Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studies Included in the HBM4EU Aligned Studies
2.2. Biological Specimens Collected
2.3. Exposure Assessment
| Study | Country | Phthalates and HEXAMOLL® DINCH | BFRs | OPFRs | PFASs | Cd | Bisphenols | PAH |
|---|---|---|---|---|---|---|---|---|
| Children | ||||||||
| NEBII | NO | 300 | 300 | 300 | - | - | - | - |
| OCC | DK | 300 | 0 | 291 | - | - | - | - |
| InAirQ | HU | 262 | 0 | 0 | - | - | - | - |
| PCB cohort | SK | 300 | 0 | 300 | - | - | - | - |
| POLAES | PL | 300 | 0 | 0 | - | - | - | - |
| SLO CRP | SL | 149 | 130 | 147 | - | - | - | - |
| CROME | EL | 161 | 55 | 0 | - | - | - | - |
| NAC II | IT | 300 | 0 | 0 | - | - | - | - |
| ESTEBAN | FR | 286 | 226 | 299 | - | - | - | - |
| GerES V-sub(unweighted) | DE | 300 | 0 | 300 | - | - | - | - |
| 3 × G | BE | 133 | 0 | 133 | - | - | - | - |
| SPECIMEn-NL | NL | 89 | 0 | 0 | - | - | - | - |
| Total | 2880 | 711 | 1770 | - | - | - | - | |
| Teenagers | ||||||||
| NEB II | NO | 181 | - | - | 177 | - | - | - |
| Riksmaten Adolescents 2016–2017 | SE | 300 | - | - | 300 | - | - | - |
| POLAES | PL | 281 | - | - | 0 | - | - | - |
| CELSPAC: TE | CZ | 300 | - | - | 0 | - | - | - |
| PCB cohort (follow-up) | SK | 287 | - | - | 292 | - | - | - |
| SLO CRP | SL | 96 | - | - | 94 | - | - | - |
| CROME | EL | 150 | - | - | 52 | - | - | - |
| BEA | ES | 300 | - | - | 299 | - | - | - |
| ESTEBAN | FR | 304 | - | - | 143 | - | - | - |
| FLEHS IV | BE | 300 | - | - | 300 | - | - | - |
| GerES V-sub(unweighted) | DE | 300 | - | - | 300 | - | - | - |
| Total | 2799 | - | - | 1957 | - | - | - | |
| Adults | ||||||||
| Diet_HBM | IS | - | - | - | - | 203 | 203 | 203 |
| FinHealth | FI | - | - | - | - | 0 | 300 | 0 |
| POLAES | PL | - | - | - | - | 228 | 228 | 228 |
| (C)ELSPAC: YA | CZ | - | - | - | - | 300 | 290 | 300 |
| HBM survey in adults in Croatia | HR | - | - | - | - | 300 | 300 | 300 |
| INSEF-ExpoQuim | PT | - | - | - | - | 295 | 296 | 296 |
| ESTEBAN | FR | - | - | - | - | 393 | 163 | 201 |
| HBM4EU study for Switzerland | CH | - | - | - | - | 0 | 300 | 300 |
| ESB | DE | - | - | - | - | 289 | 180 | 331 |
| Oriscav-Lux2 | LU | - | - | - | - | 210 | 209 | 210 |
| CPHMINIPUB (parents)/DYMS | DK | - | - | - | - | 292 | 287 | 240 |
| Total | - | - | - | - | 2510 | 2756 | 2609 |
3. Results
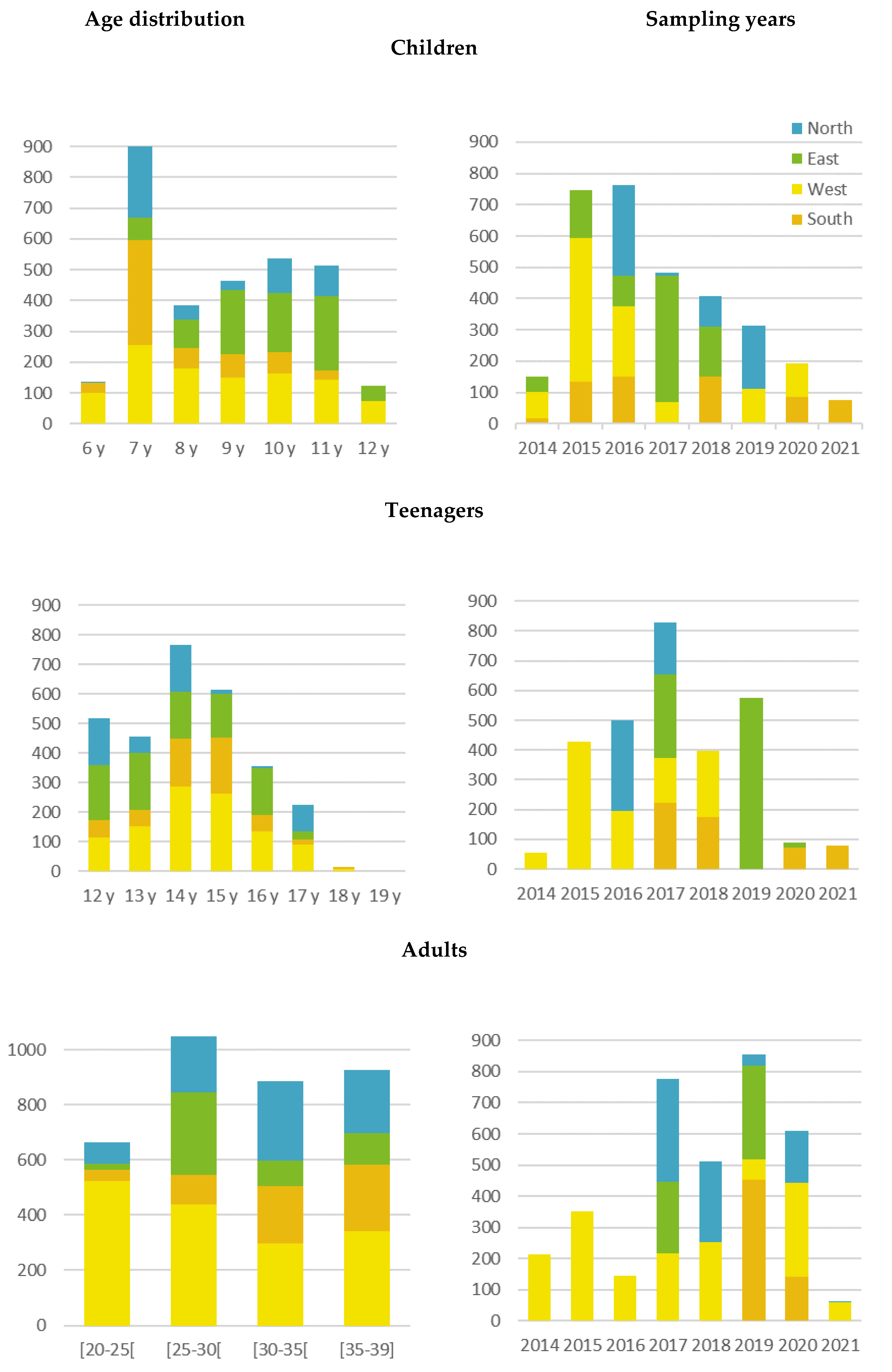
3.1. Geographical Distribution of the Sample
3.2. Baseline Characteristics of Study Participants
| Characteristics | Northern EU | Eastern EU | Southern EU | Western EU | EU Total | EU Reference |
|---|---|---|---|---|---|---|
| No. of participants | 600 | 862 | 610 | 1065 | 3137 | |
| Age (years) | ||||||
| Median (p25–p75) | 7 (7–10) | 10 (9–11) | 7 (7–9) | 8 (7–10) | 9 (7–10) | |
| Min-max | 6–11 | 7–12 | 6 – 11 | 6–12 | 6–12 | |
| Sampling period (year) | ||||||
| Median (p25–p75) | 2017.5 (2016–2019) | 2017 (2016–2017) | 2018 (2016–2020) | 2015 (2015–2017) | 2016 (2015–2018) | |
| Min-max | 2016–2019 | 2014–2018 | 2014–2021 | 2014–2020 | 2014–2021 | |
| Sex N (%) | ||||||
| Girl | 275 (45.8%) | 448 (52.0%) | 315 (51.6%) | 514 (48.3%) | 1552 (49.5%) | 48.68% |
| Boy | 325 (54.2%) | 414 (48.0%) | 295 (48.4%) | 548 (51.5%) | 1582 (50.4%) | 51.32% |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 3 (0.3%) | 3 (0.1%) | |
| Residential degree of urbanization N (%) | ||||||
| Cities | 386 (64.3%) | 464 (53.8%) | 318 (52.1%) | 215 (20.2%) | 1383 (44.1%) | 41.70% |
| Towns/suburbs | 134 (22.3%) | 221 (25.6%) | 80 (13.1%) | 476 (44.7%) | 911 (29.0%) | 31.00% |
| Rural area | 80 (13.3%) | 176 (20.4%) | 212 (34.8%) | 374 (35.1%) | 842 (26.8%) | 27.30% |
| Missing | 0 (0%) | 1 (0.1%) | 0 (0%) | 0 (0%) | 1 (0.03%) | |
| Educational level of the household N (%) | ||||||
| ISCED 0–2 | 41 (6.8%) | 19 (2.2%) | 36 (5.9%) | 34 (3.2%) | 130 (4.1%) | 26.00% |
| ISCED 3–4 | 168 (28.0%) | 393 (45.6%) | 200 (32.8%) | 342 (32.1%) | 1103 (35.2%) | 46.10% |
| ISCED ≥ 5 | 378 (63.0%) | 411 (47.7%) | 367 (60.2%) | 676 (63.5%) | 1832 (58.4%) | 27.90% |
| Missing | 13 (2.2%) | 39 (4.5%) | 7 (1.1%) | 13 (1.2%) | 72 (2.3%) |
3.3. Questionnaires
3.4. Data Accessibility
4. Discussion
4.1. Strengths and Limitations
4.2. Future Plans within HBM4EU
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EEA. The European Environment—State and Outlook 2020: Knowledge for Transition to a Sustainable Europe; European Environment Agency: Copenhagen, Denmark, 2020. [Google Scholar]
- Cao, Z.; Lin, S.; Zhao, F.; Lv, Y.; Qu, Y.; Hu, X.; Yu, S.; Song, S.; Lu, Y.; Yan, H.; et al. Cohort profile: China National Human Biomonitoring (CNHBM)-A nationally representative, prospective cohort in Chinese population. Environ. Int. 2021, 146, 106252. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hwang, M.; Kim, H.; Ryu, S.; Lee, K.; Choi, K.; Paek, D. Early snapshot on exposure to environmental chemicals among Korean adults-results of the first Korean National Environmental Health Survey (2009–2011). Int. J. Hyg. Environ. Health 2016, 219, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ganzleben, C.; Antignac, J.P.; Barouki, R.; Castano, A.; Fiddicke, U.; Klanova, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg. Environ. Health 2017, 220, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Conrad, A.; Becker, K.; Kolossa-Gehring, M.; Seiwert, M.; Seifert, B. Twenty years of the German Environmental Survey (GerES): Human biomonitoring—Temporal and spatial (West Germany/East Germany) differences in population exposure. Int. J. Hyg. Environ. Health 2007, 210, 271–297. [Google Scholar] [CrossRef]
- Schoeters, G.; Den Hond, E.; Colles, A.; Loots, I.; Morrens, B.; Keune, H.; Bruckers, L.; Nawrot, T.; Sioen, I.; De Coster, S.; et al. Concept of the Flemish human biomonitoring programme. Int. J. Hyg. Environ. Health 2012, 215, 102–108. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Fillol, C.; Charles, M.A.; Denys, S. The French human biomonitoring program: First lessons from the perinatal component and future needs. Int. J. Hyg. Environ. Health 2017, 220, 64–70. [Google Scholar] [CrossRef]
- Cerna, M.; Krskova, A.; Cejchanova, M.; Spevackova, V. Human biomonitoring in the Czech Republic: An overview. Int. J. Hyg. Environ. Health 2012, 215, 109–119. [Google Scholar] [CrossRef]
- Berglund, M. Hälsorelaterad Miljöövervakning (HÄMI); Institutet för Miljömedicin, Karolinska Institutet: Stockholm, Sweden, 2015; p. 52. [Google Scholar]
- Snoj Tratnik, J.; Falnoga, I.; Mazej, D.; Kocman, D.; Fajon, V.; Jagodic, M.; Stajnko, A.; Trdin, A.; Slejkovec, Z.; Jeran, Z.; et al. Results of the first national human biomonitoring in Slovenia: Trace elements in men and lactating women, predictors of exposure and reference values. Int. J. Hyg. Environ. Health 2019, 222, 563–582. [Google Scholar] [CrossRef]
- Stajnko, A.; Snoj Tratnik, J.; Kosjek, T.; Mazej, D.; Jagodic, M.; Erzen, I.; Horvat, M. Seasonal glyphosate and AMPA levels in urine of children and adolescents living in rural regions of Northeastern Slovenia. Environ. Int. 2020, 143, 105985. [Google Scholar] [CrossRef]
- Schindler, B.K.; Esteban, M.; Koch, H.M.; Castano, A.; Koslitz, S.; Canas, A.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Schoeters, G.; et al. The European COPHES/DEMOCOPHES project: Towards transnational comparability and reliability of human biomonitoring results. Int. J. Hyg. Environ. Health 2014, 217, 653–661. [Google Scholar] [CrossRef]
- Gilles, L.; Govarts, E.; Rambaud, L.; Vogel, N.; Castano, A.; Esteban Lopez, M.; Rodriguez Martin, L.; Koppen, G.; Remy, S.; Vrijheid, M.; et al. HBM4EU combines and harmonises human biomonitoring data across the EU, building on existing capacity—The HBM4EU survey. Int. J. Hyg. Environ. Health 2021, 237, 113809. [Google Scholar] [CrossRef] [PubMed]
- Ougier, E.; Ganzleben, C.; Lecoq, P.; Bessems, J.; David, M.; Schoeters, G.; Lange, R.; Meslin, M.; Uhl, M.; Kolossa-Gehring, M.; et al. Chemical prioritisation strategy in the European Human Biomonitoring Initiative (HBM4EU)—Development and results. Int. J. Hyg. Environ. Health 2021, 236, 113778. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.htm (accessed on 7 February 2022).
- Statistics Canada. Canadian Health Measures Survey (CHMS). Available online: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071 (accessed on 7 February 2022).
- Seo, J.W.; Kim, B.G.; Kim, Y.M.; Kim, R.B.; Chung, J.Y.; Lee, K.M.; Hong, Y.S. Trend of blood lead, mercury, and cadmium levels in Korean population: Data analysis of the Korea National Health and Nutrition Examination Survey. Environ. Monit. Assess 2015, 187, 146. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Ono, M.; Yonemoto, J.; Tamura, K.; Suda, E.; Ito, H.; Takeuchi, A.; Kawamoto, T.; et al. The Japan Environment and Children’s Study (JECS): A Preliminary Report on Selected Characteristics of Approximately 10 000 Pregnant Women Recruited During the First Year of the Study. J. Epidemiol. 2015, 25, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Den Hond, E.; Govarts, E.; Willems, H.; Smolders, R.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Seiwert, M.; Fiddicke, U.; Castano, A.; et al. First steps toward harmonized human biomonitoring in Europe: Demonstration project to perform human biomonitoring on a European scale. Environ. Health Perspect. 2015, 123, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Baken, K.A.; Lambrechts, N.; Remy, S.; Mustieles, V.; Rodriguez-Carrillo, A.; Neophytou, C.M.; Olea, N.; Schoeters, G. A strategy to validate a selection of human effect biomarkers using adverse outcome pathways: Proof of concept for phthalates and reproductive effects. Environ. Res. 2019, 175, 235–256. [Google Scholar] [CrossRef]
- Esteban Lopez, M.; Goen, T.; Mol, H.; Nubler, S.; Haji-Abbas-Zarrabi, K.; Koch, H.M.; Kasper-Sonnenberg, M.; Dvorakova, D.; Hajslova, J.; Antignac, J.P.; et al. The European human biomonitoring platform—Design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg. Environ. Health 2021, 234, 113740. [Google Scholar] [CrossRef]
- Pérez-Gómez, B.; Pastor-Barriuso, R.; Cervantes-Amat, M.; Esteban, M.; Ruiz-Moraga, M.; Aragonés, N.; Pollán, M.; Navarro, C.; Calvo, E.; Román, J.; et al. BIOAMBIENT.ES study protocol: Rationale and design of a cross-sectional human biomonitoring survey in Spain. Environ. Sci. Pollut. Res. 2013, 20, 1193–1202. [Google Scholar] [CrossRef]
- Piler, P.; Kandrnal, V.; Kukla, L.; Andryskova, L.; Svancara, J.; Jarkovsky, J.; Dusek, L.; Pikhart, H.; Bobak, M.; Klanova, J. Cohort Profile: The European Longitudinal Study of Pregnancy and Childhood (ELSPAC) in the Czech Republic. Int. J. Epidemiol. 2017, 46, 1379–1379f. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.S.; Ljubicic, M.L.; Upners, E.N.; Fischer, M.B.; Kolby, N.; Eckert-Lind, C.; Jespersen, K.; Andersson, A.M.; Frederiksen, H.; Johannsen, T.H.; et al. Cohort profile: The COPENHAGEN Minipuberty Study—A longitudinal prospective cohort of healthy full-term infants and their parents. Paediatr. Perinat. Epidemiol. 2021, 35, 601–611. [Google Scholar] [CrossRef]
- Kolossa-Gehring, M.; Becker, K.; Conrad, A.; Schroter-Kermani, C.; Schulz, C.; Seiwert, M. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int. J. Hyg. Environ. Health 2012, 215, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lermen, D.; Schmitt, D.; Bartel-Steinbach, M.; Schroter-Kermani, C.; Kolossa-Gehring, M.; von Briesen, H.; Zimmermann, H. A new approach to standardize multicenter studies: Mobile lab technology for the German Environmental Specimen Bank. PLoS ONE 2014, 9, e105401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemke, N.; Murawski, A.; Lange, R.; Weber, T.; Apel, P.; Debiak, M.; Koch, H.M.; Kolossa-Gehring, M. Substitutes mimic the exposure behaviour of REACH regulated phthalates—A review of the German HBM system on the example of plasticizers. Int. J. Hyg. Environ. Health 2021, 236, 113780. [Google Scholar] [CrossRef] [PubMed]
- Fillol, C.; Oleko, A.; Saoudi, A.; Zeghnoun, A.; Balicco, A.; Gane, J.; Rambaud, L.; Leblanc, A.; Gaudreau, É.; Marchand, P.; et al. Sébastien Denys Exposure of the French population to bisphenols, phthalates, parabens, glycol ethers, brominated flame retardants, and perfluorinated compounds in 2014–2016: Results from the Esteban study. Environ. Int. 2021, 147, 106340. [Google Scholar] [CrossRef] [PubMed]
- Balicco, A.; Oleko, A.; Szego, E.; Boschat, L.; Deschamps, V.; Saoudi, A.; Zeghnoun, A.; Fillol, C. Protocole Esteban: Une Étude transversale de santé sur l’environnement, la biosurveillance, l’activité physique et la nutrition (2014–2016). Toxicol. Anal. Clin. 2017, 29, 517–537. [Google Scholar] [CrossRef]
- Koponen, P.; Borodulin, K.; Lundqvist, A.; Sääksjärvi, K.; Koskinen, S. Terveys, Toimintakyky ja Hyvinvointi Suomessa, FinTerveys 2017-Tutkimus; Terveyden ja Hyvinvoinnin Laitos (THL): Helsinki, Finland, 2018; p. 236. [Google Scholar]
- Schoeters, G.; Verheyen, V.; Colles, A.; Remy, S.; Martin, L.R.; Govarts, E.; Nelen, V.; Hond, E.D.; De Decker, A.; Franken, C.; et al. Internal exposure of Flemish teenagers to environmental pollutants: Results of the Flemish Environment and Health Study 2016–2020 (FLEHS IV). Int. J. Hyg. Environ. Health 2022, 242, 113972. [Google Scholar] [CrossRef]
- Schulz, C.; Conrad, A.; Rucic, E.; Schwedler, G.; Reiber, L.; Peisker, J.; Kolossa-Gehring, M. The German Environmental Survey for Children and Adolescents 2014–2017 (GerES V)—Study population, response rates and representativeness. Int. J. Hyg. Environ. Health 2021, 237, 113821. [Google Scholar] [CrossRef] [PubMed]
- Szabados, M.; Csako, Z.; Kotlik, B.; Kazmarova, H.; Kozajda, A.; Jutraz, A.; Kukec, A.; Otorepec, P.; Dongiovanni, A.; Di Maggio, A.; et al. Indoor air quality and the associated health risk in primary school buildings in Central Europe—The InAirQ study. Indoor Air 2021, 31, 989–1003. [Google Scholar] [CrossRef]
- Vecchi Brumatti, L.; Rosolen, V.; Mariuz, M.; Piscianz, E.; Valencic, E.; Bin, M.; Athanasakis, E.; D’Adamo, P.; Fragkiadoulaki, E.; Calamandrei, G.; et al. Impact of Methylmercury and Other Heavy Metals Exposure on Neurocognitive Function in Children Aged 7 Years: Study Protocol of the Follow-up. J. Epidemiol. 2021, 31, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Magnus, P.; Birke, C.; Vejrup, K.; Haugan, A.; Alsaker, E.; Daltveit, A.K.; Handal, M.; Haugen, M.; Hoiseth, G.; Knudsen, G.P.; et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 2016, 45, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Kyhl, H.B.; Jensen, T.K.; Barington, T.; Buhl, S.; Norberg, L.A.; Jorgensen, J.S.; Jensen, D.F.; Christesen, H.T.; Lamont, R.F.; Husby, S. The Odense Child Cohort: Aims, design, and cohort profile. Paediatr. Perinat. Epidemiol. 2015, 29, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Alkerwi, A.; Pastore, J.; Sauvageot, N.; Coroller, G.L.; Bocquet, V.; d’Incau, M.; Aguayo, G.; Appenzeller, B.; Bejko, D.; Bohn, T.; et al. Challenges and benefits of integrating diverse sampling strategies in the observation of cardiovascular risk factors (ORISCAV-LUX 2) study. BMC Med. Res. Methodol. 2019, 19, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz-Picciotto, I.; Trnovec, T.; Kočan, A.; Charles, M.J.; Čižnar, P.; Langer, P.; Sovcikova, E.; James, R. PCBs and early childhood development in Slovakia: Study design and background. Fresenius Environ. Bull. 2003, 12, 208–214. [Google Scholar]
- Moraeus, L.; Lemming, E.W.; Hursti, U.K.; Arnemo, M.; Sipinen, J.P.; Lindroos, A.K. Riksmaten Adolescents 2016–17: A national dietary survey in Sweden—Design, methods, and participation. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorkamp, K.; Castano, A.; Antignac, J.P.; Boada, L.D.; Cequier, E.; Covaci, A.; Esteban Lopez, M.; Haug, L.S.; Kasper-Sonnenberg, M.; Koch, H.M.; et al. Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ. Int. 2021, 146, 106082. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T.; Turner, W.E.; Patterson, D.G., Jr.; Needham, L.L. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 2007, 68, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Nubler, S.; Lopez, M.E.; Castano, A.; Mol, H.; Schafer, M.; Haji-Abbas-Zarrabi, K.; Bury, D.; Koch, H.M.; Vaccher, V.; Antignac, J.P.; et al. Interlaboratory comparison investigations (ICI) and external quality assurance schemes (EQUAS) for cadmium in urine and blood: Results from the HBM4EU project. Int. J. Hyg. Environ. Health 2021, 234, 113711. [Google Scholar] [CrossRef]
- Nübler, S.; Esteban López, M.; Castano, A.; Mol, H.; Haji-Abbas-Zarrabi, K.; Schäfer, M.; Müller, J.; Hajslova, J.; Dvorakova, D.; Antignac, J.P.; et al. Interlaboratory Comparison Investigations (ICI) and External Quality Assurance Schemes (EQUAS) for Human Biomonitoring of Perfluoroalkyl Substances (PFASs) in Serum as Part of the Quality Assurance Programme Under HBM4EU; SSRN: Rochester, NY, USA, 2022. [Google Scholar] [CrossRef]
- Pack Kim, L.G.; Jirka, C.; Hanna, T.; van Irene, K.; Marta, E.; Susana, P.D.; Marina, L.; Beatriz, G.A.; Dominik, L.; Andromachi, K.; et al. A Step Towards Harmonising HBM Study Design on European Level: Documents Provided and Lessons Learnt in HBM4EU. Int. J. Hyg. Environ. Health 2022. [Google Scholar]
- EUROSTAT. Regions in the European Union Nomenclature of Territorial Units for Statistics-NUTS 2016/EU-28; Publications Office of the European Union: Luxembourg, 2018; p. 158. [Google Scholar]
- Lewis Dijkstra, H.P.; European Commission. Directorate-General for Regional and Urban Policy (DG REGIO). In A Harmonised Definition of Cities and Rural Areas: The New Degree of Urbanisation; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- European Food Safety Authority. General principles for the collection of national food consumption data in the view of a pan-European dietary survey. EFSA J. 2009, 7, 1435. [Google Scholar] [CrossRef]
- Middleton, D.R.; Watts, M.J.; Lark, R.M.; Milne, C.J.; Polya, D.A. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ. Health 2016, 15, 68. [Google Scholar] [CrossRef] [Green Version]
- LaKind, J.S.; Pollock, T.; Naiman, D.Q.; Kim, S.; Nagasawa, A.; Clarke, J. Factors affecting interpretation of national biomonitoring data from multiple countries: BPA as a case study. Environ. Res. 2019, 173, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ehresman, D.J.; Froehlich, J.W.; Olsen, G.W.; Chang, S.C.; Butenhoff, J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Methods Committee of the Lipid Research Clinics Program. Cholesterol and triglyceride concentrations in serum/plasma pairs. Clin. Chem. 1977, 23, 60–63. [Google Scholar]
- Galea, S.; Tracy, M. Participation Rates in Epidemiologic Studies. Ann. Epidemiol. 2007, 17, 643–653. [Google Scholar] [CrossRef]
- van Dijk, J.; Leopold, A.; Flerlage, H.; van Wezel, A.; Seiler, T.B.; Enrici, M.H.; Bloor, M.C. The EU Green Deal’s ambition for a toxic-free environment: Filling the gap for science-based policymaking. Integr. Environ. Assess Manag. 2021, 17, 1105–1113. [Google Scholar] [CrossRef]
- Hays, S.M.; Becker, R.A.; Leung, H.W.; Aylward, L.L.; Pyatt, D.W. Biomonitoring equivalents: A screening approach for interpreting biomonitoring results from a public health risk perspective. Regul. Toxicol. Pharmacol. 2007, 47, 96–109. [Google Scholar] [CrossRef]
- Lange, R.; Apel, P.; Rousselle, C.; Charles, S.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. The European Human Biomonitoring Initiative (HBM4EU): Human biomonitoring guidance values for selected phthalates and a substitute plasticizer. Int. J. Hyg. Environ. Health 2021, 234, 113722. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Apel, P.; Rousselle, C.; Lange, R.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. Human biomonitoring initiative (HBM4EU)—Strategy to derive human biomonitoring guidance values (HBM-GVs) for health risk assessment. Int. J. Hyg. Environ. Health 2020, 230, 113622. [Google Scholar] [CrossRef]
- Lamkarkach, F.; Ougier, E.; Garnier, R.; Viau, C.; Kolossa-Gehring, M.; Lange, R.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for cadmium and its compounds. Environ. Int. 2021, 147, 106337. [Google Scholar] [CrossRef]
- Buekers, J.; David, M.; Koppen, G.; Bessems, J.; Scheringer, M.; Lebret, E.; Sarigiannis, D.; Kolossa-Gehring, M.; Berglund, M.; Schoeters, G.; et al. Development of Policy Relevant Human Biomonitoring Indicators for Chemical Exposure in the European Population. Int. J. Environ. Res. Public Health 2018, 15, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, K.C.; Konstantinou, C.; Andrianou, X.D.; Charisiadis, P.; Kyriacou, A.; Gribble, M.O.; Christophi, C.A. A cluster-randomized crossover trial of organic diet impact on biomarkers of exposure to pesticides and biomarkers of oxidative stress/inflammation in primary school children. PLoS ONE 2019, 14, e0219420. [Google Scholar] [CrossRef] [Green Version]
- Berman, T.; Barnett-Itzhaki, Z.; Goen, T.; Hamama, Z.; Axelrod, R.; Keinan-Boker, L.; Shimony, T.; Goldsmith, R. Organophosphate pesticide exposure in children in Israel: Dietary associations and implications for risk assessment. Environ. Res. 2020, 182, 108739. [Google Scholar] [CrossRef]
- Berman, T.; Goldsmith, R.; Levine, H.; Grotto, I. Human biomonitoring in Israel: Recent results and lessons learned. Int. J. Hyg. Environ. Health 2017, 220, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Parliament, E. European Parliament resolution of 10 July 2020 on the Chemicals Strategy for Sustainability (2020/2531(RSP)). In Proceedings of the Chemicals Strategy for Sustainability, Brussels, Belgium, 10 July 2020. [Google Scholar]

| Study Acronym | Location | Level of Representativity | Region | Study Design | Sampling Time Period | Original Age Range | Total Number of Study Subjects | N Selected for HBM4EU | Reference |
|---|---|---|---|---|---|---|---|---|---|
| BEA | Spain | National | - | Cross sectional | 10/2017–02/2018 | 14–16 | 499 | 300 | [22] |
| CELSPAC: YA | Czech Republic | Regional | South Moravia | Longitudinal | 03/2019–12/2022 | 28–31 | 800 | 300 | [23] |
| CELSPAC:TE | Czech Republic | Regional | South Moravia | Cross sectional | 2019–2020 | 12–17 | 365 | 300 | - |
| CPHMINIPUB (parents)/DYMS | Denmark | Regional | The Capital Region of Denmark | Cross sectional | 03/2017– 02/2019 | 20–39 | 292 | 292 | [24] |
| CROME | Greece | Regional | Thessaloniki | Cross sectional | 07/2020–03/2021 | 6–18 | 560 | 161 children 150 teenagers | - |
| Diet-HBM | Iceland | National | - | Cross sectional | 10/2019–12/2020 | 20–39 | 205 | 205 | - |
| ESB | Germany | Regional | Münster, Greifswald, Halle/Saale and Ulm | Cross sectional | Earliest samples from 1981, ongoing study | 20–29 | 500 per year | 700 | [25,26,27] |
| ESTEBAN | France | National | Mainland France | Cross sectional | 04/2014– 03/2016 | 6–74 | 592 (6–11 y), 512 (12–17 y), 2503 (18–74 y) | 543 447 393 | [28,29] |
| FinHealth | Finland | National | Mainland excl. Åland | Cross sectional | 01/2017–06/2017 | ≥25 years | 300 | 300 | [30] |
| FLEHS IV | Belgium | Regional | Flanders | Cross sectional | 09/2017–06/2018 | 14–15 | 428 | 300 | [31] |
| GerES V-sub (unweighted) | Germany | National | - | Cross sectional | 01/2015–06/2017 | 2294 | 300 children 300 teenagers | [32] | |
| HBM survey in adults in Croatia | Croatia | National | - | Cross sectional | 11/2019–01/2020 | 20–39 | 300 | 300 | - |
| HBM4EU-study for Switzerland | Switzerland | Regional | Basel | Cross sectional | 02/2019– 10/2020 | 20–39 | 300 | 300 | - |
| InAirQ | Hungary | National | - | Cross sectional | 11/2017–03/2018 | 8–11 | 262 | 262 | [33] |
| INSEF-ExpoQuim | Portugal | National | - | Cross sectional | 05/2019–03/2020 | 28–39 | 296 | 296 | - |
| NAC II | Italy | Regional | Trieste | Longitudinal | 08/2014–12/2016 | 6–8 | 487 | 300 | [34] |
| NEB II | Norway | National | - | Longitudinal | 2016–2017 | 7–14 | 668 | 300 children 181 teenagers | [35] |
| OCC | Denmark | Regional | Fynn region | Longitudinal | 2018–2019 | 7 | 2449 | 300 | [36] |
| Oriscav-Lux2 | Luxembourg | National | - | Cross sectional | 06/01/2016–31/01/2018 | 25–80 | 1558 | 210 | [37] |
| PCB cohort/PCB cohort (follow-up) | Slovakia | Regional | Michalovce region | Longitudinal | 2014–2017 | 10–12, 15–17 | Original: 415, follow-up: 297 | 300 children 294 teenagers | [38] |
| POLAES | Poland | Regional | Lower Silesia | Case-control | 09/2017–12/2017 | 6–11 | 300 children 281 teenagers 228 adults | - | |
| Riksmaten Adolescents 2016–2017 | Sweden | National | - | Cross sectional | 09/2016–05/2017 | 10–21 | 1305 | 300 | [39] |
| SLO CRP | Slovenia | Regional | Mura region | Cross sectional | 01/2018–06/2018 | 7–10, 12-15 | 246 | 149 (children) 97 (teenagers) | [11] |
| SPECIMEn-NL | The Netherlands | Regional | Central-East | Cross sectional | 01/2020–03/2020 | 6–11 | 102 | 102 | - |
| 3 × G | Belgium | Regional | Dessel, Mol, Retie | Longitudinal | 01/2019–06/2021 | 6–8 | 212 | 212 | - |
| Characteristics | Northern EU | Eastern EU | Southern EU | Western EU | EU Total | EU Reference |
|---|---|---|---|---|---|---|
| No. of participants | 481 | 875 | 547 | 1047 | 2950 | |
| Age (years) | ||||||
| Median (p25–p75) | 14 (12–14) | 14 (13–15) | 14 (14–15) | 14 (13–15) | 14 (13–15) | |
| Min-max | 12–17 | 12–17 | 12–18 | 12–18 | 12–18 | |
| Missing | 0 (0%) | 0 (0%) | 4 (0.7%) | 0 (0%) | 4 (0.1%) | |
| Sampling period (years) | ||||||
| Median (p25–p75) | 2016 (2016–2017) | 2019 (2017–2019) | 2018 (2017–2020) | 2016 (2015–2017) | 2017 (2016–2019) | |
| Min-max | 2016–2017 | 2017–2020 | 2017–2021 | 2014–2018 | 2014–2021 | |
| Sex N (%) | ||||||
| Girl | 254 (52.8%) | 423 (48.3%) | 274 (50.1%) | 541 (51.7%) | 1492 (50.6%) | 48.38% |
| Boy | 227 (47.2%) | 452 (51.7%) | 273 (49.9%) | 506 (48.3%) | 1458 (49.4%) | 51.62% |
| Residential degree of urbanization N (%) | ||||||
| Cities | 155 (32.2%) | 380 (43.4%) | 337 (61.6%) | 240 (22.9%) | 1112 (37.7%) | 41.70% |
| Towns/suburbs | 219 (45.5%) | 187 (21.4%) | 65 (11.9%) | 450 (43.0%) | 921 (31.2%) | 31.00% |
| Rural area | 106 (22.0%) | 283 (32.3%) | 145 (26.5%) | 357 (34.1%) | 891 (30.2%) | 27.30% |
| Missing | 1 (0.2%) | 25 (2.9%) | 0 (0%) | 0 (0%) | 26 (0.9%) | |
| Educational level of the household N (%) | ||||||
| ISCED 0–2 | 23 (4.8%) | 20 (2.3%) | 69 (12.6%) | 68 (6.5%) | 180 (6.1%) | 26.00% |
| ISCED 3–4 | 114 (23.7%) | 420 (48.0%) | 151 (27.6%) | 404 (38.6%) | 1089 (36.9%) | 46.10% |
| ISCED ≥5 | 329 (68.4%) | 396 (45.3%) | 307 (56.1%) | 575 (54.9%) | 1607 (54.5%) | 27.90% |
| Missing | 15 (3.1%) | 39 (4.5%) | 20 (3.7%) | 0 (0%) | 74 (2.5%) |
| Characteristics | Northern EU | Eastern EU | Southern EU | Western EU | Total | Reference EU |
|---|---|---|---|---|---|---|
| No. of participants | 795 | 528 | 596 | 1603 | 3522 | |
| Age (years) | ||||||
| Median (p25–p75) | 31 (28–35) | 28 (27–34) | 33 (30–37) | 27 (24–33) | 30 (26–35) | |
| Min-max | 20–39 | 20–39 | 20–39 | 20–39 | 20–39 | |
| Sampling period (year) | ||||||
| Median (p25–p75) | 2018 (2017–2019) | 2019 (2017–2019) | 2019 (2019–2019) | 2017 (2015–2019) | 2018 (2017–2019) | |
| Min-max | 2017–2021 | 2017–2019 | 2019–2020 | 2014–2021 | 2014–2021 | |
| Sex N (%) | ||||||
| Women | 393 (49.4%) | 313 (59.3%) | 330 (55.4%) | 818 (51.0%) | 1854 (52.6%) | 49.35% |
| Men | 402 (50.6%) | 215 (40.7%) | 266 (44.6%) | 785 (49.0%) | 1668 (47.4%) | 50.65% |
| Residential degree of urbanization N (%) | ||||||
| Cities | 645 (81.1%) | 443 (83.9%) | 254 (42.6%) | 1191 (74.3%) | 2533 (71.9%) | 41.80% |
| Towns/suburbs | 86 (10.8%) | 30 (5.7%) | 151 (25.3%) | 233 (14.5%) | 500 (14.2%) | 38.20% |
| Rural area | 59 (7.4%) | 43 (8.1%) | 191 (32.0%) | 179 (11.2%) | 472 (13.4%) | 20.00% |
| Missing | 5 (0.6%) | 12 (2.3%) | 0 (0%) | 0 (0%) | 17 (0.5%) | |
| Educational level of participant N (%) | ||||||
| ISCED 0–2 | 153 (19.2%) | 2 (0.4%) | 60 (10.1%) | 22 (1.4%) | 237 (6.7%) | 16.20% |
| ISCED 3–4 | 222 (27.9%) | 165 (31.2%) | 215 (36.1%) | 254 (15.8%) | 856 (24.3%) | 44.80% |
| ISCED ≥5 | 407 (51.2%) | 360 (68.2%) | 321 (53.9%) | 1323 (82.5%) | 2411 (68.5%) | 39% |
| Missing | 13 (1.6%) | 1 (0.2%) | 0 (0%) | 4 (0.2%) | 18 (0.5%) | |
| Smoking behavior N (%) | ||||||
| Non smoker | 679 (85.4%) | 459 (86.9%) | 425 (71.3%) | 1303 (81.3%) | 2866 (81.4%) | |
| Smoker | 105 (13.2%) | 66 (12.5%) | 163 (27.3%) | 283 (17.7%) | 617 (17.5%) | 22.9% |
| Missing | 11 (1.4%) | 3 (0.6%) | 8 (1.3%) | 17 (1.1%) | 39 (1.1%) |
| Study Acronym | Location | Level of Representativity | Region | Study Design | Sampling Time Period | Original Age Range | Total Number of Study Subjects | N Selected for HBM4EU | References |
|---|---|---|---|---|---|---|---|---|---|
| ORGANIKO | Cyprus | Regional | Limassol | Cross-over | 01/2017–04/2017 | 10–12 | 191 | 166 | [60] |
| RAV-MABAT | Israel | National | Cross-sectional | 2015–2016 | 4–11 year and 18–64 years | Children 103 Adults 194 | Children 103 Adults 194 | [61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilles, L.; Govarts, E.; Rodriguez Martin, L.; Andersson, A.-M.; Appenzeller, B.M.R.; Barbone, F.; Castaño, A.; Coertjens, D.; Den Hond, E.; Dzhedzheia, V.; et al. Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. Int. J. Environ. Res. Public Health 2022, 19, 6787. https://doi.org/10.3390/ijerph19116787
Gilles L, Govarts E, Rodriguez Martin L, Andersson A-M, Appenzeller BMR, Barbone F, Castaño A, Coertjens D, Den Hond E, Dzhedzheia V, et al. Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. International Journal of Environmental Research and Public Health. 2022; 19(11):6787. https://doi.org/10.3390/ijerph19116787
Chicago/Turabian StyleGilles, Liese, Eva Govarts, Laura Rodriguez Martin, Anna-Maria Andersson, Brice M. R. Appenzeller, Fabio Barbone, Argelia Castaño, Dries Coertjens, Elly Den Hond, Vazha Dzhedzheia, and et al. 2022. "Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants" International Journal of Environmental Research and Public Health 19, no. 11: 6787. https://doi.org/10.3390/ijerph19116787
APA StyleGilles, L., Govarts, E., Rodriguez Martin, L., Andersson, A.-M., Appenzeller, B. M. R., Barbone, F., Castaño, A., Coertjens, D., Den Hond, E., Dzhedzheia, V., Eržen, I., López, M. E., Fábelová, L., Fillol, C., Franken, C., Frederiksen, H., Gabriel, C., Haug, L. S., Horvat, M., ... Schoeters, G. (2022). Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. International Journal of Environmental Research and Public Health, 19(11), 6787. https://doi.org/10.3390/ijerph19116787















