Hematologic System Dysregulation in Critically Ill Septic Patients with Anemia—A Retrospective Cohort Study
Abstract
1. Introduction
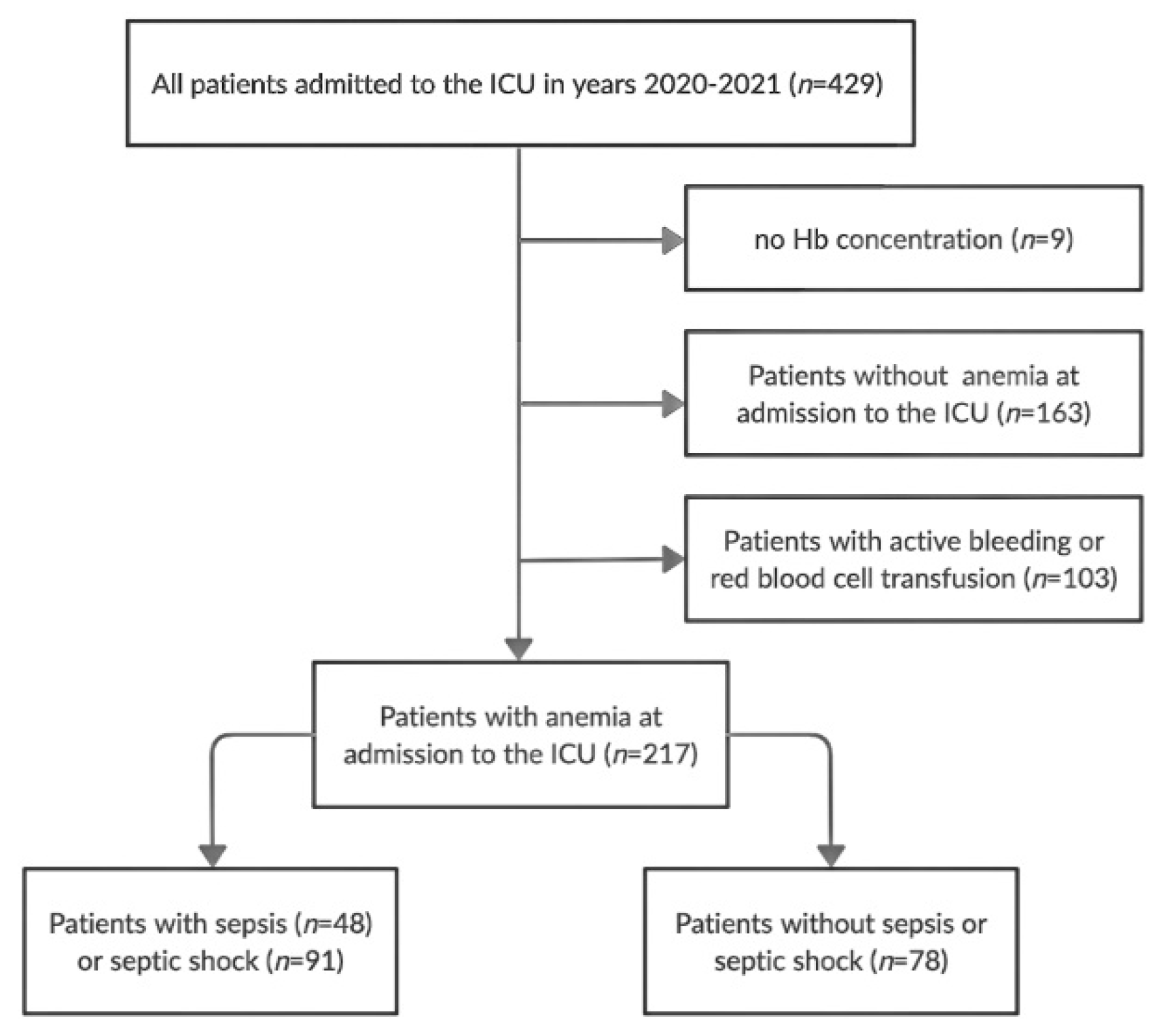
2. Materials and Methods
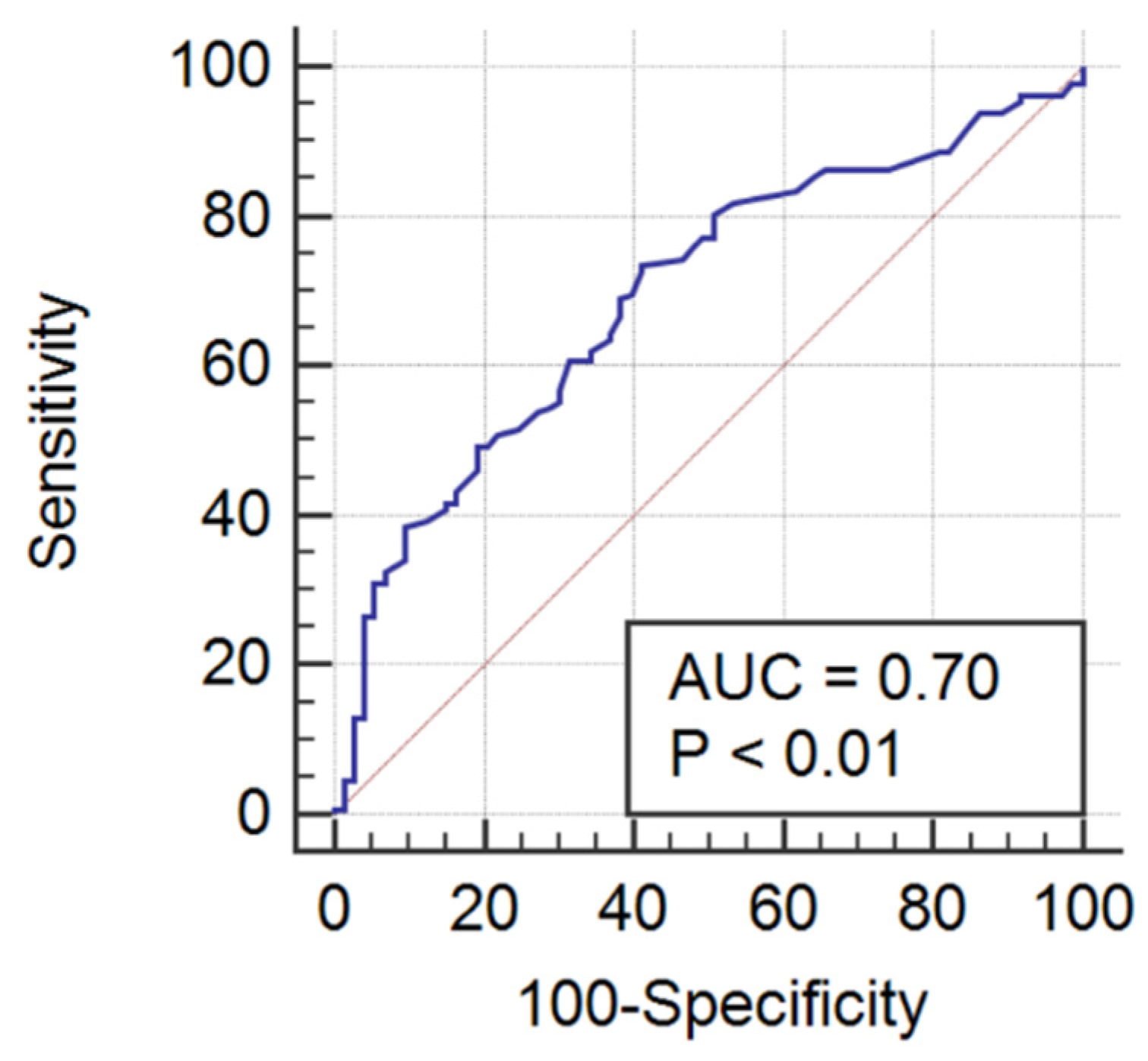
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Islam, M.; Nasrin, T.; Walther, B.A.; Wu, C.C.; Yang, H.-C.; Li, Y.-C. Prediction of sepsis patients using machine learning approach: A meta-analysis. Comput. Methods Programs Biomed. 2019, 170, 1–9. [Google Scholar] [CrossRef]
- Piagnerelli, M.; Boudjeltia, K.Z.; Brohee, D.; Vincent, J.L.; Vanhaeverbeek, M. Modifications of red blood cell shape and glycoproteins membrane content in septic patients. Adv. Exp. Med. Biol. 2003, 510, 109–114. [Google Scholar]
- De Oliveira, Y.P.A.; Pontes-de-Carvalho, L.C.; Couto, R.D.; Noronha-Dutra, A.A. Oxidative stress in sepsis. Possible production of free radicals through an erythrocyte-mediated positive feedback mechanism. BJID 2017, 21, 19–26. [Google Scholar] [CrossRef]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef]
- Evans, T.C.; Jehle, D. The red blood cell distribution width. J. Emerg. Med. 1991, 9 (Suppl. 1), 71–74. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, K.; Lee, J.H.; Kang, C.; Kim, T.; Park, H.-M.; Kang, K.W.; Kim, J.; Rhee, J.E. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am. J. Emerg Med. 2013, 31, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Sadaka, F.; O’Brien, J.; Prakash, S. Red cell distribution width and outcome in patients with septic shock. J. Intensive Care Med. 2013, 28, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fontana, V.; Spadaro, S.; Bond, O.; Cavicchi, F.Z.; Annoni, F.; Donadello, K.; Vincent, J.L.; De Backer, D.; Taccone, F.S. No relationship between red blood cell distribution width and microcirculatory alterations in septic patients. Clin. Hemorheol. Microcirc. 2017, 66, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, G.; Occhipinti, G.; De Gasperi, A.; Vincent, J.L.; Piagnerelli, M. Early alterations of red blood cell rheology in critically ill patients. Crit. Care Med. 2009, 37, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Czempik, P.F.; Krzych, Ł.J. Anemia of critical illness—A narrative review. Acta Haematol. Pol. 2022, 54, 1–24. [Google Scholar] [CrossRef]
- Czempik, P.F.; Pluta, M.P.; Krzych, Ł.J. Ferritin and transferrin saturation cannot be used for diagnosis of iron-deficiency anaemia in critically ill patients. Acta Haematol. Pol. 2021, 52, 566–570. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [CrossRef]
- Kolls, J.K. Oxidative stress in sepsis: A redox redux. J. Clin. Investig. 2006, 17, 860–863. [Google Scholar] [CrossRef]
- Pierce, C.N.; Larson, D.F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005, 20, 83–90. [Google Scholar] [CrossRef]
- Krishna, V.; Pillai, G.; Velickakathu Sukumaran, S. Red Cell Distribution Width as a Predictor of Mortality in Patients with Sepsis. Cureus 2021, 13, e12912. [Google Scholar] [CrossRef]
- Fukuta, H.; Ohte, N.; Mukai, S.; Saeki, T.; Asada, K.; Wakami, K.; Kimura, G. Elevated plasma levels of B-type natriuretic peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int. Heart J. 2009, 50, 301–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.T.; Kim, E.J.; Han, J.H.; Han, J.S.; Choi, J.Y.; Han, S.H.; Yoo, T.-H.; Kim, Y.S.; Kang, S.-W.; et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 2013, 17, R282. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, T.S.; Weuve, J.; Pfeffer, M.A.; Beckman, J.A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 2009, 169, 588–594. [Google Scholar] [CrossRef]
- Ani, C.; Ovbiagele, B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J. Neurol. Sci. 2009, 277, 103–108. [Google Scholar] [CrossRef]
- Ye, Z.; Smith, C.; Kullo, I.J. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am. J. Cardiol. 2011, 107, 1241–1245. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Chopra, V.; Gupta, A.; Battala, V.R.; Poludasu, S.; Eng, C.; Marmur, J.D. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int. J. Cardiol. 2010, 141, 141–146. [Google Scholar] [CrossRef]
- Patel, K.V.; Ferrucci, L.; Ershler, W.B.; Longo, D.L.; Guralnik, J.M. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch. Intern. Med. 2009, 169, 515–523. [Google Scholar] [CrossRef]
- Wang, F.; Pan, W.; Pan, S.; Ge, J.; Wang, S.; Chen, M. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann. Med. 2011, 43, 40–46. [Google Scholar] [CrossRef]
- Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Red cell distribution width and all-cause mortality in critically ill patients. Crit. Care Med. 2011, 39, 1913–1921. [Google Scholar] [CrossRef]
- Ellahony, D.M.; El-Mekkawy, M.S.; Farag, M.M. A Study of Red Cell Distribution Width in Neonatal Sepsis. Pediatric Emerg. Care 2020, 36, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.A.; Mathew, J.; Kang, B.; DeBari, V.A.; Khan, M.A. Broadening of the red blood cell distribution width is associated with increased severity of illness in patients with sepsis. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 278–282. [Google Scholar] [PubMed]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hampole, C.V.; Mehrotra, A.K.; Thenappan, T.; Gomberg-Maitland, M.; Shah, S.J. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am. J. Cardiol. 2009, 104, 868–872. [Google Scholar] [CrossRef]
- Omiya, K.; Sato, H.; Sato, T.; Wykes, L.; Hong, M.; Hatzakorzian, R.; Kristof, A.S.; Schricker, T. Albumin and fibrinogen kinetics in sepsis: A prospective observational study. Crit. Care 2021, 25, 436. [Google Scholar] [CrossRef]
- Matsubara, T.; Yamakawa, K.; Umemura, Y.; Gando, S.; Ogura, H.; Shiraishi, A.; Kushimoto, S.; Abe, T.; Tarui, T.; Hagiwara, A.; et al. Significance of plasma fibrinogen level and antithrombin activity in sepsis: A multicenter cohort study using a cubic spline model. Thromb. Res. 2019, 181, 17–23. [Google Scholar] [CrossRef]
- Prucha, M.; Bellingan, G.; Zazula, R. Sepsis biomarkers. Clin. Chim. Acta 2015, 440, 97–103. [Google Scholar] [CrossRef]
- Han, Y.Q.; Yan, L.; Zhang, L.; Ouyang, P.H.; Li, P.; Lippi, G.; Hu, Z.D. Performance of D-dimer for predicting sepsis mortality in the intensive care unit. Biochem. Med. 2021, 31, 020709. [Google Scholar] [CrossRef]
- Lyons, P.G.; Micek, S.T.; Hampton, N.; Kollef, M.H. Sepsis-Associated Coagulopathy Severity Predicts Hospital Mortality. Crit. Care Med. 2018, 46, 736–742. [Google Scholar] [CrossRef]
- van Vught, L.A.; Uhel, F.; Ding, C.; Van’t Veer, C.; Scicluna, B.P.; Peters-Sengers, H.; Klein Klouwenberg, P.M.C.; Nürnberg, P.; Cremer, O.L.; Schultz, M.J.; et al. Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis. J. Thromb. Haemost. 2021, 19, 1049–1063. [Google Scholar] [CrossRef]
- Dempfle, C.E.; Lorenz, S.; Smolinski, M.; Wurst, M.; West, S.; Houdijk, W.P.; Quintel, M.; Borggrefe, M. Utility of activated partial thromboplastin time waveform analysis for identification of sepsis and overt disseminated intravascular coagulation in patients admitted to a surgical intensive care unit. Crit. Care Med. 2004, 32, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Chopin, N.; Floccard, B.; Sobas, F.; Illinger, J.; Boselli, E.; Benatir, F.; Levrat, A.; Guillaume, C.; Crozon, J.; Négrier, C.; et al. Activated partial thromboplastin time waveform analysis: A new tool to detect infection? Crit. Care Med. 2006, 34, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.Y.; Yang, F.; Chen, Z.; Yu, Q.; Shi, H.; Huang, S.; Zhao, X.; Xiu, L.; Li, X.; et al. Platelets as a prognostic marker for sepsis: A cohort study from the MIMIC-III database. Medicine 2020, 99, e23151. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Reference Range [Male/Female] |
|---|---|
| Hemoglobin [g/dL] | 13.5–16.5/11.5–15.0 |
| Hct [%] | 40–53/36–46 |
| MCV [fL] | 84–98 |
| MCH [pg] | 27–31 |
| MCHC [pg] | 32–36 |
| RDW [%] | 11–16 |
| RDW-SD [fL] | 36.3–47.3/38.9–50.0 |
| Fibrinogen [mg/dL] | 200.0–393.0 |
| PT [s] | 9.4–12.5 |
| INR | 0.80–1.20 |
| aPTT [s] | 25.4–36.9 |
| D-dimers [ng/mL] | <500.0 |
| TT [s] | 10.3–16.6 |
| PLT [103/µL] | 130–400 |
| Characteristic | All Patients | Septic Patients | Non-Septic Patients | p |
|---|---|---|---|---|
| (n = 217) | (n = 139) | (n = 78) | ||
| Age [years] | 65 (58–73) | 66 (58–74) | 67 (56.5–71.2) | 0.57 |
| Sex, female/male [n, %] | 98(45.2)/ | 62(44.6)/ | 36(46.2)/ | 0.83 |
| 119(54.8) | 77(55.4) | 42(53.8) | ||
| Severity of disease: | ||||
| - SAPS II [points] | 44 (32–59) | 49 (36–62) | 39 (29–49) | <0.01 |
| - APACHE II points] | 18 (13–24) | 20(14–26) | 16 (12–21) | <0.01 |
| - SOFA II [points] | 8 (6–12) | 10 (6–13) | 7 (4–9) | <0.01 |
| Organ injuries: | ||||
| - acute respiratory failure [n, %] | 199 (91.7) | 127 (91.4) | 72 (92.3) | 0.81 |
| - acute kidney injury [n, %] | 99 (45.6) | 86 (61.9) | 13 (16.7) | <0.01 |
| - acute liver injury [n, %] | 46 (21.2) | 41 (29.5) | 5 (6.4) | <0.01 |
| - shock [n, %] | 122 (56.2) | 91 (65.5) | 31 (39.7) | <0.01 |
| Selected laboratory tests: | ||||
| - lactate [mmol/L] | 2.5 (1.7–4.1) | 2.8 (1.9–4.7) | 1.9 (1.8–2.3) | <0.01 |
| - PCT [ng/mL] | 1.2 (0.33–7.18) | 3.2 (1.18–17.0) | 0.2 (0.1–0.4) | <0.01 |
| - CRP [mg/L] | 127.6 (57.9–228.5) | 192 (98.9–266.0) | 61.2 (32.5–115.0) | <0.01 |
| - Creatinine [mg/dL] | 1.3 (0.8–2.2) | 1.5 (0.8–2.7) | 0.9 (0.8–1.1) | <0.01 |
| - BUN [mg/dL] | 32.3 (19.4–49.6) | 38.3 (23.1–57.4) | 23.5 (17.1–36.6) | <0.01 |
| - Bilirubin [mg/dL] | 0.7 (0.5–1.3) | 0.8 (0.5–1.6) | 0.6 (0.4–0.8) | <0.01 |
| All-cause ICU mortality [n, %] | 96 (44.2) | 75 (53.9) | 20 (25.6) | <0.01 |
| Parameter | All Patients | Septic Patients | Non-Septic Patients | p |
|---|---|---|---|---|
| Hb [g/dL] | 10.2 (9.1–11.2) | 10.0 (9.1–11.1) | 10.5 (9.2–11.3) | 0.13 |
| Hct [%] | 30.6 (27.8–34.0) | 30.2 (27.4–33.8) | 31.6 (28.3–35.0) | 0.05 |
| MCV [fL] | 89.4 (85.7–94.4) | 89.4 (86.5–94.6) | 89.4 (84.0–94.0) | 0.39 |
| MCH [pg] | 29.9 (28.4–31.1) | 29.9 (28.7–31.1) | 29.8 (28.0–31.2) | 0.48 |
| MCHC [pg] | 33.1 (31.7–34.0) | 33.1 (31.7–34.1) | 33.1 (31.3–34.0) | 0.54 |
| RDW [%] | 15.3 (13.9–17.2) | 15.7 (14.2–17.2) | 14.8 (13.6–16.5) | 0.02 |
| RDW-SD [fL] | 49.9 (45.9–55.8) | 51.0 (46.9–56.9) | 48.9 (43.8–53.1) | 0.02 |
| Fibrinogen [mg/dL] | 480.0 (326.2–647.0) | 518.5 (344.5–674.5) | 402.0 (280.2–604.0) | 0.02 |
| PT [s] | 15.0 (13.1–17.8) | 15.9 (13.8–18.8) | 13.5 (12.6–15.6) | <0.01 |
| INR | 1.3 (1.1–1.6) | 1.4 (1.2–1.6) | 1.2 (1.1–1.4) | <0.01 |
| aPTT [s] | 36.7 (30.2–43.1) | 37.7 (31.7–44.3) | 33.2 (29.8–38.7) | <0.01 |
| D-dimers [mcg/mL] | 5175.0 (2265.2–7626.2) | 5681.0 (2765.0–12,771.7) | 3449.5 (1445.5–6190.5) | <0.01 |
| TT [s] | 17.6 (15.9–21.0) | 17.8 (15.8–20.4) | 17.5 (16.9–21.1) | 0.62 |
| PLT [103/µL] | 212.0 (140.7–316.5) | 195.0 (128.0–315.2) | 239.0 (176.0–320.0) | 0.04 |
| Parameter | Survivors | Non-Survivors | p |
|---|---|---|---|
| Hb [g/dL] | 10.0 (9.1–11.0) | 10.0 (8.7–11.1) | 0.95 |
| Hct [%] | 30.1 (27.6–33.0) | 30.3 (27.2–34.6) | 0.62 |
| MCV [fL] | 88.4 (85.9–95.6) | 91.2 (86.8–94.5) | 0.21 |
| MCH [pg] | 29.8 (28.9–31.4) | 29.9 (28.5–31.1) | 0.61 |
| MCHC [pg] | 33.3 (32.2–34.2) | 32.9 (31.6–33.9) | 0.08 |
| RDW [%] | 15.3 (14.0–17.4) | 15.9 (14.4–17.2) | 0.38 |
| RDW-SD [fL] | 50.4 (46.1–57.1) | 51.3 (47.2–56.6) | 0.40 |
| Fibrinogen [mg/dL] | 574.5 (335.0–770.0) | 505.5 (357.5–635.5) | 0.41 |
| PT [s] | 14.8 (13.2–16.3) | 17.7 (14.5–19.6) | <0.01 |
| INR | 1.3 (1.1–1.4) | 1.5 (1.3–1.7) | <0.01 |
| aPTT [s] | 37.0 (30.0–44.3) | 38.4 (32.8–44.6) | 0.25 |
| D-dimers [mcg/mL] | 5157.0 (2351.0–7321.5) | 6513.5 (4199.0–13,339.5) | 0.07 |
| TT [s] | 16.8 (15.1–19.5) | 18.1 (15.9–22.8) | 0.09 |
| PLT [103/µL] | 203.0 (137.0–344.5) | 189.0 (109.0–304.7) | 0.39 |
| Parameter | SOFA Score | p | ICU Length of Stay | p |
|---|---|---|---|---|
| Hb [g/dL] | −0.06 | 0.45 | −0.09 | 0.30 |
| Hct [%] | −0.03 | 0.69 | −0.08 | 0.36 |
| MCV [fL] | 0.17 | 0.05 | 0.06 | 0.94 |
| MCH [pg] | 0.07 | 0.43 | −0.06 | 0.49 |
| MCHC [pg] | −0.13 | 0.14 | −0.03 | 0.69 |
| RDW [%] | 0.06 | 0.47 | −0.03 | 0.69 |
| RDW-SD [fL] | 0.15 | 0.07 | −0.005 | 0.95 |
| Fibrinogen [md/dL] | −0.11 | 0.23 | 0.08 | 0.39 |
| PT [s] | 0.32 | <0.01 | −0.05 | 0.55 |
| INR | 0.32 | <0.01 | −0.06 | 0.53 |
| aPTT [s] | 0.16 | 0.08 | −0.08 | 0.38 |
| D-dimers [mcg/mL] | 0.31 | <0.01 | 0.09 | 0.32 |
| TT [s] | 0.30 | <0.01 | −0.09 | 0.41 |
| PLT [103/µL] | −0.17 | 0.04 | 0.19 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czempik, P.F.; Herzyk, J.; Wilczek, D.; Krzych, Ł.J. Hematologic System Dysregulation in Critically Ill Septic Patients with Anemia—A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 6626. https://doi.org/10.3390/ijerph19116626
Czempik PF, Herzyk J, Wilczek D, Krzych ŁJ. Hematologic System Dysregulation in Critically Ill Septic Patients with Anemia—A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(11):6626. https://doi.org/10.3390/ijerph19116626
Chicago/Turabian StyleCzempik, Piotr F., Jan Herzyk, Dawid Wilczek, and Łukasz J. Krzych. 2022. "Hematologic System Dysregulation in Critically Ill Septic Patients with Anemia—A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 11: 6626. https://doi.org/10.3390/ijerph19116626
APA StyleCzempik, P. F., Herzyk, J., Wilczek, D., & Krzych, Ł. J. (2022). Hematologic System Dysregulation in Critically Ill Septic Patients with Anemia—A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 19(11), 6626. https://doi.org/10.3390/ijerph19116626








