A Novel Heterozygous Mutation of the CYP17A1 Gene in a Child with a Micropenis and Isolated 17,20-Lyase Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report
2.2. Physical and Clinical Evaluation
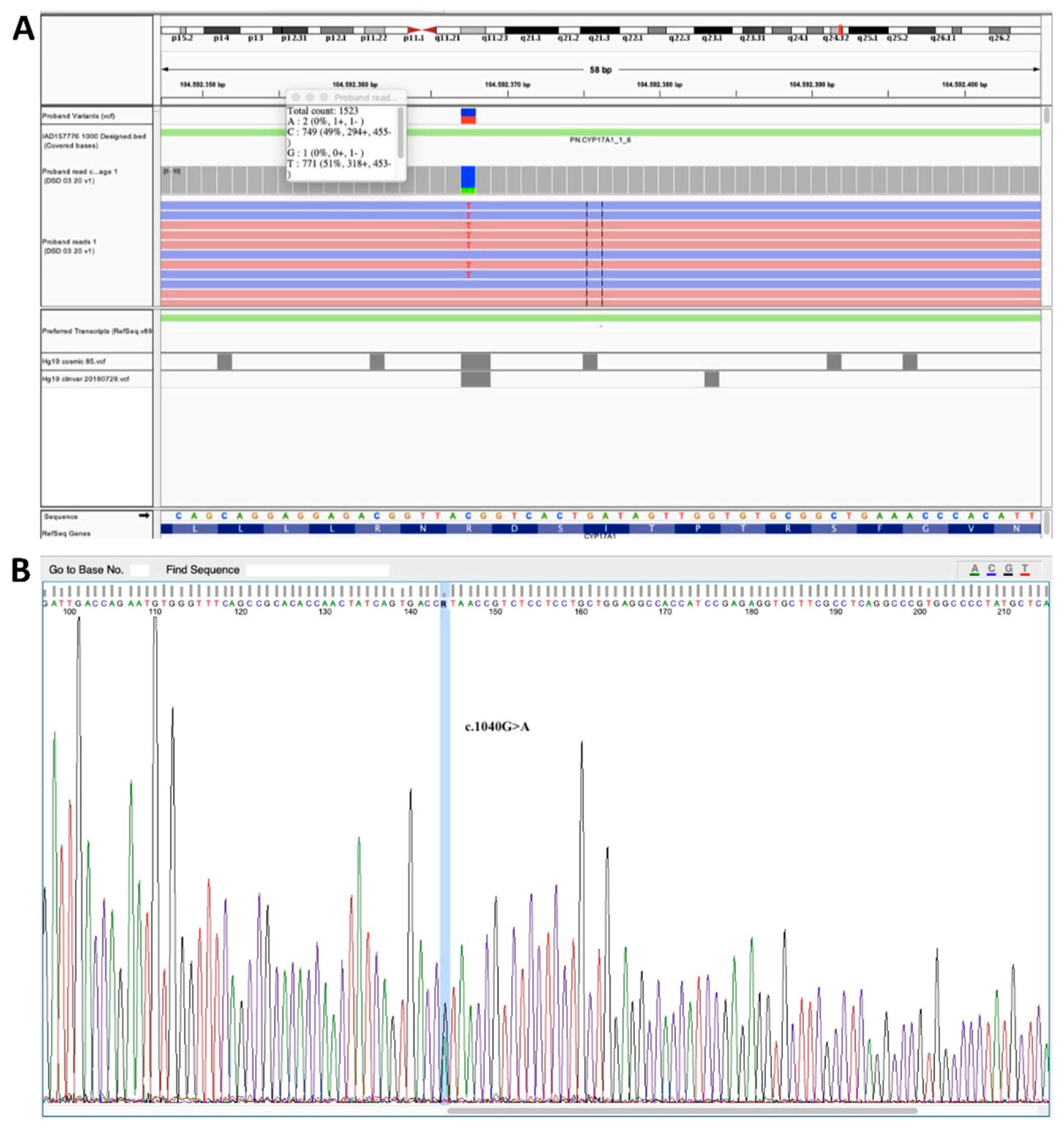
2.3. Genetic Testing
2.4. Serum Steroid Profiling by Liquid Chromatography Coupled to Tandem Mass Spectrometry
2.5. Stimulation Tests
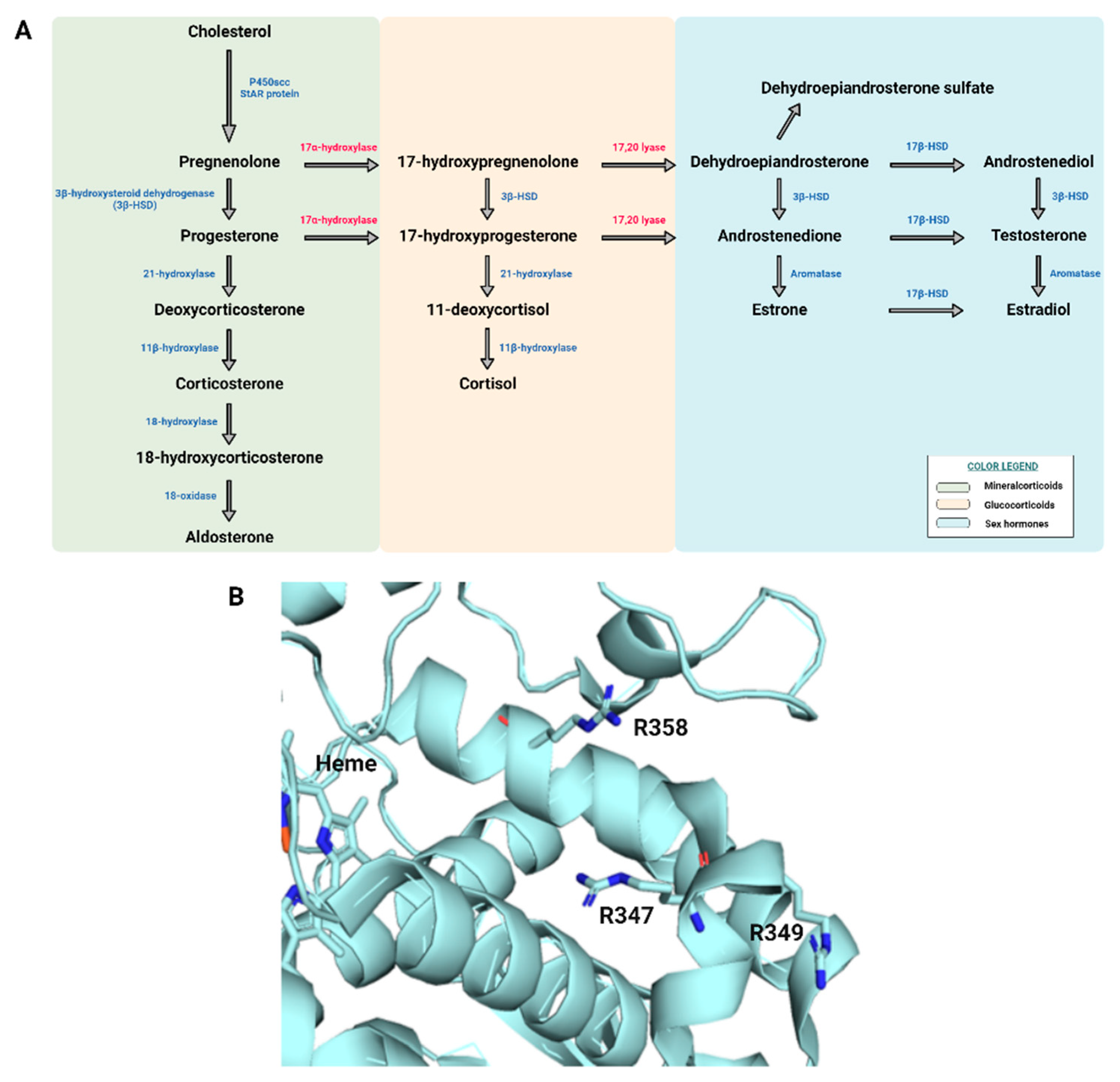
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, P.A.; Houk, C.P.; Ahmed, S.F.; Hughes, I.A.; International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics 2006, 118, e488–e500. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; De Ferran, K.; Bhangoo, A.; Ten, S.; Lahlou, N.; Audran, F.; Servant, N.; Poulat, F.; Philibert, P.; Sultan, C. Isolated ‘idiopathic’ micropenis: Hidden genetic defects? Int. J. Androl. 2011, 34, e518–e525. [Google Scholar] [CrossRef]
- Hiort, O.; Birnbaum, W.; Marshall, L.; Wünsch, L.; Werner, R.; Schröder, T.; Döhnert, U.; Holterhus, P.M. Management of disorders of sex development. Nat. Rev. Endocrinol. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Lai, F.; Srinivasan, S.; Wiley, V. Evaluation of a two-tier screening pathway for congenital adrenal hyperplasia in the New South Wales Newborn screening programme. Int. J. Neonatal. Screen 2020, 6, 63. [Google Scholar] [CrossRef]
- Lacey, J.M.; Minutti, C.Z.; Magera, M.J.; Tauscher, A.L.; Casetta, B.; McCann, M.; Lymp, J.; Hahn, S.H.; Rinaldo, P.; Matern, D. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin. Chem. 2004, 50, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Calton, L.; Brown, H.A.; Gillingwater, S.; Wallace, A.M.; Petrucci, F.; Ciavardelli, D.; Urbani, A.; Sacchetta, P.; Morris, M. Confirmation of congenital adrenal hyperplasia by adrenal steroid profiling of filter paper dried blood samples using ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2011, 49, 677–684. [Google Scholar] [CrossRef]
- Rossi, C.; Calton, L.; Hammond, G.; Brown, H.A.; Wallace, A.M.; Sacchetta, P.; Morris, M. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta 2010, 411, 222–228. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904, Erratum in Pediatrics 2018, 142, e20181739. [Google Scholar] [CrossRef]
- Flynn, J.T.; Daniels, S.R.; Hayman, L.L.; Maahs, D.M.; McCrindle, B.W.; Mitsnefes, M.; Zachariah, J.P.; Urbina, E.M.; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension 2014, 63, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Cicalini, I.; Tumini, S.; Guidone, P.I.; Pieragostino, D.; Zucchelli, M.; Franchi, S.; Lisi, G.; Lelli Chiesa, P.; Stuppia, L.; Laurenzi, V.; et al. Serum steroid Profiling by liquid chromatography-tandem mass spectrometry for the rapid confirmation and early treatment of congenital adrenal hyperplasia: A neonatal case report. Metabolites 2019, 9, 284. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Zucchelli, M.; di Ioia, M.; Onofrj, M.; Federici, L.; Del Boccio, P.; Pieragostino, D. Metabolomic signature in sera of multiple sclerosis patients during pregnancy. Int. J. Mol. Sci. 2018, 19, 3589. [Google Scholar] [CrossRef]
- Pieragostino, D.; Agnifili, L.; Cicalini, I.; Calienno, R.; Zucchelli, M.; Mastropasqua, L.; Sacchetta, P.; Del Boccio, P.; Rossi, C. Tear film steroid profiling in dry eye disease by liquid chromatography tandem mass spectrometry. Int. J. Mol. Sci. 2017, 18, 1349. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J. Steroid Biochem. Mol. Biol. 2017, 165, 71–78. [Google Scholar] [CrossRef]
- Biason-Lauber, A.; Boscaro, M.; Mantero, F.; Balercia, G. Defects of steroidogenesis. J. Endocrinol. Investig. 2010, 33, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. The syndrome of 17,20 lyase deficiency. J. Clin. Endocrinol. Metab. 2012, 97, 59–67. [Google Scholar] [CrossRef]
- Xu, S.; Hu, S.; Yu, X.; Zhang, M.; Yang, Y. 17α hydroxylase/17,20 lyase deficiency in congenital adrenal hyperplasia: A case report. Mol. Med. Rep. 2017, 15, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, A.; Baronio, F.; Ortolano, R.; Menabo, S.; Baldazzi, L.; Di Natale, V.; Vissani, S.; Cassio, A. Congenital adrenal hyperplasias presenting in the newborn and young infant. Front. Pediatr. 2020, 8, 593315. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.H.; Auchus, R.J.; Mendonça, B.B.; Miller, W.L. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat. Genet. 1997, 17, 201–205. [Google Scholar] [CrossRef]
- Van Den Akker, E.L.; Koper, J.W.; Boehmer, A.L.; Themmen, A.P.; Verhoef-Post, M.; Timmerman, M.A.; Otten, B.J.; Drop, S.L.; De Jong, F.H. Differential inhibition of 17alpha-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J. Clin. Endocrinol. Metab. 2002, 87, 5714–5721. [Google Scholar] [CrossRef]
- Sato, T.; Takezaki, S.; Goto, T.; Ishikawa, S.; Oura, K.; Takahata, A.; Shiraishi, H.; Maruo, Y.; Sato, N.; Suganuma, T.; et al. Atypical familial mediterranean fever in a Japanese boy with heterozygous MEFV p.Ser503Cys Exon 5 Variant. Case Rep. Pediatrics 2021, 2021, 6650226. [Google Scholar] [CrossRef] [PubMed]
- Zerkaoui, M.; Laarabi, F.Z.; Ajhoun, Y.; Chkirate, B.; Sefiani, A. A novel single variant in the MEFV gene causing Mediterranean fever and Behçet’s disease: A case report. J. Med. Case Rep. 2018, 12, 53. [Google Scholar] [CrossRef][Green Version]
- Wu, D.; Shen, M.; Zeng, X. Familial Mediterranean fever in Chinese adult patients. Rheumatology 2018, 57, 2140–2144. [Google Scholar] [CrossRef]
- Lee-Robichaud, P.; Akhtar, M.E.; Akhtar, M. Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b5. Biochem. J. 1998, 332, 293–296. [Google Scholar] [CrossRef]
- Biason-Lauber, A.; Leiberman, E.; Zachmann, M. A single amino acid substitution in the putative redox partner-binding site of P450c17 as cause of isolated 17,20-lyase deficiency. J. Clin. Endocrinol. Metab. 1997, 82, 3807–3812. [Google Scholar] [CrossRef][Green Version]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Auyeung, B.; Ahluwalia, J.; Thomson, L.; Taylor, K.; Hackett, G.; O’donnell, K.J.; Baron-Cohen, S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol. Autism. 2012, 3, 17. [Google Scholar] [CrossRef]
- Janšáková, K.; Hill, M.; Čelárová, D.; Celušáková, H.; Repiská, G.; Bičíková, M.; Máčová, L.; Ostatníková, D. Alteration of the steroidogenesis in boys with autism spectrum disorders. Transl. Psychiatry 2020, 10, 340. [Google Scholar] [CrossRef]
| Gene | OMIM | Refseq | Gene | OMIM | Refseq | ||
|---|---|---|---|---|---|---|---|
| 1 | AKR1C2 | 600450 | NM_001354.5 | 26 | HSD17B4 | 601860 | NM_000414.3 |
| 2 | AKR1C4 | 600451 | NM_001818.3 | 27 | HSD3B2 | 613890 | NM_000198.3 |
| 3 | ANOS1 | 300836 | NM_000216.3 | 28 | INSL3 | 146738 | NM_005543.4 |
| 4 | AR | 313700 | NM_000044.3 | 29 | LEP | 164160 | NM_000230.2 |
| 5 | ATRX | 300032 | NM_000489.3 | 30 | LHCGR | 152790 | NM_000233.3 |
| 6 | AMHR2 | 600956 | NM_020547.3 | 31 | MAMLD1 | 300120 | NM_005491.3 |
| 7 | BMP15 | 300247 | NM_005448.2 | 32 | MAP3K1 | 600982 | NM_005921.2 |
| 8 | CHD7 | 608892 | NM_017780.3 | 33 | NR0B1 | 300473 | NM_000475.4 |
| 9 | DMRT1 | 601898 | NM_004122.2 | 34 | NR3C1 | 138040 | NM_001018077.1 |
| 10 | CYB5A | 613218 | NM_001914.3 | 35 | NR5A1 | 184757 | NM_004959.4 |
| 11 | CYP11A1 | 118485 | NM_000781.3 | 36 | POR | 124015 | NM_000941.2 |
| 12 | CYP11B1 | 610613 | NM_000497.4 | 37 | PROK2 | 607002 | NM_00112128.1 |
| 13 | CYP17A1 | 609300 | NM_000102.4 | 38 | PROKR2 | 607623 | NM_144773.3 |
| 14 | CYP19A1 | 107910 | NM_000103.3 | 39 | RXFP2 | 606655 | NM_130806.3 |
| 15 | DHH | 605423 | NM_021044 | 40 | SOX9 | 608160 | NM_000346.3 |
| 16 | FGF8 | 600483 | NM_006119.4 | 41 | SRD5A2 | 607306 | NM_000348.3 |
| 17 | FGFR1 | 136350 | NM_023110.2 | 42 | WDR11 | 606417 | NM_018117.11 |
| 18 | FGFR2 | 176943 | NM_000141.4 | 43 | SRY | 480000 | NM_003140.3 |
| 19 | FSHB | 136530 | NM_000510.2 | 44 | WT1 | 607102 | NM_024426.4 |
| 20 | FSHR | 136435 | NM_000145.4 | 45 | STAR | 600612 | NM_000349.2 |
| 21 | GATA4 | 600576 | NM_002052.3 | 46 | TAC3 | 162330 | NM_001178054.1 |
| 22 | GNRH1 | 152760 | NM_001083111.1 | 47 | ZFPM2 | 603693 | NM_012082.3 |
| 23 | GNRHR | 138850 | NM_000406.2 | 48 | PROP1 | 601538 | NM_006261.4 |
| 24 | HESX1 | 601802 | NM_003865.2 | 49 | RSPO1 | 609595 | NM_001038633.3 |
| 25 | HSD17B3 | 605573 | NM_000197.1 | 50 | DMRT2 | 602424 | NM_021951.2 |
| Laboratory Test | Result | Reference Range |
|---|---|---|
| FSH (IU/L) | 0.9 | 0.95–11.95 |
| LH (IU/L) | 0.0 | 0.57–12.07 |
| ACTH (ng/mL) | 12.7–38.1 | 4.7–48.8 |
| 17 beta-Estradiol (pg/mL) | <10 | 11–44 |
| Cortisol (ng/mL) | 75.8 | 10–330 |
| Corticosterone (ng/mL) | 2.4 | 0.8–18.6 |
| 11-Deoxycortisol (ng/mL) | 0.4 | 0.1–1.56 |
| Androstenedione (ng/mL) | 0.1 | 0.06–2.6 |
| 17α-Hydroxy-Progesterone (ng/mL) | 0.4 | 0.03–2.65 |
| Testosterone (ng/mL) | / | 0.03–9.7 |
| Progesterone (ng/mL) | / | 0.07–12.94 |
| DHEA (ng/mL) | / | 0.09–19 |
| DHEA-S (ng/mL) | 400 | 50−4420 |
| Laboratory Test | T0 (before Stimulus) | T1 (4th Day after Stimulus) | Reference Range |
|---|---|---|---|
| Testosterone (ng/mL) | <0.2 | <0.2 | 2.12–7.42 |
| Dihydrotestosterone (pg/mL) | 45 | 62 | 250–990 |
| DHEA (ng/mL) | <0.3 | <0.3 | 0.3–1.9 |
| Laboratory Test | T0’ (before Stimulus) | T1’ (60 min after Stimulus) | Reference Range |
|---|---|---|---|
| Cortisol ng/mL | 43.85 | 207.85 | 10–330 |
| Corticosterone ng/mL | 1.20 | 39.15 | 0.8–18.6 |
| 11-Deoxycortisol ng/mL | 0.25 | 1.35 | 0.1–1.56 |
| Androstenedione ng/mL | 0.15 | 0.20 | 0.06–2.6 |
| 17α-Hydroxy-Progesterone ng/mL | 0.30 | 4.85 | 0.03–2.65 |
| Testosterone ng/mL | / | / | 0.03–9.7 |
| Progesterone ng/mL | 0.05 | 1.90 | 0.07–12.94 |
| DHEA ng/mL | / | 0.25 | 0.09–19 |
| DHEA-Sng/mL | 482.75 | 453.2 | 50–4420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltarelli, M.A.; Ferrante, R.; Marcello, F.D.; David, D.; Valentinuzzi, S.; Pilenzi, L.; Federici, L.; Rossi, C.; Stuppia, L.; Tumini, S. A Novel Heterozygous Mutation of the CYP17A1 Gene in a Child with a Micropenis and Isolated 17,20-Lyase Deficiency. Int. J. Environ. Res. Public Health 2022, 19, 6880. https://doi.org/10.3390/ijerph19116880
Saltarelli MA, Ferrante R, Marcello FD, David D, Valentinuzzi S, Pilenzi L, Federici L, Rossi C, Stuppia L, Tumini S. A Novel Heterozygous Mutation of the CYP17A1 Gene in a Child with a Micropenis and Isolated 17,20-Lyase Deficiency. International Journal of Environmental Research and Public Health. 2022; 19(11):6880. https://doi.org/10.3390/ijerph19116880
Chicago/Turabian StyleSaltarelli, Maria Alessandra, Rossella Ferrante, Francesca Di Marcello, Daniela David, Silvia Valentinuzzi, Lucrezia Pilenzi, Luca Federici, Claudia Rossi, Liborio Stuppia, and Stefano Tumini. 2022. "A Novel Heterozygous Mutation of the CYP17A1 Gene in a Child with a Micropenis and Isolated 17,20-Lyase Deficiency" International Journal of Environmental Research and Public Health 19, no. 11: 6880. https://doi.org/10.3390/ijerph19116880
APA StyleSaltarelli, M. A., Ferrante, R., Marcello, F. D., David, D., Valentinuzzi, S., Pilenzi, L., Federici, L., Rossi, C., Stuppia, L., & Tumini, S. (2022). A Novel Heterozygous Mutation of the CYP17A1 Gene in a Child with a Micropenis and Isolated 17,20-Lyase Deficiency. International Journal of Environmental Research and Public Health, 19(11), 6880. https://doi.org/10.3390/ijerph19116880







