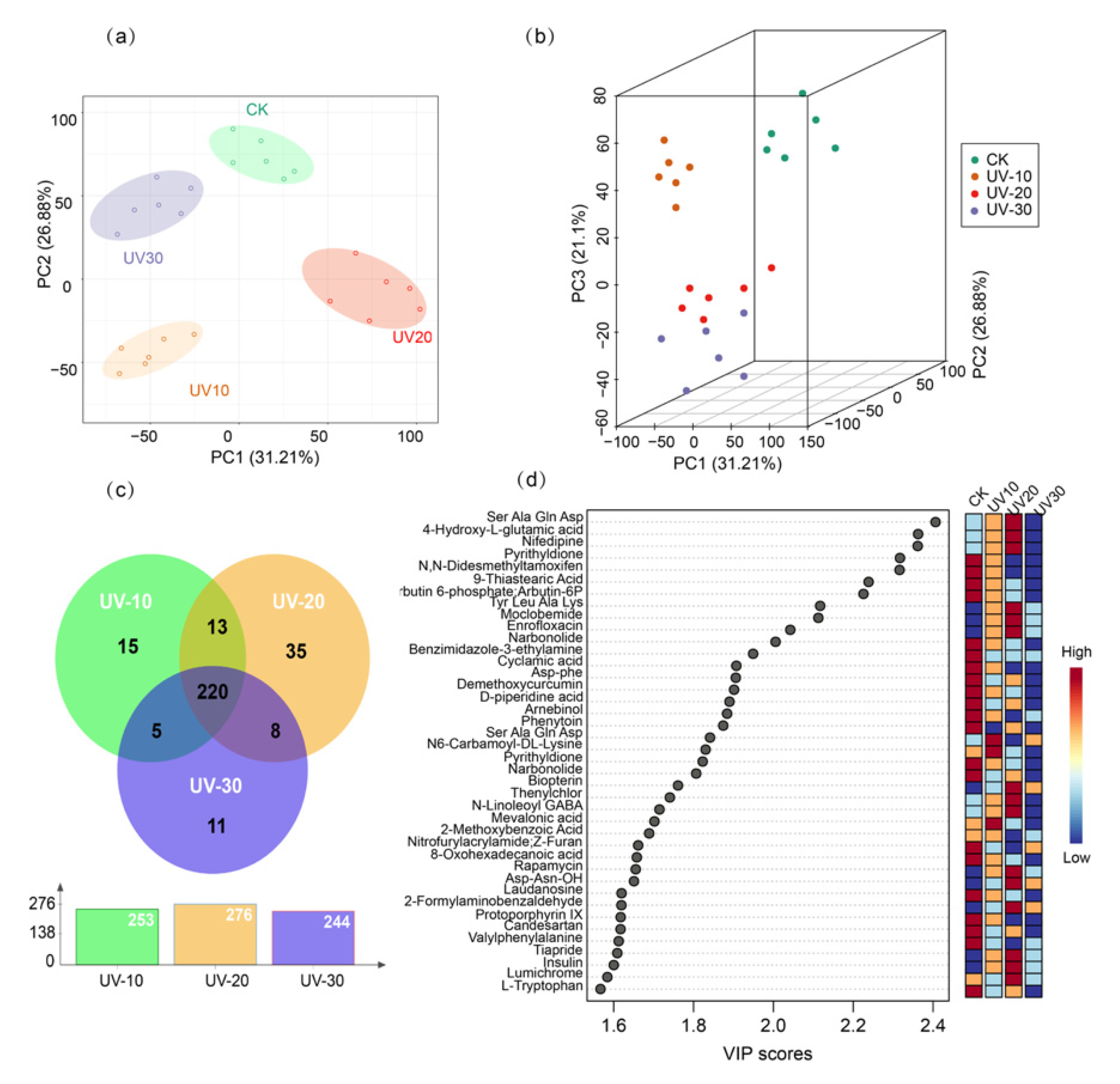
2.3.1. PCA and PLS-DA Analysis of the BSF with and without UV Treatment
2.3.2. OPLS-DA Analysis of BSF with Different UV Induction Times
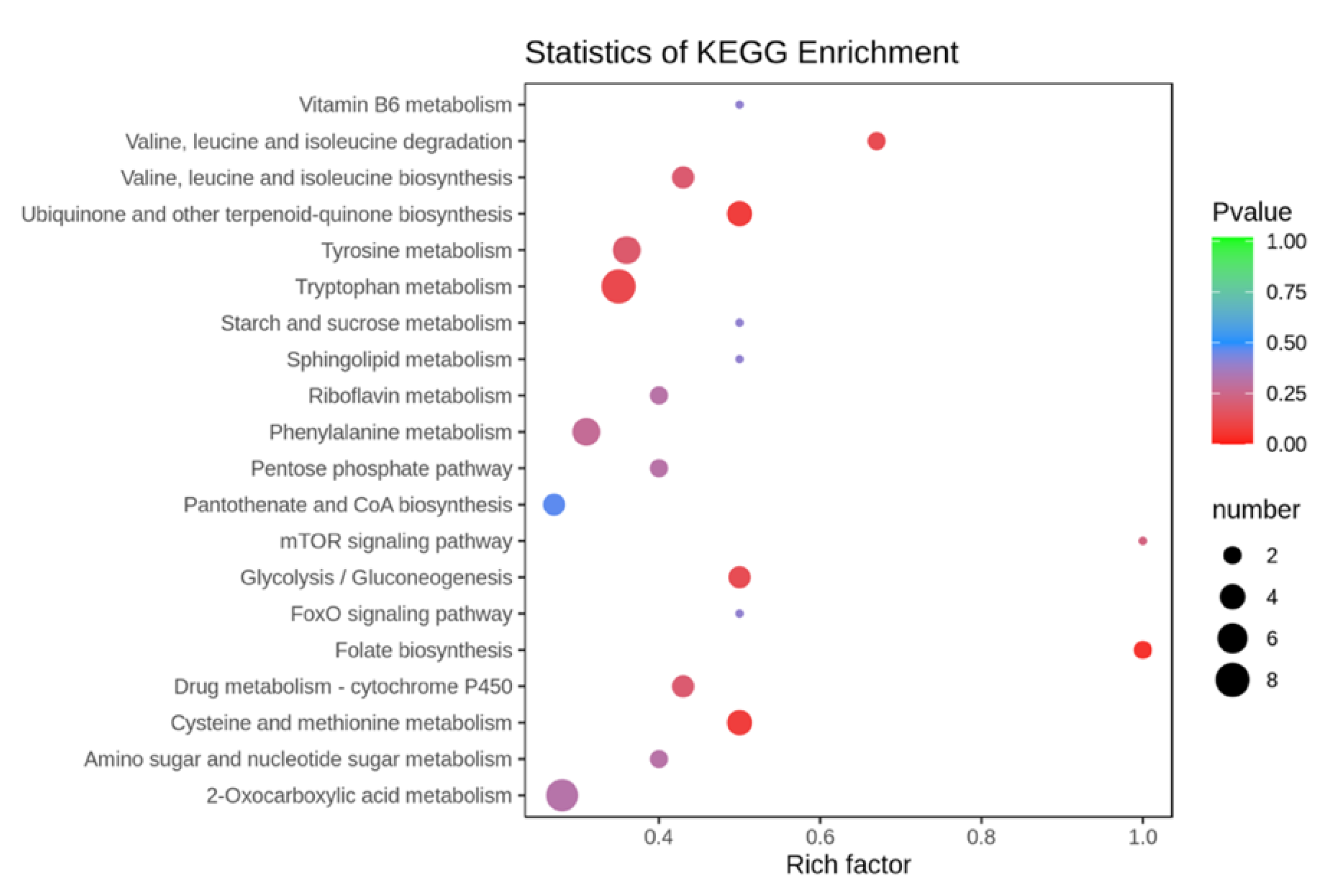
2.3.3. Effect of UV Induction on the Enriched Metabolic Pathway of BSF
3. Discussion
3.1. Environmental Signaling Effects of UV-Induced BSF
3.2. Effect of UV Induction on the Digestion of Sugars and Starch in Food Waste by BSF
3.3. Effect of UV Induction on the Metabolism Cofactors and Bioactive Compounds in Food Waste by BSF
3.4. Effect of UV Induction on Amino Acids and Peptides in BSF
3.5. Application Potential of Bioactive Compounds in UV Induced BSF
4. Materials and Methods
4.1. Materials
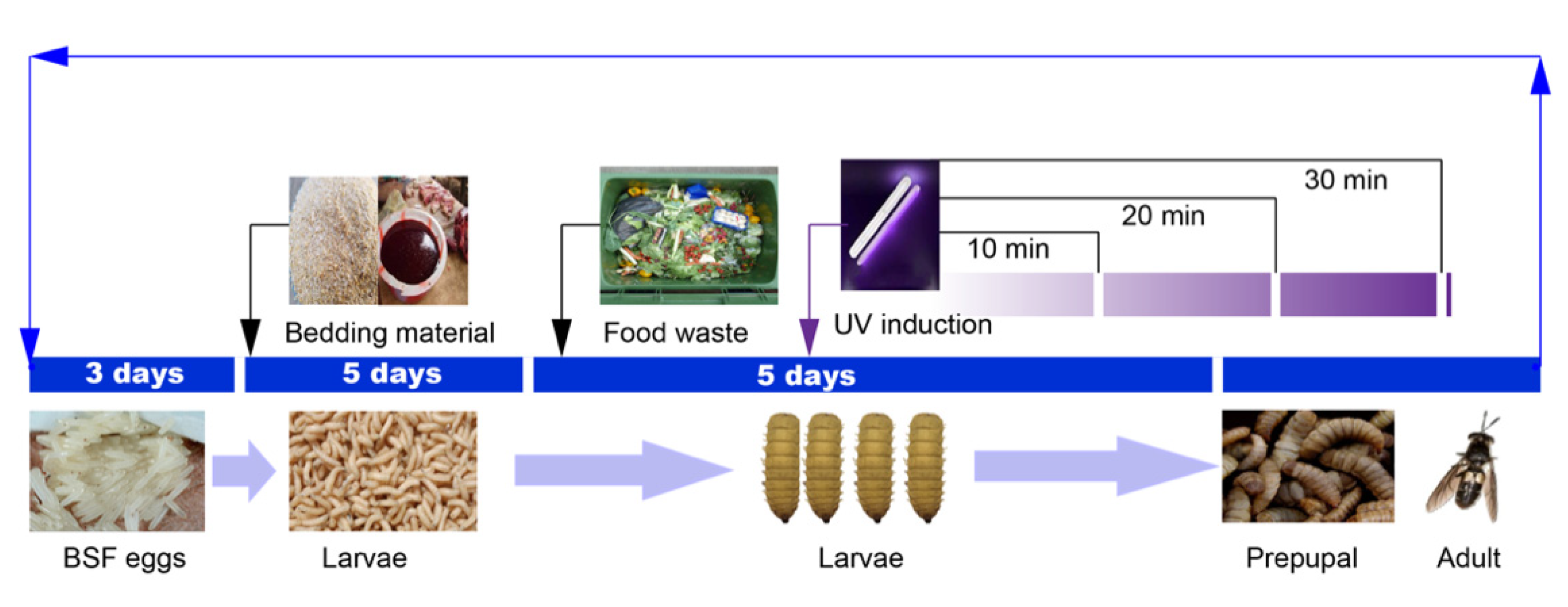
4.2. Breeding Process
4.3. UV Induction
4.4. Antioxidant Assays
4.5. Antibacterial Experiment
4.6. Untargeted Metabolomic Analysis
4.6.1. Extraction of BSF
4.6.2. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis and Data Processing
4.6.3. Identification of Metabolites
4.6.4. Data Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- To, S.; Coughenour, C.; Pharr, J. The Environmental Impact and Formation of Meals from the Pilot Year of a Las Vegas Convention Food Rescue Program. Int. J. Environ. Res. Public Health 2019, 16, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Singh, A.; Srikanth, B.; Kumari, K. Determining the Black Soldier fly larvae performance for plant-based food waste reduction and the effect on Biomass yield. Waste Manag. 2021, 130, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, C.-H.; Kim, M.-J.; Chung, H. Effects of microplastics and salinity on food waste processing by black soldier fly (Hermetia illucens) larvae. J. Ecol. Environ. 2020, 44, 7. [Google Scholar] [CrossRef] [Green Version]
- Lavine, M.; Strand, M. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Oonincx, D.; Van Keulen, P.; Finke, M.; Baines, F.; Vermeulen, M.; Bosch, G. Evidence of vitamin D synthesis in insects exposed to UVb light. Sci. Rep. 2018, 8, 10807. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Y.; Liu, Q.; Tan, X.; Xie, X.; Xia, Q.; Zhao, P. GC/MS-based metabolomics analysis reveals active fatty acids biosynthesis in the Filippi’s gland of the silkworm, Bombyx mori, during silk spinning. Insect Biochem. Mol. Biol. 2019, 105, 1–9. [Google Scholar] [CrossRef]
- Melis, R.; Braca, A.; Sanna, R.; Spada, S.; Mulas, G.; Fadda, M.L.; Sassu, M.M.; Serra, G.; Anedda, R. Metabolic response of yellow mealworm larvae to two alternative rearing substrates. Metabolomics 2019, 15, 113. [Google Scholar] [CrossRef]
- Krautz, R.; Arefin, B.; Theopold, U. Damage signals in the insect immune response. Front. Plant Sci. 2014, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.-Y.; Zhang, C.-Y.; Zhu, F.; Wang, X.-P.; Lei, C.-L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef]
- Erion, R.; Sehgal, A. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walterscheid, J.P.; Nghiem, D.X.; Kazimi, N.; Nutt, L.K.; McConkey, D.J.; Norval, M.; Ullrich, S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 17420–17425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, W.; Ma, W.-H.; Qiu, L.; Zhu, Z.-H.; Lei, C.-L. The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect Physiol. 2012, 58, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Mohan, M.; Taneja, T.K.; Sahdev, S.; Mohareer, K.; Begum, R.; Athar, M.; Sah, N.K.; Hasnain, S.E. Antioxidants prevent UV-induced apoptosis by inhibiting mitochondrial cytochrome c release and caspase activation in Spodoptera frugiperda (Sf9) cells. Cell Biol. Int. 2003, 27, 483–490. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Molecular basis and regulatory mechanisms underlying fungal insecticides’ resistance to solar ultraviolet irradiation. Pest Manag. Sci. 2022, 78, 30–42. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Fu, B.; Tong, S.-M.; Ying, S.-H.; Feng, M.-G. Two photolyases repair distinct DNA lesions and reactivate UVB-inactivated conidia of an insect mycopathogen under visible light. Appl. Environ. Microbiol. 2019, 85, e02459-18. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Adar, S. The cartography of UV-induced DNA damage formation and DNA repair. Photochem. Photobiol. 2017, 93, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S. Computational approaches to metabolomics. In Bioinformatics Methods in Clinical Research; Springer: Berlin/Heidelberg, Germany, 2010; pp. 283–313. [Google Scholar]
- Cevallos-Cevallos, J.M.; Jines, C.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J.; Ochoa, D.E.; Flores-Cedeno, J.A. GC-MS metabolite profiling for specific detection of dwarf somaclonal variation in banana plants. Appl. Plant Sci. 2018, 6, e01194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otvos, L.O.I.; Rogers, M.E.; Consolvo, P.J.; Condie, B.A.; Lovas, S.; Bulet, P.; Blaszczyk-Thurin, M. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 2000, 39, 14150–14159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tomberlin, J.; Cai, M.; Xiao, X.; Zheng, L.; Yu, Z. Research and industrialisation of Hermetia illucens L. in China. J. Insects Food Feed 2020, 6, 5–12. [Google Scholar] [CrossRef]
- Surendra, K.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.-H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Fang, G.; Cai, M.; Kou, Z.; Xu, J.; Cao, Y.; Bai, L.; Zhang, Y.; Jiang, Y.; Luo, X. Genomic landscape and genetic manipulation of the black soldier fly Hermetia illucens, a natural waste recycler. Cell Res. 2020, 30, 50–60. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Zhang, J.; Li, J.-H.; Tomerlin, J.K.; Xiao, X.-P.; Rehman, K.; Cai, M.-M.; Zheng, L.-Y.; Yu, Z.-N. Black soldier fly: A new vista for livestock and poultry manure management. J. Integr. Agric. 2021, 20, 1167–1179. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Mahadevan, S.; Shah, S.L.; Marrie, T.J.; Slupsky, C.M. Analysis of metabolomic data using support vector machines. Anal. Chem. 2008, 80, 7562–7570. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Abou El Asrar, R.; Cools, D.; Broeck, J.V. Role of peptide hormones in insect gut physiology. Curr. Opin. Insect Sci. 2020, 41, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, F.; Dong, Z.; Hua, X.; Xia, Q. Label-free quantitative phosphoproteomic profiling of cellular response induced by an insect cytokine paralytic peptide. J. Proteom. 2017, 154, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-W.; Storey, K.B. mTOR Signaling in Metabolic Stress Adaptation. Biomolecules 2021, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Sabatini, D.M. A central role for mTOR in lipid homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, B.K.; Lamming, D.W. The mechanistic target of rapamycin: The grand conducTOR of metabolism and aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef] [Green Version]
- Prete, P.E. Growth effects of Phaenicia sericata larval extracts on fibroblasts: Mechanism for wound healing by maggot therapy. Life Sci. 1997, 60, 505–510. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a multi-signalling guardian angel in human diseases: When, where and how does it exert its protective effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Knight, D.; Harvey, P.J.; Iliadi, K.G.; Klose, M.K.; Iliadi, N.; Dolezelova, E.; Charlton, M.P.; Zurovec, M.; Boulianne, G.L. Equilibrative nucleoside transporter 2 regulates associative learning and synaptic function in Drosophila. J. Neurosci. 2010, 30, 5047–5057. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Biochemistry and Molecular Biology of Digestion; Academic Press: Cambridge, MA, USA, 2012; pp. 365–418. [Google Scholar]
- Pimentel, A.C.; Barroso, I.G.; Ferreira, J.M.; Dias, R.O.; Ferreira, C.; Terra, W.R. Molecular machinery of starch digestion and glucose absorption along the midgut of Musca domestica. J. Insect Physiol. 2018, 109, 11–20. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Feng, S.; Zou, W.; Guo, K.; Fan, C.; Si, S.; Peng, L. Analysis of five rice 4-coumarate: Coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2013, 430, 1151–1156. [Google Scholar] [CrossRef]
- Hunter, D.J.; Torkelson, J.L.; Bodnar, J.; Mortazavi, B.; Laurent, T.; Deason, J.; Thephavongsa, K.; Zhong, J. The Rickettsia endosymbiont of Ixodes pacificus contains all the genes of de novo folate biosynthesis. PLoS ONE 2015, 10, e0144552. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The B vitamin nutrition of insects: The contributions of diet, microbiome and horizontally acquired genes. Curr. Opin. Insect Sci. 2017, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Dai, W.; Qi, L.; Zhang, Q.; Zhang, C. Identification and functional analysis of a delta class glutathione S-transferase gene associated with insecticide detoxification in Bradysia odoriphaga. J. Agric. Food Chem. 2019, 67, 9979–9988. [Google Scholar] [CrossRef] [PubMed]
- Miyata, U.; Arakawa, K.; Takei, M.; Asami, T.; Asanbou, K.; Toshima, H.; Suzuki, Y. Identification of an aromatic aldehyde synthase involved in indole-3-acetic acid biosynthesis in the galling sawfly (Pontania sp.) and screening of an inhibitor. Insect Biochem. Mol. Biol. 2021, 137, 103639. [Google Scholar] [CrossRef]
- Uçkan, F.; Özbek, R.; Ergin, E. Effects of Indol-3-Acetic Acid on the biology of Galleria mellonella and its endoparasitoid Pimpla turionellae. Belg. J. Zool. 2015, 145, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 79. [Google Scholar] [CrossRef]
- Rocic, P.; Schwartzman, M.L. 20-HETE in the regulation of vascular and cardiac function. Pharmacol. Ther. 2018, 192, 74–87. [Google Scholar] [CrossRef]
- Sudheer, S.; Gangwar, P.; Usmani, Z.; Sharma, M.; Sharma, V.K.; Sana, S.S.; Almeida, F.; Dubey, N.K.; Singh, D.P.; Dilbaghi, N. Shaping the gut microbiota by bioactive phytochemicals: An emerging approach for the prevention and treatment of human diseases. Biochimie 2021, 193, 38–63. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Doelle, K.; Smith, R. External morphology of Hermetia illucens Stratiomyidae: Diptera (L. 1758) based on electron microscopy. Annu. Res. Rev. Biol. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
- Zielińska, E.; Ba Raniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Category | Compounds | Index | Formula | Precursor (g/mol) | Abundance | Log2FC | |||
|---|---|---|---|---|---|---|---|---|---|
| CK | UV-10 | UV-20 | UV-30 | ||||||
| Peptides | (-)-α-Kainic acid | MW0103805 | C10H15NO4 | 212.09 | 37,437.47 | 56,806.2 | 39,603.8 | 47,494.9 | 1.006 |
| Tyr-Leu | MEDL00365 | C15H22N2O4 | 292.13 | 719.85 | 4842.0 | 865.97 | 4709.35 | 1.210 | |
| Isoleucyl-Phenylalanine | MW0107571 | C15H22N2O3 | 277.15 | 47,560.84 | 47,878.7 | 15,486 | 31,611.15 | 1.625 | |
| Hydroxyprolyl-Leucine | MW0107402 | C11H20N2O4 | 243.13 | 10,048.15 | 18,385.8 | 11,287.9 | 35,568.13 | 2.314 | |
| Lysyl-Methionine | MW0108102 | C11H23N3O3S | 276.12 | 898.69 | 264,387 | 59,785 | 217,602.57 | 4.491 | |
| Asn Thr Gln Glu | MW0145900 | C18H30N6O10 | 489.20 | 157,233.05 | 3447.24 | 1390.97 | 2182.42 | 1.245 | |
| Ile Val Leu Glu | MW0151650 | C22H40N4O7 | 471.29 | 5930.95 | 1703.68 | 10,064.1 | 4905.41 | 1.766 | |
| Ac-Yvad-cho | MW0144352 | C23H32N4O8 | 491.21 | 20,644.29 | 272,409.3 | 68,677.8 | 64,529.24 | 1.506 | |
| Ser Ala Gln Asp | MW0156626 | C15H25N5O9 | 418.16 | 14,331.34 | 22,733.4 | 11,507.4 | 20,810.78 | 1.036 | |
| Ac-DEVD-CHO | MW0144237 | C20H30N4O11 | 501.18 | 56,303.65 | 84,072.9 | 91,302.4 | 77,289.35 | 2.309 | |
| DL-Leucine | MEDP0752 | C6H13NO2 | 130.08 | 38,200.09 | 35,940.6 | 28,033.1 | 47,911.62 | 1.480 | |
| Gly-Phe | MEDN1029 | C11H14N2O3 | 221.09 | 43,628.87 | 643,738 | 3,744,324 | 2,342,296.48 | 1.301 | |
| L-Valine | MEDL00009 | C5H11NO2 | 116.07 | 92,603.12 | 7481.62 | 15,518.6 | 4595.11 | 1.193 | |
| 2-Amino-3-phosphonopropionic acid | MW0104504 | C3H8NO5P | 189.98 | 4635.25 | 64,681.7 | 24,372.4 | 59,307.2 | 1.139 | |
| Alkaloids | Piperolactam A | MW0000400 | C16H11NO3 | 264.06 | 1507.38 | 4319.89 | 1329.52 | 1737.47 | 1.908 |
| Graveolinine | MW0124270 | C17H13NO3 | 278.08 | 17,550.98 | 209,423 | 46,621.5 | 136,055.46 | 1.610 | |
| Epigallocatechin | MEDL02039 | C15H14O7 | 305.05 | 5480.58 | 187,292 | 952,427 | 614,951.93 | 1.129 | |
| Withaferin A | MW0103132 | C28H38O6 | 469.25 | 63,941.05 | 36,776.2 | 14,410.1 | 98,156.15 | 2.826 | |
| Methylarmepavine | MW0115762 | C20H25NO3 | 326.16 | 15,201.71 | 198,100 | 27,575.8 | 251,073.24 | 1.227 | |
| Demethoxycurcumin | MEDL01854 | C20H18O5 | 378.13 | 11,249.69 | 53,841.3 | 28,938.3 | 77,343.14 | 2.773 | |
| Hesperetin | MEDL02106 | C16H14O6 | 301.07 | 288,642.25 | 27,143.7 | 5930.95 | 8098.36 | 1.436 | |
| Vitamin K | MW0103120 | C31H46O2 | 449.32 | 26,053.19 | 34,390 | 97,277.2 | 22,556.79 | -1.222 | |
| Fatty acids | Caffeic Acid | MEDP0302 | C9H8O4 | 179.03 | 65,537.89 | 24,450.57 | 58,851.4 | 31,987.33 | 1.907 |
| Ganosporeric acid A | MW0053470 | C30H38O8 | 525.25 | 13,253.72 | 103,550 | 17,550.9 | 82,876.28 | −1.126 | |
| 13,14-dihydro-15-keto-tetranor PGF1 | MW0141363 | C16H28O5 | 299.18 | 66,761.84 | 6987.71 | 1777.06 | 3524.61 | 1.283 | |
| (3R)-3-Hydroxydodecanoic acid | MW0103903 | C12H24O3 | 215.16 | 176,791.1 | 167,876 | 37,938.4 | 60,404.19 | 1.987 | |
| 20-HETE | MEDP1153 | C20H32O3 | 319.21 | 12,600.82 | 26,700.4 | 18,676.8 | 58,888.53 | 1.346 | |
| Mandelic Acid | MEDN0334 | C8H8O3 | 134.03 | 2371.76 | 3676.24 | 1132.86 | 4007.93 | −3.041 | |
| Isovaleric acid | MW0054155 | C5H10O2 | 102.06 | 8064.84 | 33,994 | 49,511.4 | 34,828.08 | 3.111 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Guo, Y.; Muhmood, A.; Lv, Z.; Zeng, B.; Qiu, Y.; Zhang, L.; Wang, P.; Ren, L. Food Waste Management Employing UV-Induced Black Soldier Flies: Metabolomic Analysis of Bioactive Components, Antioxidant Properties, and Antibacterial Potential. Int. J. Environ. Res. Public Health 2022, 19, 6614. https://doi.org/10.3390/ijerph19116614
Lu J, Guo Y, Muhmood A, Lv Z, Zeng B, Qiu Y, Zhang L, Wang P, Ren L. Food Waste Management Employing UV-Induced Black Soldier Flies: Metabolomic Analysis of Bioactive Components, Antioxidant Properties, and Antibacterial Potential. International Journal of Environmental Research and Public Health. 2022; 19(11):6614. https://doi.org/10.3390/ijerph19116614
Chicago/Turabian StyleLu, Jiaxin, Yuwen Guo, Atif Muhmood, Zheng Lv, Bei Zeng, Yizhan Qiu, Luxi Zhang, Pan Wang, and Lianhai Ren. 2022. "Food Waste Management Employing UV-Induced Black Soldier Flies: Metabolomic Analysis of Bioactive Components, Antioxidant Properties, and Antibacterial Potential" International Journal of Environmental Research and Public Health 19, no. 11: 6614. https://doi.org/10.3390/ijerph19116614
APA StyleLu, J., Guo, Y., Muhmood, A., Lv, Z., Zeng, B., Qiu, Y., Zhang, L., Wang, P., & Ren, L. (2022). Food Waste Management Employing UV-Induced Black Soldier Flies: Metabolomic Analysis of Bioactive Components, Antioxidant Properties, and Antibacterial Potential. International Journal of Environmental Research and Public Health, 19(11), 6614. https://doi.org/10.3390/ijerph19116614






