The Cost-Effectiveness Analysis of an Integrated Mental Health Care Programme in Germany
Abstract
:1. Introduction
2. Materials and Methods
- Being between 18 and 80 years old;
- Being diagnosed with a mental illness of the ICD-10 categories F20–F69 and F91–F94 during the last 12 months;
- Having either had a psychiatric inpatient admission or an ambulatory prescription of antipsychotic, anxiolytic or antidepressant drugs during the last 12 months prior to enrolment;
- Not being eligible for receiving benefits from statutory long-term care insurance;
- Being a member of one of the NWpG-participating health insurances (only for the intervention group).
2.1. Intervention
2.2. Outcomes and Assessment
2.3. Cost Estimation
2.4. Statistical Analysis
3. Results
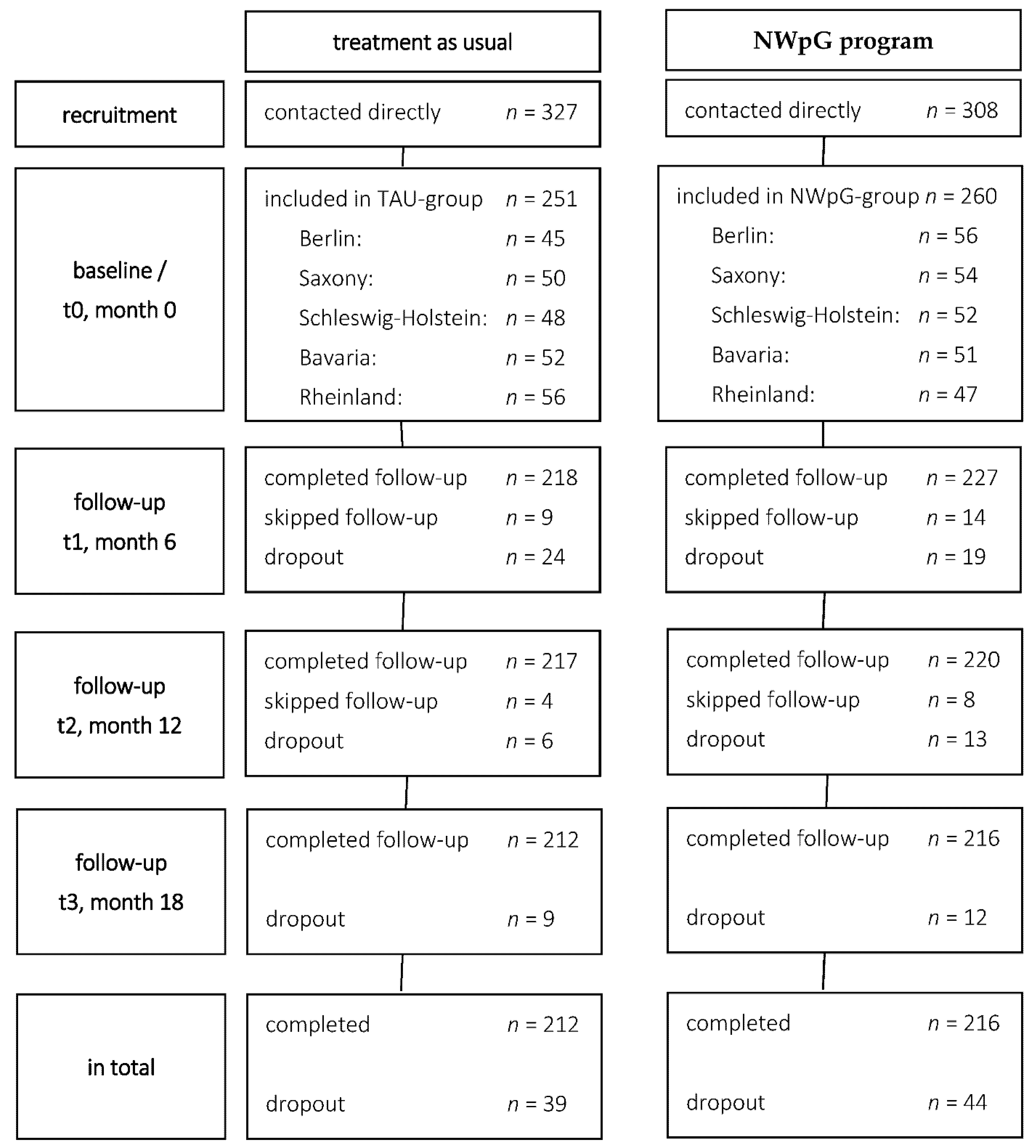
3.1. Study Population and Study Flow
3.2. Service Use
3.3. Unadjusted Health Care Costs
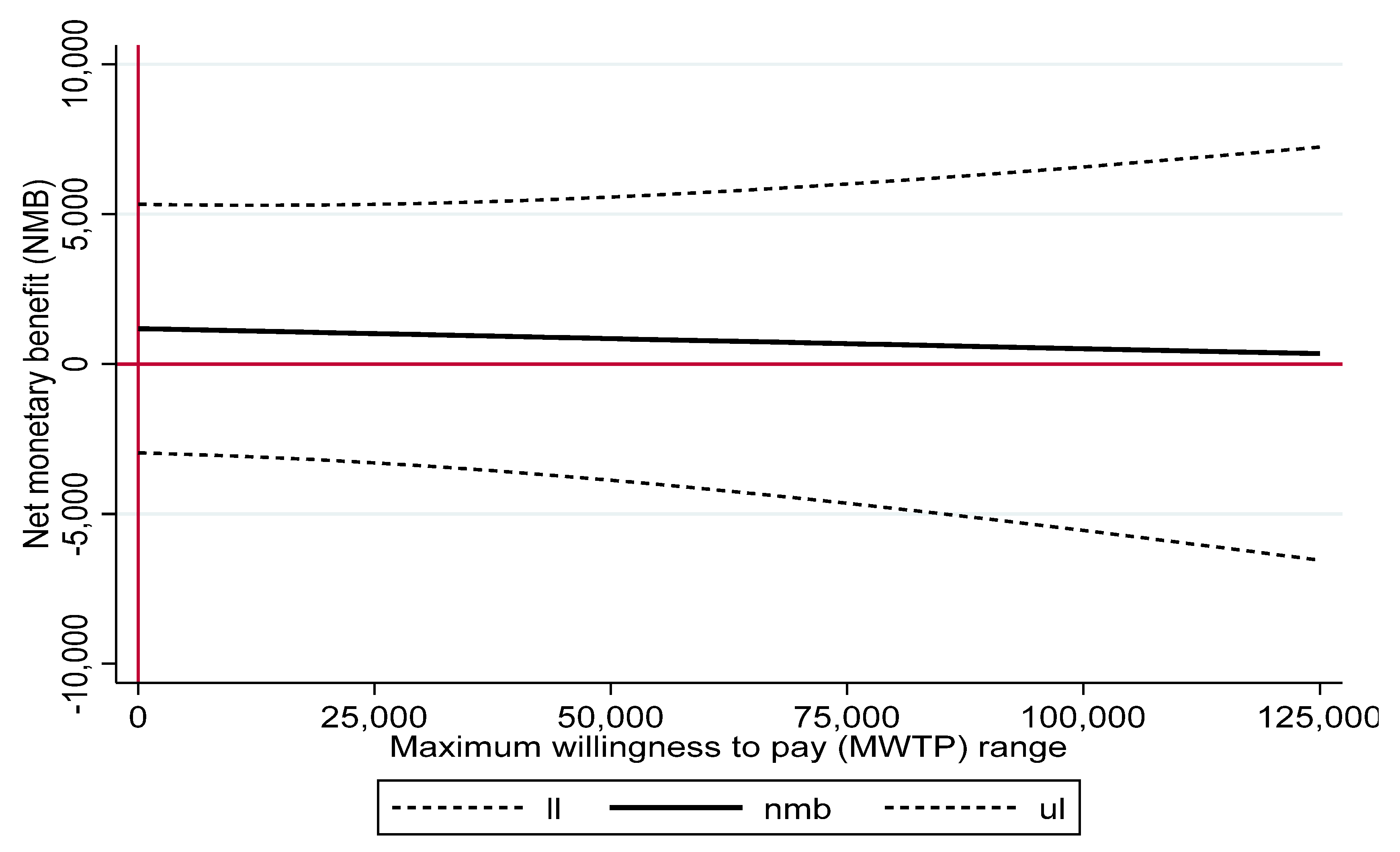
3.4. PS-Adjusted Health Care Costs, QALYs and Cost–Utility Ratios and Net Benefit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salize, H.-J.; Kilian, R. Gesundheitsökonomie in der Psychiatrie: Konzepte, Methoden, Analysen, 1st ed.; Kohlhammer: Stuttgart, Germany, 2010; ISBN 978-3170198395. [Google Scholar]
- Heider, D.; Bernert, S.; Koenig, H.-H.; Matschinger, H.; Hogh, T.; Brugha, T.S.; Bebbington, P.E.; Azorin, M.; Angermeyer, M.C.; Toumi, M. Direct medical mental health care costs of schizophrenia in France, Germany and the United Kingdom—findings from the European Schizophrenia Cohort (EuroSC). Eur. Psychiatry 2009, 24, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Salize, H.-J.; McCabe, R.; Bullenkamp, J.; Hansson, L.; Lauber, C.; Martinez-Leal, R.; Reinhard, I.; Roessler, W.; Svensson, B.; Torres-Gonzalez, F.; et al. Cost of treatment of schizophrenia in six European countries. Schizophr. Res. 2009, 111, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, S. The economic burden of schizophrenia in Germany: A population-based retrospective cohort study using genetic matching. Eur. Psychiatry 2014, 29, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bramesfeld, A.; Ungewitter, C.; Boettger, D.; El Jurdi, J.; Losert, C.; Kilian, R. What promotes and inhibits cooperation in mental health care across disciplines, services and service sectors? A qualitative study. Epidemiol. Psychiatr. Sci. 2012, 21, 63–72. [Google Scholar] [CrossRef]
- Puschner, B.; Baumgartner, I.; Loos, S.; Volker, K.A.; Ramacher, M.; Sohla, K.; Grempler, J.; Becker, T.; Kilian, R. Kosteneffektivitat bedarfsorientierter Entlassungsplanung bei Menschen mit hoher Inanspruchnahme psychiatrischer Versorgung. Psychiatr. Prax. 2012, 39, 381–387. [Google Scholar] [CrossRef]
- Salize, H.-J.; Roessler, W.; Becker, T. Mental health care in Germany: Current state and trends. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 92–103. [Google Scholar] [CrossRef]
- Brookhart, A.M.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stuermer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Latimer, E.A. Economic impacts of assertive community treatment: A review of the literature. Can. J. Psychiatry 1999, 44, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Rosen, A.; Mueser, K.T.; Teesson, M. Assertive community treatment—issues from scientific and clinical literature with implications for practice. J. Rehabil. Res. Dev. 2007, 44, 813–825. [Google Scholar] [CrossRef]
- Knapp, M.; Beecham, J.; McDaid, D.; Matosevic, T.; Smith, M. The economic consequences of deinstitutionalisation of mental health services: Lessons from a systematic review of European experience. Health Soc. Care Community 2011, 19, 113–125. [Google Scholar] [CrossRef]
- Marshall, M.; Lockwood, A. Assertive community treatment for people with severe mental disorders. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Irving, C.B.; Park, B.; Marshall, M. Intensive case management for severe mental illness. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [Green Version]
- Burns, T.; Catty, J.; Dash, M.; Roberts, C.; Lockwood, A.; Marshall, M. Use of intensive case management to reduce time in hospital in people with severe mental illness: Systematic review and meta-regression. BMJ 2007, 335, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieterich, M.; Irving, C.B.; Bergman, H.; Khokhar, M.A.; Park, B.; Marshall, M. Intensive case management for severe mental illness. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Stein, L.I.; Test, M.A. Alternative to mental hospital treatment: I. Conceptual model, treatment program, and clinical evaluation. Arch. Gen. Psychiatry 1980, 37, 392–397. [Google Scholar] [CrossRef]
- Bauer, E.; Kleine-Budde, K.; Stegbauer, C.; Kaufmann-Kolle, P.; Goetz, K.; Bestmann, B.; Szecsenyi, J.; Bramesfeld, A. Structures and processes necessary for providing effective home treatment to severely mentally ill persons: A naturalistic study. BMC Psychiatry 2016, 16, 242. [Google Scholar] [CrossRef] [Green Version]
- Karow, A.; Reimer, J.; Koenig, H.-H.; Heider, D.; Bock, T.; Huber, C.G.; Schoettle, D.; Meister, K.; Rietschel, L.; Ohm, G.; et al. Cost-effectiveness of 12-month therapeutic assertive community treatment as part of integrated care versus standard care in patients with schizophrenia treated with quetiapine immediate release (ACCESS trial). J. Clin. Psychiatry 2012, 73, e402–e408. [Google Scholar] [CrossRef]
- Becker, T.; Kilian, R.; Koesters, M. Policies, guideline implementation and practice change—how can the process be understood? Epidemiol. Psychiatr. Sci. 2016, 26, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Stierlin, A.S.; Helmbrecht, M.J.; Herder, K.; Prinz, S.; Rosenfeld, N.; Walendzik, J.; Holzmann, M.; Dinc, U.; Schutzwohl, M.; Becker, T.; et al. Does one size really fit all? The effectiveness of a non-diagnosis-specific integrated mental health care program in Germany in a prospective, parallel-group controlled multi-centre trial. BMC Psychiatry 2017, 17, 283. [Google Scholar] [CrossRef]
- Stierlin, A.S.; Herder, K.; Helmbrecht, M.J.; Prinz, S.; Walendzik, J.; Holzmann, M.; Becker, T.; Schuetzwohl, M.; Kilian, R. Effectiveness and efficiency of integrated mental health care programmes in Germany: Study protocol of an observational controlled trial. BMC Psychiatry 2014, 14, 163. [Google Scholar] [CrossRef] [Green Version]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013, 16, 231–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, H.-H.; Roick, C.; Angermeyer, M.C. Validity of the EQ-5D in assessing and valuing health status in patients with schizophrenic, schizotypal or delusional disorders. Eur. Psychiatry 2007, 22, 177–187. [Google Scholar] [CrossRef]
- König, H.-H.; Born, A.; Günther, O.; Matschinger, H.; Heinrich, S.; Riedel-Heller, S.G.; Angermeyer, M.C.; Roick, C. Validity and responsiveness of the EQ-5D in assessing and valuing health status in patients with anxiety disorders. Health Qual. Life Outcomes 2010, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochtdreis, T.; Brettschneider, C.; Hajek, A.; Schierz, K.; Hoyer, J.; Koenig, H.-H. Mapping the Beck Depression Inventory to the EQ-5D-3L in Patients with Depressive Disorders. J. Ment. Health Policy Econ. 2016, 19, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Goni, J.M.; Rivero-Arias, O. eq5d: A command to calculate index values for the EQ-5D quality-of-life instrument. Stata J. 2011, 11, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Szende, A.; Devlin, N.; Oppe, M. EQ-5D Value Sets: Inventory, Comparative Review and User Guide; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1402055102. [Google Scholar]
- Roick, C.; Kilian, R.; Matschinger, H.; Bernert, S.; Mory, C.; Angermeyer, M.C. Die deutsche Version des Client Sociodemographic and Service Receipt Inventory—Ein Instrument zur Erfassung psychiatrischer Versorgungskosten. Psychiatr. Prax. 2001, 28 (Suppl. S2), S84–S90. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, D.; Knapp, M.R.; Knudsen, H.C.; Amaddeo, F.; Gaite, L.; van Wijngaarden, B. Client Socio-Demographic and Service Receipt Inventory—European Version: Development of an instrument for international research. EPSILON Study 5. European Psychiatric Services: Inputs Linked to Outcome Domains and Needs. Br. J. Psychiatry Suppl. 2000, 177, s28–s33. [Google Scholar] [CrossRef]
- Bock, J.-O.; Brettschneider, C.; Seidl, H.; Bowles, D.; Holle, R.; Greiner, W.; König, H.H. Ermittlung standardisierter Bewertungssätze aus gesellschaftlicher Perspektive für die gesundheitsökonomische Evaluation. Gesundheitswesen 2015, 77, 53–61. [Google Scholar] [CrossRef]
- Frasch, K.; Weiser, P.; Becker, T.; Laengle, G.; Steinert, T.; Niederreiner, C.; Pfiffner, C.; Jaeger, S.; Bayer, W.; Eschweiler, G.W.; et al. Psychotropic drug treatment, clinical characteristics and cognitive processing speed in patients with schizophrenia: Results from the ELAN study. Pharmacopsychiatry 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Schwabe, U.; Paffrath, D. Arzneiverordnungs-Report 2015: Aktuelle Daten, Kosten, Trends und Kommentare; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662471852. [Google Scholar]
- Khandker, S.R.; Koolwal, G.B.; Samad, H.A. Handbook on Impact Evaluation: Quantitative Methods and Practices; World Bank: Washington, DC, USA, 2010; ISBN 978-0-8213-8028-4. [Google Scholar]
- Zellner, A. An Efficient Method of Estimating Seemingly Unrelated Regressions and Tests for Aggregation Bias. J. Am. Stat. Assoc. 1962, 57, 348. [Google Scholar] [CrossRef]
- Greene, W.H. Econometric Analysis, 5th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2003; ISBN 0-13-066189-9. [Google Scholar]
- Willan, A.R. Statistical Analysis of Cost-Effectiveness Data; John Wiley: Chichester, UK; Hoboken, NJ, USA, 2006; ISBN 978-0-470-85626-0. [Google Scholar]
- Glick, H.A. Economic Evaluation in Clinical Trials, 2nd ed.; Oxford University Press: Oxford, UK, 2014; ISBN 978-0199685028. [Google Scholar]
- Stata Corporation. Stata Statistical Software, Version 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Glick, H.A. Programs to Compute Confidence Intervals for Cost Effectiveness-Ratios. Available online: https://drhenryglick.com/sampling-uncertainty-for-cost-effectiveness-analysis/ (accessed on 30 May 2022).
- Karow, A.; Brettschneider, C.; Helmut König, H.; Correll, C.U.; Schöttle, D.; Lüdecke, D.; Rohenkohl, A.; Ruppelt, F.; Kraft, V.; Gallinat, J.; et al. Better care for less money: Cost-effectiveness of integrated care in multi-episode patients with severe psychosis. Acta Psychiatr. Scand. 2020, 141, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hastrup, L.H.; Kronborg, C.; Bertelsen, M.; Jeppesen, P.; Jorgensen, P.; Petersen, L.; Thorup, A.; Simonsen, E.; Nordentoft, M. Cost-effectiveness of early intervention in first-episode psychosis: Economic evaluation of a randomised controlled trial (the OPUS study). Br. J. Psychiatry 2013, 202, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Gardner, J. Assertive outreach could be cost-effective. Br. J. Psychiatry 2000, 177, 564. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Stierlin, A.S.; Meixner, F.; Kohlmann, A.; Schumacher, M.; Hänsel, A.; Pouwels, M.; Bias, N.; Hartl, S.; Reichstein, J.; Prestin, E.; et al. Effectiveness and cost-effectiveness of a community-based mental health care programme (GBV) for people with severe mental illness in Germany: Study protocol for a randomised controlled trial. Trials 2020, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Soltmann, B.; Neumann, A.; March, S.; Weinhold, I.; Häckl, D.; Kliemt, R.; Baum, F.; Romanos, M.; Schwarz, J.; von Peter, S.; et al. Multiperspective and Multimethod Evaluation of Flexible and Integrative Psychiatric Care Models in Germany: Study Protocol of a Prospective, Controlled Multicenter Observational Study (PsychCare). Front. Psychiatry 2021, 12, 659773. [Google Scholar] [CrossRef] [PubMed]


| Total | TAU | NWpG | p a | ||||
|---|---|---|---|---|---|---|---|
| Sociodemographic | |||||||
| Age in years; m (sd) | 46.47 | (11.61) | 47.15 | (11.01) | 45.82 | (12.14) | 0.195 |
| Female; n (%) | 353 | (69.1%) | 167 | (66.5%) | 186 | (71.5%) | 0.221 |
| Living alone; n (%) | 235 | (46.0%) | 127 | (50.6%) | 108 | (41.5%) | 0.040 |
| Employed; n (%) | 175 | (35.1%) | 58 | (23.5%) | 117 | (46.4%) | <0.001 |
| Social welfare reception; n (%) | 26 | (5.1%) | 20 | (8.0%) | 6 | (2.3%) | 0.004 |
| Health insurance company affiliation, TK; n (%) | 214 | (41.9%) | 32 | (12.7%) | 182 | (70.0%) | <0.001 |
| Medical history and treatment | |||||||
| Duration of illness in years; m (sd) | 12.47 | (11.63) | 14.24 | (11.85) | 10.77 | (11.18) | 0.001 |
| Number of hospitalisations; m (sd) | 2.89 | (4.64) | 4.06 | (6.06) | 1.77 | (2.10) | <0.001 |
| Diagnosis | 0.017 | ||||||
| F20–F29; n (%) | 67 | (13.1%) | 43 | (17.1%) | 24 | (9.2%) | |
| F30–F39; n (%) | 317 | (62.0%) | 140 | (55.8%) | 177 | (68.1%) | |
| F40–F48; n (%) | 98 | (19.2%) | 53 | (21.1%) | 45 | (17.3%) | |
| Multiple mental diagnoses; n (%) | 248 | (48.5%) | 135 | (53.8%) | 113 | (43.5%) | 0.020 |
| Prescription of pharmaceuticals; n (%) | 379 | (74.2%) | 199 | (79.3%) | 180 | (69.2%) | 0.009 |
| Assisted living; n (%) | 36 | (7.1%) | 31 | (12.4%) | 5 | (1.9%) | <0.001 |
| Legal guardian; n (%) | 31 | (6.1%) | 23 | (9.2%) | 8 | (3.1%) | 0.004 |
| Outcomes | |||||||
| Empowerment—EPAS total; m (sd) | 3.42 | (0.60) | 3.42 | (0.62) | 3.42 | (0.59) | 0.959 |
| Impairment—HONOS total; m (sd) | 10.66 | (5.30) | 10.53 | (5.12) | 10.79 | (5.48) | 0.576 |
| Number of needs; m (sd) | 4.70 | (2.63) | 5.07 | (2.70) | 4.34 | (2.51) | 0.002 |
| Proportion of met needs; m (sd) | 59.5% | (31.8%) | 62.9% | (30.4%) | 56.2% | (32.8%) | 0.017 |
| Satisfaction score; m (sd) | 24.22 | (4.43) | 24.51 | (4.53) | 23.94 | (4.33) | 0.154 |
| WHOQOL-BREF; m (sd) | 48.86 | (22.04) | 48.63 | (22.64) | 49.08 | (21.49) | 0.818 |
| EQ-5D; m (sd) | 0.77 | (0.25) | 0.74 | (0.25) | 0.79 | (0.24) | 0.028 |
| Total | TAU | NWpG | Difference NWpG-TAU | p | |
|---|---|---|---|---|---|
| M (95% CI a) | M (95% CI a) | M (95% CI a) | M (95% CI a) | ||
| Direct costs | 9583.53 | 10,908.01 | 8288.25 | −2619.76 | 0.002 |
| (8781.25 to 10,385.80) | (9488.82 to 12,327.19) | (7416.09 to 9160.42) | (−4276.02 to −963.49) | ||
| Indirect costs | 12,173.57 | 14,953.53 | 9448.72 | −5500.42 | <0.001 |
| (10,704.39 to 13,642.74) | (12,662.93 to 17,244.15) | (7526.98 to 11,370.43) | (−8350.15 to −2659.51) | ||
| Total psychiatric costs (direct and indirect) | 21,763.47 | 25,767.18 | 17,826,71 | −7940.50 | <0.001 |
| (19,940.70 to 23,586.24) | (11,455.41 to 14,311.79) | (15,607.98 to 20,045.44) | (−5762.91 to −2177.58) | ||
| Somatic treatment | 122.50 | 93.80 | 150.58 | 56.80 | 0.214 |
| (76.08 to 168.94) | (39.55 to 148.02) | (80.47 to 220.71) | (−32.83 to 146.41) | ||
| Average 12-month total costs of illness over 24 months | 21,997.82 | 26,111.33 | 18,014.39 | −8096.92 | <0.001 |
| (20,179.89 to 23,815.76) | (23,247.23 to 28,975.42) | (15,695.95 to 20,332.83) | (−11,735.75 to −4458.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller-Stierlin, A.S.; Dinc, U.; Herder, K.; Walendzik, J.; Schuetzwohl, M.; Becker, T.; Kilian, R. The Cost-Effectiveness Analysis of an Integrated Mental Health Care Programme in Germany. Int. J. Environ. Res. Public Health 2022, 19, 6814. https://doi.org/10.3390/ijerph19116814
Mueller-Stierlin AS, Dinc U, Herder K, Walendzik J, Schuetzwohl M, Becker T, Kilian R. The Cost-Effectiveness Analysis of an Integrated Mental Health Care Programme in Germany. International Journal of Environmental Research and Public Health. 2022; 19(11):6814. https://doi.org/10.3390/ijerph19116814
Chicago/Turabian StyleMueller-Stierlin, Annabel Sandra, Uemmueguelsuem Dinc, Katrin Herder, Julia Walendzik, Matthias Schuetzwohl, Thomas Becker, and Reinhold Kilian. 2022. "The Cost-Effectiveness Analysis of an Integrated Mental Health Care Programme in Germany" International Journal of Environmental Research and Public Health 19, no. 11: 6814. https://doi.org/10.3390/ijerph19116814






