Optic Flow Speed and Retinal Stimulation Influence Microsaccades
Abstract
1. Introduction
2. Materials and Methods
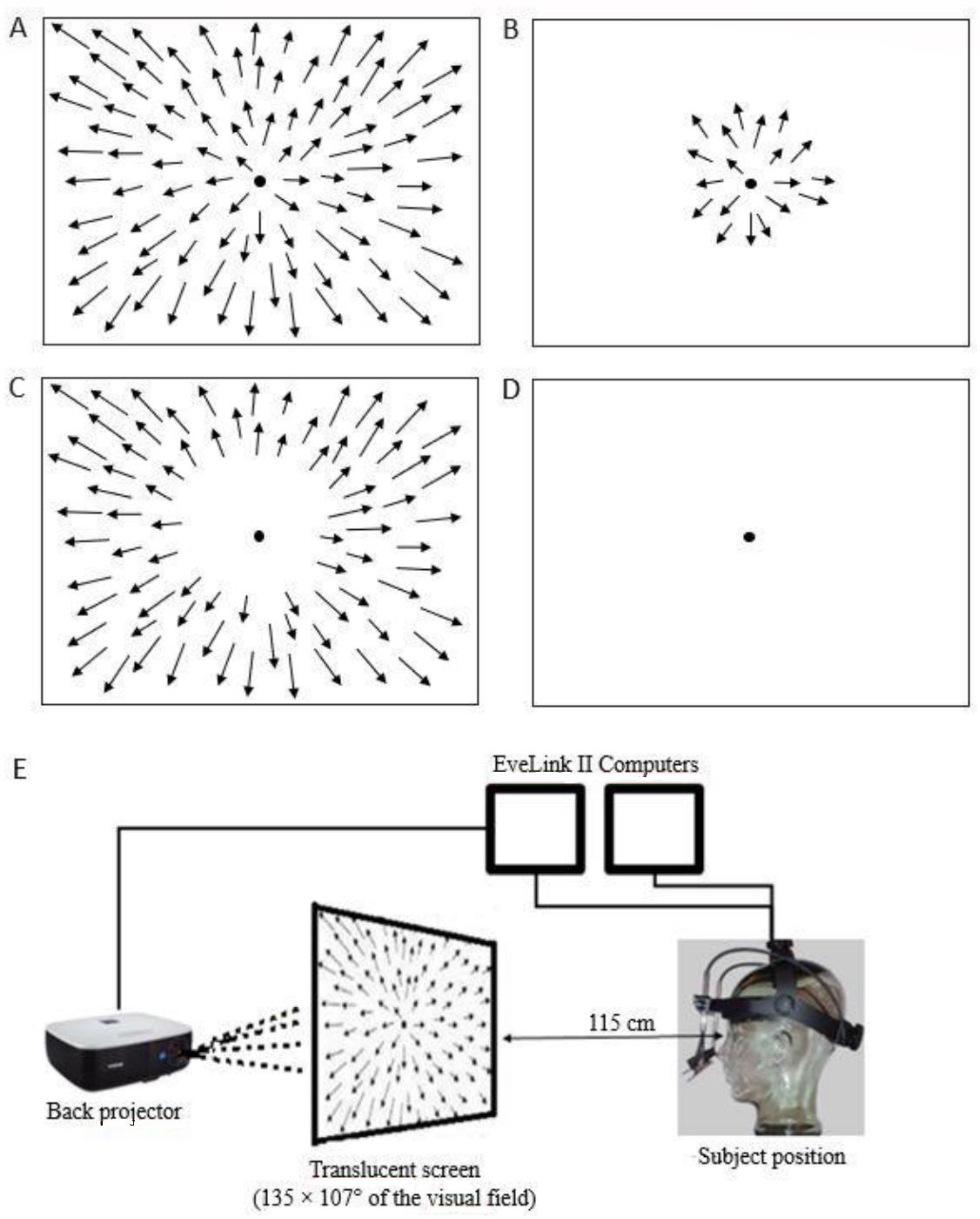
2.1. Optic Flow Stimuli
2.2. Eye Movements and Eye Position Recordings
2.3. Data Analysis
3. Results
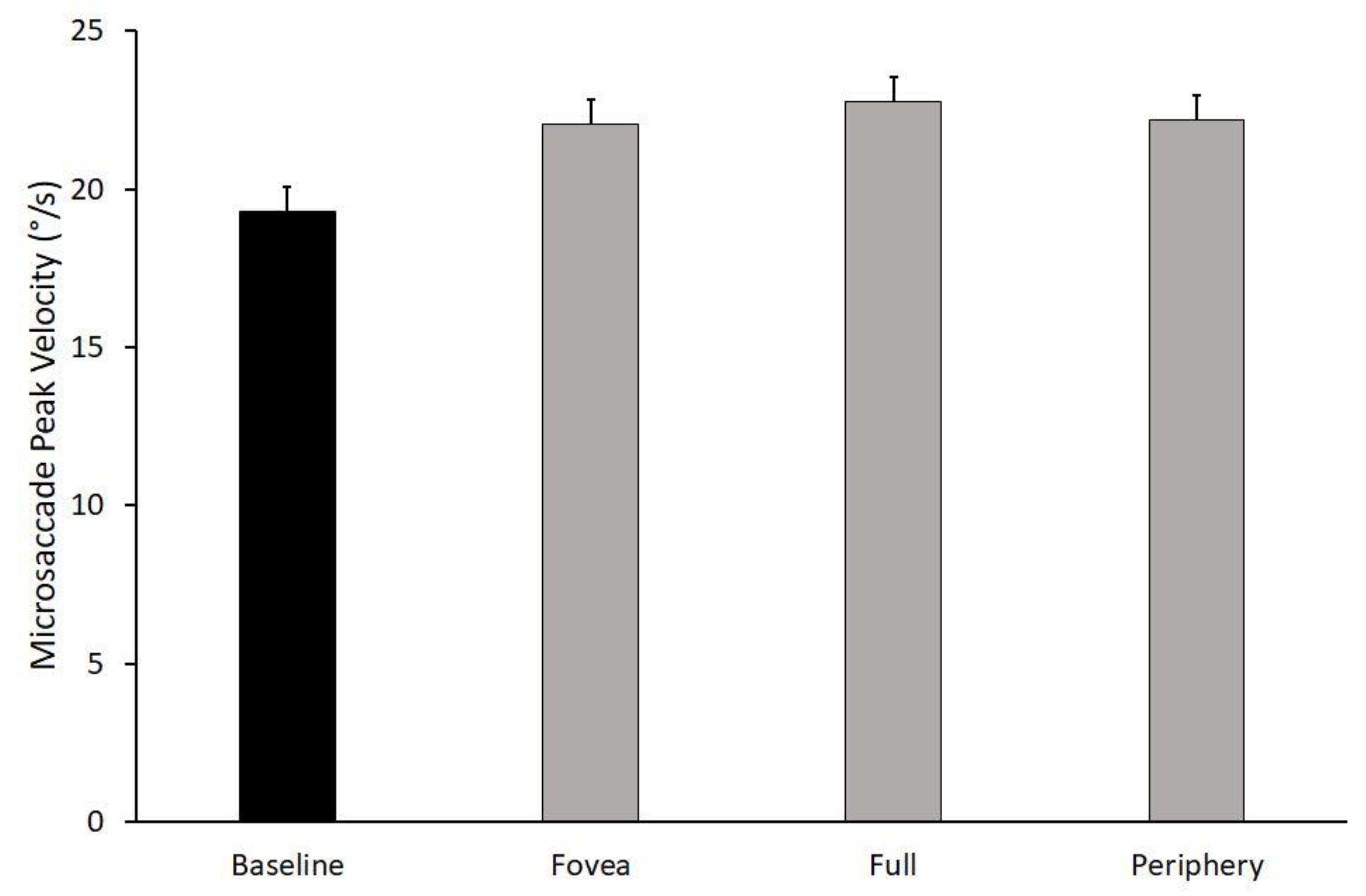
3.1. Microsaccades Peak Velocity
3.2. Microsaccades Latency
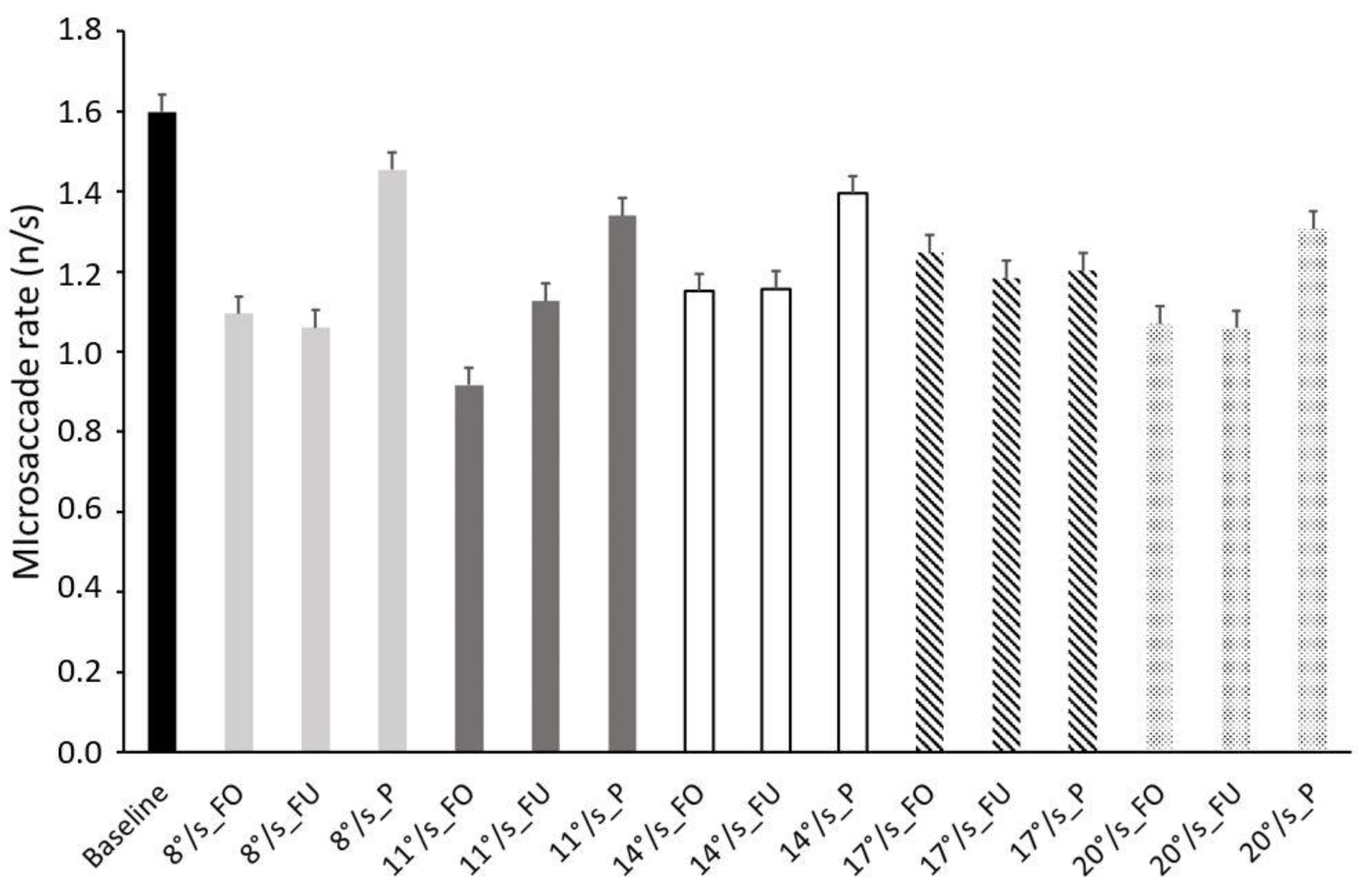
3.3. Microsaccades Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, R.A.; Bracewell, R.M.; Barash, S.; Gnadt, J.W.; Fogassi, L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a of macaque. J. Neurosci. 1990, 10, 1176–1196. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Essick, G.; Siegel, R.M. Neurons of area 7a activated by both stimuli and oculomotor behavior. Exp. Brain Res. 1987, 67, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, L.; Biscaldi, M.; Corbetta, M.; Peru, A.; Tassinari, G.; Berlucchi, G. Oculomotor activity and visual spatial attention. Behav. Brain Res. 1995, 71, 81–88. [Google Scholar] [CrossRef]
- Corbetta, M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; Ollinger, J.M.; Drury, H.A.; Linenweber, M.R.; Petersen, S.E.; Raichle, M.E.; Van Essen, D.C.; et al. A common network of functional areas for attention and eye movements. Neuron 1998, 21, 761–773. [Google Scholar] [CrossRef]
- Bremmer, F.; Graf, W.; Ben Hamed, S.; Duhamel, J.-R. Eye position encoding in the macaque ventral intraparietal area (VIP). Neuroreport 1999, 10, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, C.Y. Eye position-dependent activation of neurones in striate cortex of macaque. Neuroreport 1997, 8, 1405–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otero-Millan, J.; Troncoso, X.G.; Macknik, S.L.; Serrano-Pedraza, I.; Martinez-Conde, S. Saccades and microsaccades during visual fixation, exploration, and search: Foundations for a common saccadic generator. J. Vis. 2008, 8, 21. [Google Scholar] [CrossRef]
- Piras, A.; Malagoli Lanzoni, I.; Raffi, M.; Persiani, M.; Squatrito, S. The within-task criterion to determine successful and unsuccessful table tennis players. Int. J. Sports Sci. Coach. 2016, 11, 523–531. [Google Scholar] [CrossRef]
- Raffi, M.; Squatrito, S.; Maioli, M.G. Gaze and smooth pursuit signals interact in parietal area 7m of the behaving monkey. Exp. Brain Res 2007, 182, 35–46. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Gray, F.; Brunet, P. Infarcts of both inferior parietal lobules with impairment of visually guided eye movements, peripheral visual inattention and optic ataxia. Brain 1986, 109, 81–97. [Google Scholar] [CrossRef]
- Martinez-Conde, S.; Macknik, S.L.; Hubel, D.H. The role of fixational eye movements in visual perception. Nat. Rev. Neurosci. 2004, 5, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S.; Macknik, S.L.; Troncoso, X.G.; Hubel, D.H. Microsaccades: A neurophysiological analysis. Trends Neurosci. 2009, 32, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, A.S.; Costela, F.M.; Martinez-Conde, S.; Macknik, S.L.; Goldinger, S.D. Microsaccades reflect the dynamics of misdirected attention in magic. J. Eye Mov. Res. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Meoni, A.; Piras, A. Analysis of microsaccades during extended practice of a visual discrimination task in the macaque monkey. Neurosci. Lett. 2021, 743, 135581. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Timmis, M.; Trofè, A.; Raffi, M. Understanding the underlying mechanisms of Quiet Eye: The role of microsaccades, small saccades and pupil-size before final movement initiation in a soccer penalty kick. Eur. J. Sport Sci. 2021, 21, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Raffi, M.; Malagoli Lanzoni, I.; Persiani, M.; Squatrito, S. Microsaccades and prediction of a motor act outcome in a dynamic sport situation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4520–4530. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Perazzolo, M.; Malagoli Lanzoni, I.; Squatrito, S. Microsaccades and interest areas during free-viewing sport task. J. Sports Sci. 2019, 37, 980–987. [Google Scholar] [CrossRef]
- Yu, G.; Yang, M.; Yu, P.; Dorris, M.C. Time compression of visual perception around microsaccades. J. Neurophysiol. 2017, 118, 416–424. [Google Scholar] [CrossRef]
- Dimigen, O.; Valsecchi, M.; Sommer, W.; Kliegl, R. Human microsaccade-related visual brain responses. J. Neurosci. 2009, 29, 12321–12331. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Persiani, M.; Perazzolo, M.; Squatrito, S. Effect of heading perception on microsaccade dynamics. Behav. Brain Res. 2016, 312, 246–252. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Chen, J.T.; Tseng, P.; Wang, C.A. Role of the frontal eye field in human microsaccade responses: A TMS study. Biol. Psychol. 2021, 165, 108202. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, R.; McPeek, R.M. Microsaccades and attention in a high-acuity visual alignment task. J. Vis. 2021, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Siegel, R.M. Optic flow selectivity in the anterior superior temporal polysensory area, STPa, of the behaving monkey. J. Neurosci. 1999, 19, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.; Lakshminarasimhan, K.J.; DeAngelis, G.C.; Angelaki, D.E. Visual and Vestibular Selectivity for Self-Motion in Macaque Posterior Parietal Area 7a. Cereb Cortex 2019, 29, 3932–3947. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamed, S.; Page, W.; Duffy, C.; Pouget, A. MSTd neuronal basis functions for the population encoding of heading direction. J. Neurophysiol. 2003, 90, 549–558. [Google Scholar] [CrossRef]
- Bremmer, F.; Duhamel, J.-R.; Ben Hamed, S.; Graf, W. Heading encoding in the macaque ventral intraparietal area (VIP). Eur. J. Neurosci. 2002, 16, 1554–1568. [Google Scholar] [CrossRef]
- Duffy, C.J.; Wurtz, R.H. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large field stimuli. J. Neurophysiol. 1991, 65, 1329–1345. [Google Scholar] [CrossRef]
- Duffy, C.J.; Wurtz, R.H. Sensitivity of MST neurons to optic flow stimuli. II. Mechanism of response selectivity revealed by small-field stimuli. J. Neurophysiol. 1991, 65, 1346–1359. [Google Scholar] [CrossRef]
- Raffi, M.; Carrozzini, C.; Maioli, M.G.; Squatrito, S. Multimodal representation of optic flow in area PEc of macaque monkey. Neuroscience 2010, 171, 1241–1255. [Google Scholar] [CrossRef]
- Raffi, M.; Maioli, M.G.; Squatrito, S. Optic flow direction coding in area PEc of the behaving monkey. Neuroscience 2011, 194, 136–149. [Google Scholar] [CrossRef]
- Raffi, M.; Persiani, M.; Piras, A.; Squatrito, S. Optic flow neurons in area PEc integrate eye and head position signals. Neurosci. Lett. 2014, 568, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Siegel, R.M. A functional architecture of optic flow in the inferior parietal lobule of the behaving monkey. PLoS ONE 2007, 2, e200. [Google Scholar] [CrossRef] [PubMed]
- Read, H.L.; Siegel, R.M. Modulation of responses to optic flow in area 7a by retinotopic and oculomotor cues in monkeys. Cereb Cortex 1997, 7, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Wylie, D.R.W.; Glover, R.G.; Aitchison, J.D. Optic flow input to the hippocampal formation from the accessory optic system. J. Neurosci. 1999, 19, 5514–5527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, T.; Heuer, H.W.; Britten, K.H. Parietal area VIP neuronal responses to heading stimuli are encoded in head-centered coordinates. Neuron 2004, 42, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.L.; Silson, E.H.; Gouws, A.D.; Morland, A.B.; McKeefry, D.J. Differential processing of the direction and focus of expansion of optic flow stimuli in areas MST and V3A of the human visual cortex. J. Neurophysiol. 2017, 117, 2209–2217. [Google Scholar] [CrossRef]
- Smith, A.T.; Wall, M.B.; Williams, A.L.; Singh, K.D. Sensitivity to optic flow in human cortical areas MT and MST. Eur. J. Neurosci. 2006, 23, 561–569. [Google Scholar] [CrossRef]
- Hoppes, C.W.; Sparto, P.J.; Whitney, S.L.; Furman, J.M.; Huppert, T.J. Functional near-infrared spectroscopy during optic flow with and without fixation. PLoS ONE 2018, 13, e0193710. [Google Scholar] [CrossRef]
- Duffy, C.J.; Wurtz, R.H. Medial superior temporal area neurons respond to speed patterns in optic flow. J. Neurosci. 1997, 17, 2839–2851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phinney, R.E.; Siegel, R.M. Speed selectivity for optic flow in area 7a of the behaving macaque. Cereb Cortex 2000, 10, 413–421. [Google Scholar] [CrossRef][Green Version]
- Kountouriotis, G.K.; Mole, C.D.; Merat, N.; Wilkie, R.M. The need for speed: Global optic flow speed influences steering. R. Soc. Open Sci. 2016, 3, 160096. [Google Scholar] [CrossRef] [PubMed]
- Durgin, F.H.; Gigone, K. Enhanced optic flow speed discrimination while walking: Contextual tuning of visual coding. Perception 2007, 36, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Prokop, T.; Schubert, M.; Berger, W. Visual influence on human locomotion: Modulation to changes in optic flow. Exp. Brain Res. 1997, 114, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Prokop, T.; Brocke, F.; Berger, W. Visual kinesthesia and locomotion in Parkinson’s disease. Mov. Disord. 2005, 20, 141–150. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A.; Persiani, M.; Perazzolo, M.; Squatrito, S. Angle of gaze and optic flow direction modulate body sway. J. Electromyogr. Kinesiol. 2017, 35, 61–68. [Google Scholar] [CrossRef]
- Zadra, J.R.; Proffitt, D.R. Optic flow is calibrated to walking effort. Psychon. Bull. Rev. 2016, 23, 1491–1496. [Google Scholar] [CrossRef]
- Bruggeman, H.; Zosh, W.; Warren, W.H. Optic flow drives human visuo-locomotor adaptation. Curr. Biol. 2007, 17, 2035–2040. [Google Scholar] [CrossRef]
- Salinas, M.M.; Wilken, J.M.; Dingwell, J.B. How humans use visual optic flow to regulate stepping during walking. Gait Posture 2017, 57, 15–20. [Google Scholar] [CrossRef]
- Warren, W.H.; Kay, B.A.; Zosh, W.D.; Duchon, A.P.; Sahuc, S. Optic flow is used to control human walking. Nat. Neurosci. 2001, 4, 213–216. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Petit, L.; Ellmore, T.M.; Ingeholm, J.; Haxby, J.V. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage 2001, 14, 310–321. [Google Scholar] [CrossRef]
- Boyer, T.W.; Wang, M. Direct gaze, eye movements, and covert and overt social attention processes. Atten. Percept. Psychophys. 2018, 80, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Flechsenhar, A.; Larson, O.; End, A.; Gamer, M. Investigating overt and covert shifts of attention within social naturalistic scenes. J. Vis. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R.; Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 2003, 43, 1035–1045. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Clark, J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 2002, 42, 2533–2545. [Google Scholar] [CrossRef]
- Elias, L.J.; Bryden, M.P.; Bulman-Fleming, M.B. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 1998, 36, 37–43. [Google Scholar] [CrossRef]
- Levin, H.S.; High, W.M.; Williams, D.H.; Eisenberg, H.M.; Amparo, E.G.; Guinto, F.C.; Ewert, J. Dichotic listening and manual performance in relation to magnetic resonance imaging after closed head injury. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1162–1169. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A.; Persiani, M.; Squatrito, S. Importance of optic flow for postural stability of male and female young adults. Eur. J. Appl. Physiol. 2014, 114, 71–83. [Google Scholar] [CrossRef]
- Zuber, B.L.; Semmlow, J.L.; Stark, L. Frequency characteristics of the saccadic eye movement. Biophys. J. 1968, 8, 1288–1298. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Castro, J.L.; Macknik, S.L.; Martinez-Conde, S. Unsupervised clustering method to detect microsaccades. J. Vis. 2014, 14, 18. [Google Scholar] [CrossRef]
- Raffi, M.; Trofè, A.; Perazzolo, M.; Meoni, A.; Piras, A. Sensory Input Modulates Microsaccades during Heading Perception. Int. J. Environ. Res. Public Health 2021, 18, 2865. [Google Scholar] [CrossRef]
- Troncoso, X.G.; Macknik, S.L.; Martinez-Conde, S. Microsaccades counteract perceptual filling-in. J. Vis. 2008, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, M.; Castelli, L.; Scatturin, P.; Galfano, G. Working memory load modulates microsaccadic rate. J. Vis. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Hermens, F.; Zanker, J.M.; Walker, R. Microsaccades and preparatory set: A comparison between delayed and immediate, exogenous and endogenous pro- and anti-saccades. Exp. Brain Res. 2010, 201, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Matsuo, Y.; Zha, L.; Munoz, D.P.; Kobayashi, Y. Fixational saccades reflect volitional action preparation. J. Neurophysiol. 2013, 110, 522–535. [Google Scholar] [CrossRef]
- Xue, L.; Huang, D.; Wang, T.; Hu, Q.; Chai, X.; Li, L.; Chen, Y. Dynamic modulation of the perceptual load on microsaccades during a selective spatial attention task. Sci. Rep. 2017, 7, 16496. [Google Scholar] [CrossRef]
- Siegenthaler, E.; Costela, F.M.; McCamy, M.B.; Di Stasi, L.L.; Otero-Millan, J.; Sonderegger, A.; Groner, R.; Macknik, S.; Martinez-Conde, S. Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. Eur. J. Neurosci. 2014, 39, 287–294. [Google Scholar] [CrossRef]
- Gao, X.; Yan, H.; Sun, H.J. Modulation of microsaccade rate by task difficulty revealed through between- and within-trial comparisons. J. Vis. 2015, 15, 3. [Google Scholar] [CrossRef]
- Valsecchi, M.; Betta, E.; Turatto, M. Visual oddballs induce prolonged microsaccadic inhibition. Exp. Brain Res. 2007, 177, 196–208. [Google Scholar] [CrossRef]
- Valsecchi, M.; Turatto, M. Microsaccadic responses in a bimodal oddball task. Psychol. Res. 2009, 73, 23–33. [Google Scholar] [CrossRef]
- Betta, E.; Turatto, M. Are you ready? I can tell by looking at your microsaccades. NeuroReport 2006, 17, 1001–1004. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A. Investigating the crucial role of optic flow in postural control: Central vs. peripheral visual field. Appl. Sci. 2019, 9, 934. [Google Scholar] [CrossRef]
- Laubrock, J.; Engbert, R.; Kliegl, R. Microsaccade dynamics during covert attention. Vis. Res. 2005, 45, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Laubrock, J.; Engbert, K.; Kliegl, R. Fixational eye movements predict the perceived direction of ambiguous apparent motion. J. Vis. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, S.; Sadda, S.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res 2019, 68, 83–109. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Chen, C.Y.; Tian, X. Vision, perception, and attention through the lens of microsaccades: Mechanisms and implications. Front. Syst. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef]
- Wang, S.; Fukuchi, M.; Koch, C.; Tsuchiya, N. Spatial attention is attracted in a sustained fashion toward singular points in the optic flow. PLoS ONE 2012, 7, e41040. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffi, M.; Trofè, A.; Meoni, A.; Gallelli, L.; Piras, A. Optic Flow Speed and Retinal Stimulation Influence Microsaccades. Int. J. Environ. Res. Public Health 2022, 19, 6765. https://doi.org/10.3390/ijerph19116765
Raffi M, Trofè A, Meoni A, Gallelli L, Piras A. Optic Flow Speed and Retinal Stimulation Influence Microsaccades. International Journal of Environmental Research and Public Health. 2022; 19(11):6765. https://doi.org/10.3390/ijerph19116765
Chicago/Turabian StyleRaffi, Milena, Aurelio Trofè, Andrea Meoni, Luca Gallelli, and Alessandro Piras. 2022. "Optic Flow Speed and Retinal Stimulation Influence Microsaccades" International Journal of Environmental Research and Public Health 19, no. 11: 6765. https://doi.org/10.3390/ijerph19116765
APA StyleRaffi, M., Trofè, A., Meoni, A., Gallelli, L., & Piras, A. (2022). Optic Flow Speed and Retinal Stimulation Influence Microsaccades. International Journal of Environmental Research and Public Health, 19(11), 6765. https://doi.org/10.3390/ijerph19116765








