Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship
Abstract
:1. Introduction
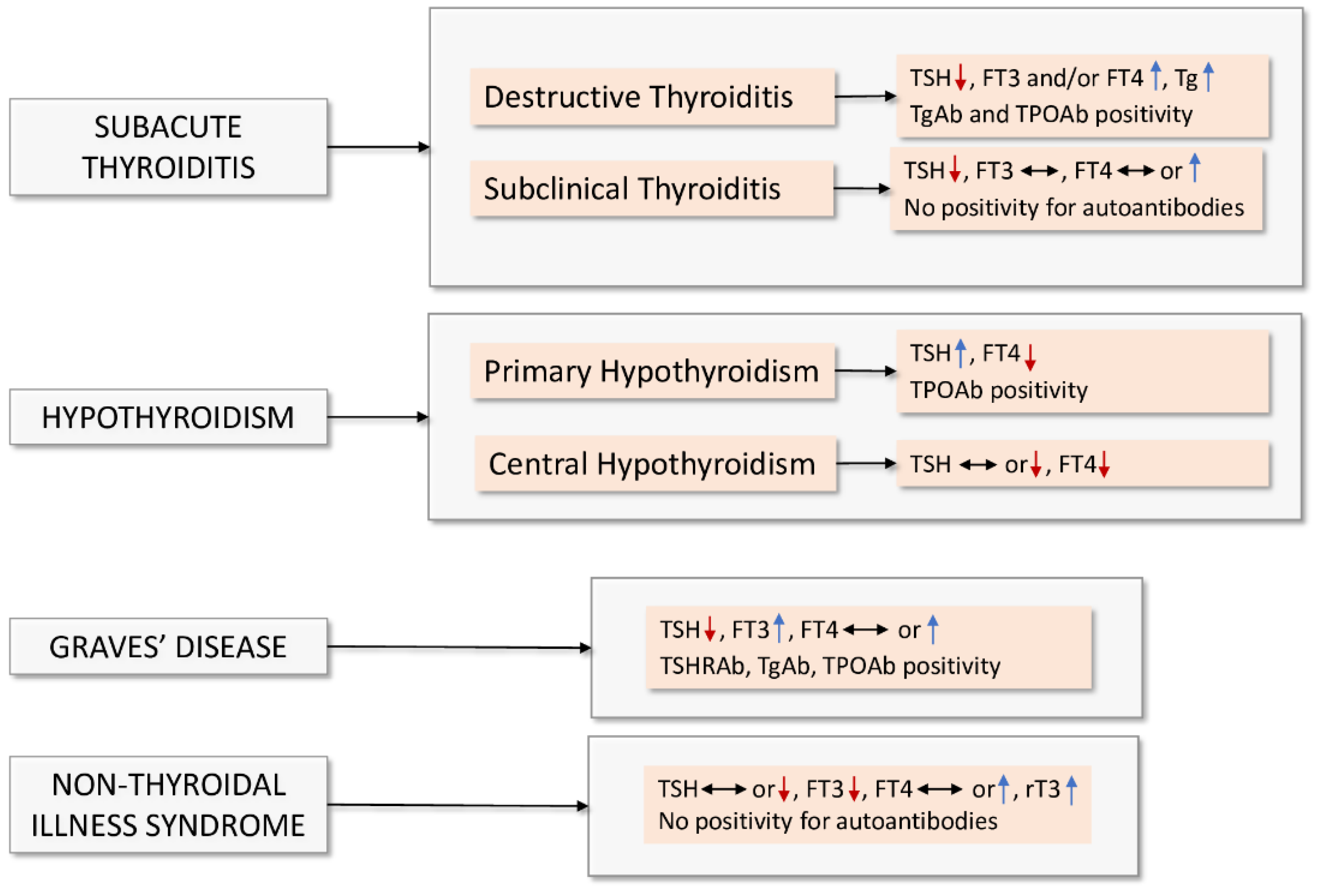
2. Bidirectional Interaction between Thyroid Dysfunction and COVID-19
3. Role of Selenium in COVID-19: Clinical Significance and Implications for Therapy
The Relationship between Selenium Status and COVID-19 Vaccination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| CRP | C-reactive protein |
| DIO | Deiodinase |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GD | Graves’ disease |
| GPx | Glutathione peroxidase |
| ICU | Intensive care unit |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| NF-kB | Nuclear factor-kB |
| NTIS | Non-thyroidal illness syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SAT | Subacute thyroiditis |
| Se | Selenium |
| Sec | Selenocysteine |
| SELENOP | Selenoprotein P |
| SeNPs | Selenium nanoparticles |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TNF-α | Tumor necrosis factor-alpha |
| TrxR | Thioredoxin reductases |
| TSH | Thyroid-stimulating hormone |
| Zn | Zinc |
References
- World Health Organization. Coronavirus Disease (COVID-19). Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 13 February 2022).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, G. H2S as a potential defense against COVID-19? Am. J. Physiol. Cell Physiol. 2020, 319, C244–C249. [Google Scholar] [CrossRef] [PubMed]
- Louis, T.J.; Qasem, A.; Abdelli, L.S.; Naser, S.A. Extra-Pulmonary Complications in SARS-CoV-2 Infection: A Comprehensive Multi Organ-System Review. Microorganisms 2022, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tian, Y.; Li, Z.; Zhu, J.; Wei, T.; Lei, J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology 2021, 162, bqab004. [Google Scholar] [CrossRef]
- Duntas, L.H.; Jonklaas, J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J. Endocr. Soc. 2021, 5, bvab076. [Google Scholar] [CrossRef]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; Report of a Joint FAO/WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2004; 341p. [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, A.R.; Lu, Q.B.; Zhang, X.A.; Zhang, Z.J.; Guan, X.G.; Che, T.L.; Yang, Y.; Li, H.; Liu, W.; et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021, 21, 452. [Google Scholar] [CrossRef]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Helmer, P.; Sudowe, S.; Sun, Q.; Hackler, J.; Roeder, D.; Lotz, C.; Meybohm, P.; et al. Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS. Nutrients 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Nardi, S.; Santoro, V.; Schiavon, M. The Relevance of Plant-Derived Se Compounds to Human Health in the SARS-CoV-2 (COVID-19) Pandemic Era. Antioxidants 2021, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Viana, E.T.; Gonçalves, T.F.; Mateus-Silva, J.R.; Vieira, R.P. Therapeutic use of intravenous selenium in respiratory and immunological diseases: Evidence based on reviews focused on clinical trials. Adv. Respir. Med. 2022, 90, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Liu, X.; Yang, T.; Cui, K.; Kong, L.; Yang, C.; Zhang, Z. Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design. Acta Pharm. Sin. B 2021, 11, 3060–3091. [Google Scholar] [CrossRef]
- Rehman, A.; John, P.; Bhatti, A. Biogenic Selenium Nanoparticles: Potential Solution to Oxidative Stress Mediated Inflammation in Rheumatoid Arthritis and Associated Complications. Nanomaterials 2021, 11, 2005. [Google Scholar] [CrossRef]
- Soltani, L.; Darbemamieh, M. Anti-proliferative, apoptotic potential of synthesized selenium nanoparticles against breast cancer cell line (MCF7). Nucleosides Nucleotides Nucleic Acids 2021, 40, 926–941. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.F.; Pi, J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef]
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B.; Yu, Y.L.; Chen, C.; Li, Y.F. Using nano-selenium to combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Jirillo, E.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Triggiani, V. Thyroid and COVID-19: A review on pathophysiological, clinical and organizational aspects. J. Endocrinol. Investig. 2021, 44, 1801–1814. [Google Scholar] [CrossRef]
- Desailloud, R.; Hober, D. Viruses and thyroiditis: An update. Virol. J. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K.; Kwek, D.S.; Ng, A.W.; Ong, K.C.; Kaw, G.J.; Lee, L.S. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin. Endocrinol. 2005, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, S.; Xu, C.H.; Zhang, J.; Xu, Y.; Zhu, H.; Peh, S.C.; Korteweg, C.; McNutt, M.A.; Gu, J. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, S.; Zhang, J.; Zhu, H.; Xu, Y.; Ma, Q.; McNutt, M.A.; Korteweg, C.; Gu, J. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem. Cell Biol. 2010, 88, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Asfuroglu Kalkan, E.; Ates, I. A case of subacute thyroiditis associated with Covid-19 infection. J. Endocrinol. Investig. 2020, 43, 1173–1174. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After Sars-COV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 105, dgaa276. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Cappellani, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Is Subacute Thyroiditis an Underestimated Manifestation of SARS-CoV-2 Infection? Insights From a Case Series. J. Clin. Endocrinol. Metab. 2020, 105, dgaa537. [Google Scholar] [CrossRef]
- Campos-Barrera, E.; Alvarez-Cisneros, T.; Davalos-Fuentes, M. Subacute Thyroiditis Associated with COVID-19. Case Rep. Endocrinol. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Chakraborty, U.; Ghosh, S.; Chandra, A.; Ray, A.K. Subacute thyroiditis as a presenting manifestation of COVID-19: A report of an exceedingly rare clinical entity. BMJ Case Rep. 2020, 13, e239953. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, S.; Dentali, F.; Tanda, M.L. SARS-CoV-2: A potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Investig. 2020, 43, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Campennì, A.; Siracusa, M.; Frazzetto, G.; Gullo, D. Subacute thyroiditis in a patient infected with SARS-COV-2: An endocrine complication linked to the COVID-19 pandemic. Hormones 2021, 20, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Semikov, V.I.; Aghayan, D.L.; Shulutko, A.M.; Khorobrykh, T.V.; Aleksandrov, Y.K.; Mansurova, G.T.; Kazaryan, A.M. Subacute thyroiditis after SARS-CoV-2 infection. Clin. Case Rep. 2021, 9, e05109. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Charlap, E.; Kim, A. Subacute Thyroiditis from COVID-19 Infection: A Case Report and Review of Literature. Eur. Thyroid J. 2021, 9, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Sohrabpour, S.; Heidari, F.; Karimi, E.; Ansari, R.; Tajdini, A.; Heidari, F. Subacute Thyroiditis in COVID-19 Patients. Eur. Thyroid J. 2021, 9, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Mattar, S.A.M.; Koh, S.J.Q.; Rama Chandran, S.; Cherng, B.P.Z. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020, 13, e237336. [Google Scholar] [CrossRef] [PubMed]
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in patients with COVID-19: The THYRCOV study. Eur. J. Endocrinol. 2020, 183, 381–387. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Lu, F.; Zhao, L.; Shi, L.; Xu, F. Low T3 syndrome is a strong predictor of poor outcomes in patients with community-acquired pneumonia. Sci. Rep. 2016, 6, 22271. [Google Scholar] [CrossRef]
- Su, W.; Zhao, X.Q.; Wang, M.; Chen, H.; Li, H.W. Low T3 syndrome improves risk prediction of in-hospital cardiovascular death in patients with acute myocardial infarction. J. Cardiol. 2018, 72, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Maiden, M.J.; Torpy, D.J. Thyroid Hormones in Critical Illness. Crit. Care Clin. 2019, 35, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Yan, P.; Huang, Q.; Shuai, T.; Liu, J.; Zhu, L.; Lu, J.; Shi, X.; Yang, K.; Liu, J. A prognostic role for non-thyroidal illness syndrome in chronic renal failure:a systematic review and meta-analysis. Int. J. Surg. 2019, 70, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, S.; Fan, X.; Jiang, Y.; Wu, L.; Li, F.; Wang, J.; Chen, S. Low-free triiodothyronine is associated with poor prognosis of portal hypertension in cirrhosis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.; Cannavaro, D.; Dazzi, D.; Covelli, D.; Mantovani, G.; Muscatello, A.; Ferrante, E.; Orsi, E.; Resi, V.; Longari, V.; et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020, 8, 739–741. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef]
- Vassiliadi, D.A.; Ilias, I.; Pratikaki, M.; Jahaj, E.; Vassiliou, A.G.; Detsika, M.; Ampelakiotou, K.; Koulenti, M.; Manolopoulos, K.N.; Tsipilis, S.; et al. Thyroid hormone alterations in critically and non-critically ill patients with SARS-CoV-2 infection. Endocr. Connect. 2021, 10, 646–655. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Xu, W. Thyroid Function Analysis in 50 Patients with COVID-19: A Retrospective Study. Thyroid 2021, 31, 8–11. [Google Scholar] [CrossRef]
- Gao, W.; Guo, W.; Guo, Y.; Shi, M.; Dong, G.; Wang, G.; Ge, Q.; Zhu, J.; Zhou, X. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J. Endocrinol. Investig. 2021, 44, 1031–1040. [Google Scholar] [CrossRef]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C.; et al. Thyroid Function Before, During, and After COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e803–e811. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1429–1430. [Google Scholar] [CrossRef]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.D.M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S.T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2021, 44, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Lazartigues, E.; Qadir, M.M.F.; Mauvais-Jarvis, F. Endocrine Significance of SARS-CoV-2’s Reliance on ACE2. Endocrinology 2020, 161, bqaa108. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020, 177, 104759. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Lin, H.Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Bretz, J.D.; Phelps, E.; Mezosi, E.; Arscott, P.L.; Utsugi, S.; Baker, J.R., Jr. A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J. Immunol. 2002, 168, 2470–2474. [Google Scholar] [CrossRef] [Green Version]
- Boelen, A.; Kwakkel, J.; Platvoet-ter Schiphorst, M.; Mentrup, B.; Baur, A.; Koehrle, J.; Wiersinga, W.M. Interleukin-18, a proinflammatory cytokine, contributes to the pathogenesis of non-thyroidal illness mainly via the central part of the hypothalamus-pituitary-thyroid axis. Eur. J. Endocrinol. 2004, 151, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, B.B.; Bhattacharya, P.; Gopisetty, A.; Prabhakar, B.S. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J. Interf. Cytokine Res. 2011, 31, 721–731. [Google Scholar] [CrossRef]
- Fliers, E.; Bianco, A.C.; Langouche, L.; Boelen, A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015, 3, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Campi, I.; Bulgarelli, I.; Dubini, A.; Perego, G.B.; Tortorici, E.; Torlasco, C.; Torresani, E.; Rocco, L.; Persani, L.; Fugazzola, L. The spectrum of thyroid function tests during hospitalization for SARS COV-2 infection. Eur. J. Endocrinol. 2021, 184, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Clausen, C.L.; Rasmussen, Å.K.; Johannsen, T.H.; Hilsted, L.M.; Skakkebæk, N.E.; Szecsi, P.B.; Pedersen, L.; Benfield, T.; Juul, A. Thyroid function in COVID-19 and the association with cytokine levels and mortality. Endocr. Connect. 2021, 10, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Salat, M.; Urgell, E.; Chico, A. SARS-COV-2 as a trigger for autoimmune disease: Report of two cases of Graves’ disease after COVID-19. J. Endocrinol. Investig. 2020, 43, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Blanco, S.; Pla-Peris, B.; Marazuela, M. COVID-19: A cause of recurrent Graves’ hyperthyroidism? J. Endocrinol. Investig. 2021, 44, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: Diagnostic value in organ injuries. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Camponovo, C.; Scappaticcio, L.; Bellastella, G.; Piccardo, A.; Rotondi, M. Thyroid sequelae of COVID-19: A systematic review of reviews. Rev. Endocr. Metab. Disord. 2021, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Tee, L.Y.; Harjanto, S.; Rosario, B.H. COVID-19 complicated by Hashimoto’s thyroiditis. Singap. Med. J. 2021, 62, 265. [Google Scholar] [CrossRef]
- van Gerwen, M.; Alsen, M.; Little, C.; Barlow, J.; Naymagon, L.; Tremblay, D.; Sinclair, C.F.; Genden, E. Outcomes of Patients With Hypothyroidism and COVID-19: A Retrospective Cohort Study. Front. Endocrinol. 2020, 11, 565. [Google Scholar] [CrossRef]
- Aemaz, U.; Rehman, M.; Farooq, H.; Ali, M.M.; Ebaad Ur Rehman, M.; Dar, Q.A.; Hussain, A. The Association of Subacute Thyroiditis with COVID-19: A Systematic Review. SN Compr. Clin. Med. 2021, 29, 1–13. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2021, 22, 803–815. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Olchovsky, D.; Tsvetov, G.; Shimon, I. Subacute thyroiditis: Clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J. Endocrinol. Investig. 2007, 30, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Andersen, S.L.; Boelaert, K.; Laurberg, P. Management of endocrine disease: Subclinical thyrotoxicosis: Prevalence, causes and choice of therapy. Eur. J. Endocrinol. 2017, 176, R325–R337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, E.N.; Bogazzi, F.; Martino, E.; Brogioni, S.; Pardini, E.; Pellegrini, G.; Parkes, A.B.; Lazarus, J.H.; Pinchera, A.; Braverman, L.E. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid 2003, 13, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Speer, G.; Somogyi, P. Thyroid complications of SARS and coronavirus disease 2019 (COVID-19). Endocr. J. 2021, 68, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G. Non-thyroidal illness in the ICU: A syndrome with different faces. Thyroid 2014, 24, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Mebis, L.; Debaveye, Y.; Visser, T.J.; Van den Berghe, G. Changes within the thyroid axis during the course of critical illness. Endocrinol. Metab. Clin. N. Am. 2006, 35, 807–821. [Google Scholar] [CrossRef]
- Croce, L.; Gangemi, D.; Ancona, G.; Liboà, F.; Bendotti, G.; Minelli, L.; Chiovato, L. The cytokine storm and thyroid hormone changes in COVID-19. J. Endocrinol. Investig. 2021, 44, 891–904. [Google Scholar] [CrossRef]
- McKechnie, J.L.; Blish, C.A. The Innate Immune System: Fighting on the Front Lines or Fanning the Flames of COVID-19? Cell Host Microbe 2020, 27, 863–869. [Google Scholar] [CrossRef]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, 411–417. (In Chinese) [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Tomer, Y.; Huber, A. The etiology of autoimmune thyroid disease: A story of genes and environment. J. Autoimmun. 2009, 32, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, J.; O’Callaghan, K.; Sinclair, H.; Hawke, K.; Love, A.; Hajkowicz, K.; Stewart, A.G. Risk factors, Treatment and Outcomes of Subacute Thyroiditis Secondary to COVID-19: A Systematic Review. Intern. Med. J. 2021, 52, 522–529. [Google Scholar] [CrossRef]
- Mendel, C.M.; Frost, P.H.; Kunitake, S.T.; Cavalieri, R.R. Mechanism of the heparin-induced increase in the concentration of free thyroxine in plasma. J. Clin. Endocrinol. Metab. 1987, 65, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, S.C.; Valyasevi, R.W.; Harteneck, D.A.; Dutton, C.M.; Bahn, R.S. Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves’ ophthalmopathy. Thyroid 2001, 11, 929–934. [Google Scholar] [CrossRef]
- Caron, P. Thyroid disorders and SARS-CoV-2 infection: From pathophysiological mechanism to patient management. Ann. Endocrinol. 2020, 81, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Selenoprotein metabolism and function: Evidence for more than one function for selenoprotein P. J. Nutr. 2003, 133, 1517S–1520S. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.M.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Wingler, K.; Böcher, M.; Flohé, L.; Kollmus, H.; Brigelius-Flohé, R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur. J. Biochem. 1999, 259, 149–157. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.W.; Radding, W. Understanding Selenium and Glutathione as Antiviral Factors in COVID-19: Does the Viral Mpro Protease Target Host Selenoproteins and Glutathione Synthesis? Front. Nutr. 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Baum, M.K.; Shor-Posner, G.; Lai, S.; Zhang, G.; Lai, H.; Fletcher, M.A.; Sauberlich, H.; Page, J.B. High risk of HIV-related mortality is associated with selenium deficiency. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 15, 370–374. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Maranon, D.G.; Anderson, J.R.; Maranon, A.G.; Wilusz, J. The interface between coronaviruses and host cell RNA biology: Novel potential insights for future therapeutic intervention. Wiley Interdiscip. Rev. RNA 2020, 11, e1614. [Google Scholar] [CrossRef]
- Lammi, M.J.; Qu, C. Selenium-Related Transcriptional Regulation of Gene Expression. Int. J. Mol. Sci. 2018, 19, 2665. [Google Scholar] [CrossRef] [Green Version]
- Popp, M.W.; Cho, H.; Maquat, L.E. Viral subversion of nonsense-mediated mRNA decay. RNA 2020, 26, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Fakhrolmobasheri, M.; Mazaheri-Tehrani, S.; Kieliszek, M.; Zeinalian, M.; Abbasi, M.; Karimi, F.; Mozafari, A.M. COVID-19 and Selenium Deficiency: A Systematic Review. Biol. Trace Elem. Res. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Meharg, A.A.; Li, G.; Chen, Z.; Yang, L.; Chen, S.C.; Zhu, Y.G. Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci. Rep. 2016, 6, 20953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Youn, H.S.; Lim, H.J.; Choi, Y.J.; Lee, J.Y.; Lee, M.Y.; Ryu, J.H. Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int. Immunopharmacol. 2008, 8, 495–501. [Google Scholar] [CrossRef]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA Virus Interactions: Potential Implications for SARS-CoV-2 Infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Khandelwal, N.; Simpson, J.; Taylor, G.; Rafique, S.; Whitehouse, A.; Hiscox, J.; Stark, L.A. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011, 18, 1889–1903. [Google Scholar] [CrossRef] [Green Version]
- Hiscott, J.; Nguyen, T.L.; Arguello, M.; Nakhaei, P.; Paz, S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 2006, 25, 6844–6867. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Xin, Z.T.; Liang, Y.; Ly, H.; Liang, Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 2008, 82, 9880–9889. [Google Scholar] [CrossRef] [Green Version]
- Liao, Q.J.; Ye, L.B.; Timani, K.A.; Zeng, Y.C.; She, Y.L.; Ye, L.; Wu, Z.H. Activation of NF-κB by the Full-length Nucleocapsid Protein of the SARS Coronavirus. Acta Biochim. Biophys. Sin. 2005, 37, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Ho, C.T.; Hsu, H.S.; Lin, C.H.; Li, C.I.; Li, T.C.; Liu, C.S.; Lin, C.C.; Lin, W.Y. Selenium is inversely associated with interleukin-6 in the elderly. J. Nutr. Health Aging 2013, 17, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhao, J.; Yao, H.; Zhao, X.; Fan, R.; Zhao, W.; Zhang, Z.; Xu, S. Selenium Deficiency Influences the mRNA Expression of Selenoproteins and Cytokines in Chicken Erythrocytes. Biol. Trace Elem. Res. 2016, 171, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Khoso, P.A.; Zhang, Y.; Yin, H.; Teng, X.; Li, S. Selenium Deficiency Affects Immune Function by Influencing Selenoprotein and Cytokine Expression in Chicken Spleen. Biol. Trace Elem. Res. 2019, 187, 506–516. [Google Scholar] [CrossRef]
- Manzanares, W.; Biestro, A.; Torre, M.H.; Galusso, F.; Facchin, G.; Hardy, G. High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Med. 2011, 37, 1120–1127. [Google Scholar] [CrossRef]
- Chelkeba, L.; Ahmadi, A.; Abdollahi, M.; Najafi, A.; Ghadimi, M.H.; Mosaed, R.; Mojtahedzadeh, M. The effect of parenteral selenium on outcomes of mechanically ventilated patients following sepsis: A prospective randomized clinical trial. Ann. Intensive Care 2015, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Khatiwada, S.; Subedi, A. A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19). Curr. Nutr. Rep. 2021, 10, 125–136. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Younesian, O.; Khodabakhshi, B.; Abdolahi, N.; Norouzi, A.; Behnampour, N.; Hosseinzadeh, S.; Alarzi, S.S.H.; Joshaghani, H. Decreased Serum Selenium Levels of COVID-19 Patients in Comparison with Healthy Individuals. Biol. Trace Elem. Res. 2022, 200, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.; Petrovic, M.; Lachat, C.; De Boevre, M.; Klingenberg, G.J.; Sun, Q.; De Saeger, S.; De Clercq, J.; Ide, L.; Vandekerckhove, L.; et al. Course and Survival of COVID-19 Patients with Comorbidities in Relation to the Trace Element Status at Hospital Admission. Nutrients 2021, 13, 3304. [Google Scholar] [CrossRef] [PubMed]
- Servidio, C.; Stellacci, F. Therapeutic approaches against coronaviruses acute respiratory syndrome. Pharmacol. Res. Perspect. 2021, 9, e00691. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef]
- Węglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Brul, S. Ebselen as a Highly Active Inhibitor of PLproCoV2. bioRxiv 2020. bioRxiv:2020.05.17.100768. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of Enterovirus 71 by Selenium Nanoparticles Loaded with siRNA through Bax Signaling Pathways. ACS Omega 2020, 5, 12495–12500. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mavandadnejad, F.; Yazdi, M.H.; Faghfuri, E.; Hashemi, H.; Homayouni-Oreh, S.; Farhoudi, R.; Shahverdi, A.R. Oral administration of synthetic selenium nanoparticles induced robust Th1 cytokine pattern after HBs antigen vaccination in mouse model. J. Infect. Public Health 2017, 10, 102–109. [Google Scholar] [CrossRef] [Green Version]
- El-Ramady, H.; Abdalla, N.; Elbasiouny, H.; Elbehiry, F.; Elsakhawy, T.; Omara, A.E.; Amer, M.; Bayoumi, Y.; Shalaby, T.A.; Eid, Y.; et al. Nano-biofortification of different crops to immune against COVID-19: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112500. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Elsisi, H.; Ramadan, S.M.; Sorour, H.; Magdi, M.; Abdeldayem, S.A. Novel Antiviral and Antibacterial Durable Polyester Fabrics Printed with Selenium Nanoparticles (SeNPs). Polymers 2022, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenmakers, E.; Agostini, M.; Mitchell, C.; Schoenmakers, N.; Papp, L.; Rajanayagam, O.; Padidela, R.; Ceron-Gutierrez, L.; Doffinger, R.; Prevosto, C.; et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Investig. 2010, 120, 4220–4235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chen, Z.; Zhang, H.; Chen, C.; Zeng, M.; Yunis, J.; Wei, Y.; Wan, Y.; Wang, N.; Zhou, M.; et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021, 22, 1127–1139. [Google Scholar] [CrossRef]
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C.N.; Goldson, A.J.; Dainty, J.R.; Nicoletti, C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2017, 36, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Yehia, N.; AbdelSabour, M.A.; Erfan, A.M.; Ali, Z.M.; Soliman, R.A.; Samy, A.; Mohamed Soliman, M.; Abd El-Hack, M.E.; El-Saadony, M.T.; Ahmed, K.A. Selenium nanoparticles enhance the efficacy of homologous vaccine against the highly pathogenic avian influenza H5N1 virus in chickens. Saudi J. Biol. Sci. 2022, 29, 2095–2111. [Google Scholar] [CrossRef]
- Demircan, K.; Chillon, T.S.; Sun, Q.; Heller, R.A.; Klingenberg, G.J.; Hirschbil-Bremer, I.M.; Seemann, P.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; et al. Humoral immune response to COVID-19 mRNA vaccination in relation to selenium status. Redox Biol. 2022, 50, 102242. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2022, 200, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol. Metab. 2021, 36, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J. Oxidative stress and cell death in cardiovascular disease: A post-genomic appraisal. In Post-Genomic Cardiology, 2nd ed.; Marín-García, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 471–498. [Google Scholar]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De la Vieja, A. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol. Endocrinol. 2011, 25, 1924–1935. [Google Scholar] [CrossRef] [Green Version]
- Balboni, E.; Zagnoli, F.; Filippini, T.; Fairweather-Tait, S.J.; Vinceti, M. Zinc and selenium supplementation in COVID-19 prevention and treatment: A systematic review of the experimental studies. J. Trace Elem. Med. Biol. 2022, 71, 126956. [Google Scholar] [CrossRef]

| Clues | Levels of Data | Main Features/Findings | Publication Type | Reference |
|---|---|---|---|---|
| Evidence for the presence of certain viruses or their components in SAT and autoimmune thyroid diseases | Epidemiological; serological, direct evidence | Retroviruses (HFV) and mumps: direct evidence in SAT. Retroviruses (HTLV-1, HFV, HIV, and SV40) direct evidence of GD. HTLV-1, enterovirus, rubella, mumps virus, HSV, EBV, and parvovirus: direct evidence in Hashimoto’s thyroiditis | Review | [24] |
| SARS-CoV detected in many endocrine organs, including the pituitary gland | Histological; serological | China; four patients died from SARS (three males aged 25, 38, and 57 years, and a 62-year-old female), four control patients (two males, 32 and 52 years old, and two females, 28 and 68 years) died of cardio-/cerebrovascular disease or ectopic pregnancy. | Case-control (postmortem) study | [25] |
| Histological; cellular; molecular | China: 18 patients died from suspected SARS, 22 died from confirmed SARS, 65 T lymphocyte counts in 65 confirmed and 35 misdiagnosed SARS cases | Retrospective (postmortem) study | [26] | |
| Possibility of transient subclinical thyrotoxicosis or reversible hypothyroidism within a hypothalamic-pituitary-adrenal axis dysfunction occurring associated with SARS-CoV | Molecular | Singapore; 61 survivors of SARS (aged 21 years and above, 39.9% with hypocortisolism after a 1-year follow-up) | Prospective cohort study | [27] |
| Significant damage to the follicular and parafollicular cells of the thyroid detected in SARS-CoV nonsurvivors | Histological | China; five thyroid samples from patients with SARS (four males, one female, 24–50 years old); 10 thyroid samples from controls of comparable age | Case-control study (postmortem) study | [28] |
| Significant reduction in the number of positive cells and the staining intensity of immunoreactivity for TSH in the adenohypophysis of patients with SARS-CoV compared with controls | Histological | China; five pituitary samples from patients with SARS (four males, one female, 24–51 years old); five pituitary samples from controls of comparable age | Case-control (postmortem) study | [29] |
| Both direct viral and postviral manifestations of COVID-19 associated with SARS-CoV related thyroiditis | Clinical; imaging; molecular | 41-year-old Caucasian woman (mild COVID-19) | Case study | [30] |
| Clinical; imaging; molecular | Italy; an 18-year-old woman (mild COVID-19). | Case study | [31] | |
| Clinical; imaging; molecular | Italy; four females (29–46 years), one patient hospitalized due to atrial fibrillation | Case series study | [32] | |
| Clinical; imaging; molecular | Mexico; a 37-year-old woman (mild COVID-19) | Case study | [33] | |
| Clinical; imaging; molecular | India; a 58-year-old man (mild COVID-19) | Case study | [34] | |
| Clinical; imaging; molecular | Italy; a 37-year-old woman hospitalized at the COVID-19 department | Case study | [35] | |
| Clinical; imaging; molecular | Italy; a 43-year-old woman (mild COVID-19) | Case study | [36] | |
| Clinical; imaging; molecular | Norway; a 45-year-old woman (mild COVID-19); a 45-year-old woman (mild COVID-19) | Case reports | [37] | |
| Clinical; imaging; serological; molecular | United States; a 41-year-old woman (mild COVID-19) | Case study | [38] | |
| Clinical; imaging; serological; molecular | Iran; six patients (four women, two men), 26–52 years old (IgG and IgM positive for COVID-19, history of family members’ hospitalization due to COVID-19 pneumonia in 3 out of 6 cases) | Case series | [39] | |
| Clinical; imaging; instrumental; molecular | Myanmar; a 34-year-old man (mild COVID-19) | Case study | [40] | |
| The prevalence of SAT in COVID-19 patients is higher than that in the general population | Epidemiological; molecular | Italy; 287 noncritically ill patients (193 men, median age 66 years) | Retrospective single-center study | [41] |
| NTIS is associated with critical illness and poor outcomes in patients with pneumonia, acute myocardial infarction, chronic renal failure, cirrhosis | Epidemiological; clinical; molecular | China; 503 hospitalized patients (mean age 63 years) with community-acquired pneumonia | Retrospective single-center study | [42] |
| Epidemiological; instrumental; molecular | China: 2459 patients with AMI diagnosis (529 patients with low T3 syndrome, 529 euthyroid patients, >18 years) | Prospective cohort study | [43] | |
| Epidemiological, molecular | NTIS is ubiquitous in critical illness, while T3 replacement in this condition remains controversial. | Review | [44] | |
| Epidemiological; molecular | 17 studies (14 cohort, three cross-sectional); 4593 patients with CRF, mean age 62 years | Systematic review and meta-analysis | [45] | |
| Epidemiological; molecular | China; 385 patients: with cirrhotic portal hypertension, mean age 56.5 years. | Prospective cohort study | [46] | |
| The inverse relationship between TSH and FT3 levels and clinical severity in COVID-19 patients | Epidemiological; clinical; molecular | Italy; 287 noncritically ill patients (193 men, median age 66 years | Retrospective single-center study | [41] |
| Epidemiological; clinical; molecular Clinical; imaging; molecular | Italy; 93 COVID-19 consecutive patients admitted to HICUs in 2020, 101 consecutive patients admitted to HICUs in 2019, and 52 COVID-19 patients admitted to LICU in 2020. | Prospective (COVID-19 patients); retrospective (controls) | [47] | |
| Epidemiological; clinical; molecular | Hong-Kong; 191 consecutive COVID-19 patients (84.3% mild, 12.6% moderate, 3.1% severe). | Prospective cohort study | [48] | |
| Epidemiological; clinical; molecular | Greece; 102 consecutive COVID-19 patients (41 admitted in the ICU, 46 admitted in the ward, 15 outpatients) | Prospective cohort study | [49] | |
| Epidemiological, molecular | China: 50 COVID-19 patients, 54 healthy patients/50 patients with pneumonia | Case-control study | [50] | |
| Epidemiological, clinical, molecular | China: 100 patients (66 critically ill) | Retrospective single-center study | [51] | |
| Epidemiological, clinical, molecular | United Kingdom; 456 patients (334 (73.2%) diagnosed with COVID-19, mean age 66.1 years) | Prospective cohort study | [52] | |
| Thyroid disease are associated with COVID-19 severity | Epidemiological, molecular | 6 studies (8 retrospective cohort, 2 case series); 2169 COVID-19 patients | Systematic review and meta-analysis | [53] |
| Thyroid hormones are involved in different aspects of innate and adaptive immune responses | Cellular | Genomic and nongenomic mechanisms by which T3 and T4 modulate the activity of macrophages and leukocytes; (innate immune response); natural kill cells (adaptative and innate immune response), and lymphocytes (adaptive immune response) | Review | [54] |
| Cellular | Cellular and molecular signaling pathways are involved in the cross talk between THs and innate immune functions (neutrophils, natural killer cells, monocytes–macrophages, and dendritic cells) | Review | [55] | |
| ACE2 and TMPRSS2 mRNA are highly expressed in the thyroid, suggesting a possible direct action of SARS-CoV-2 on the gland | Tissue; cellular | 15 thyroid samples were obtained from the disease-free tissue of patients who underwent thyroidectomy for a nodular goiter (12 women and three men); two primary cultures of normal thyrocytes | In vitro ex vivo study | [56] |
| Tissue | ACE2 and TMPRSS2 expression levels derived from the human protein atlas and genotype tissue expression | Review | [57] | |
| Binding of thyroid hormones to the membrane integrin receptor which could be implicated in the transmission and pathology of SARS-CoV-2 | Molecular; tridimensional models | Integrins as cell receptors of SARS-CoV-2 in one or more host species, through a conserved RGD (403–405: Arg-Gly-Asp) motif present in the receptor-binding domain of the spike proteins of all SARS-CoV-2 sequences | Review | [58] |
| Molecular | Nongenomic actions of T4 (tumor, endothelial cells) mediated by the binding of the extracellular domain of plasma membrane integrin ανβ3 | Review | [59] | |
| The state of immune activation accompanying inflammatory thyroid disease is comparable to the cytokine storm associated with COVID-19 | Histological; cellular; molecular | Eight-week-old female CBA/J mice (a strain susceptible to experimental autoimmune thyroiditis) were immunized with thyroglobulin and then injected with IFN-γ and TNF-α vs. control animals. | In vivo study | [60] |
| Molecular | IL-18−/−, IFN-γ−/−, and WT mice injected with bacterial lipopolysaccharide | In vivo study | [61] | |
| Cellular; molecular | Cytokines are implicated in the pathogenesis of autoimmune thyroid diseases, while cytokine modulation is a possible therapeutic target | Review | [62] | |
| Epidemiological, molecular | Cytokines activated during the inflammatory response are causally associated with the pathogenesis of NTIS, making NTIS part of the acute phase response | Review | [63] | |
| Inverse correlation between serum levels of TSH and inflammatory cytokines in patients with COVID-19, which may explain NTIS or overt thyrotoxicosis | Epidemiological; clinical; molecular | Italy; 287 noncritically ill patients (193 men, median age 66 years | Retrospective single-center study | [41] |
| Epidemiological; imaging; molecular | Italy; 144 consecutive patients (97 men, and 47 women, mean age 68.1 years) admitted to HICU or LICU | Prospective cohort study | [64] | |
| Epidemiological; clinical; molecular | Denmark; 116 consecutive patients hospitalized for moderate-to-severe COVID-19 disease | Retrospective single-center study | [65] | |
| Relapse of GD described in COVID-19 patients | Clinical; imaging; molecular | A 60-year-old woman with a previous diagnosis of GD at the age of 23 years; A 53-year-old woman (no previous known thyroid disease) | Case reports | [66] |
| Clinical; imaging; molecular | A 45-year-old woman with a 12-year medical history of GD; A 61-year-old woman with a history of atrial fibrillation and GD | Case reports | [67] |
| Pitfalls | Reference |
|---|---|
| No possibility to infer a causal relationship and to investigate the potential impact of thyroid dysfunction on COVID-19 outcomes due to: - retrospective design - cross-sectional design - single-center design - small sample size - association mainly relied on case reports | [41,50,51,65] [68] [41,51,52,64,65,68] [41,50,51,65,68] [30,31,32,33,34,35,36,37,38,39,40] |
| Differences: -in the studied populations (critically ill vs. noncritically ill subjects) - in the timing of thyroid function assessment with respect to the course of the disease | [41,47,48,49,50,51,52,65,68] [30,31,32,33,34,35,36,37,38,39,40,47,48,49,50,51,65,68] |
| Lack of measurement of: - thyroid hormones - thyroglobulin - autoantibodies | [47,52,65] [41,47,50,51,52,65] [47,50,51,52,65] |
| The potential confounding effect of medications used in COVID-19 (glucocorticoids, low-molecular-weight heparin) due to their impact on the HPT axis and free thyroid hormone assays | [41,50,51,69] |
| Difficult generalization of results due to lack of a control group of healthy individuals or an independent cohort of patients with non-COVID-19 pneumonia | [41,48,64,65] |
| SARS-CoV-2 was not directly detected in the thyroid tissue in all case studies of SAT | [30,31,32,33,34,35,36,37,38,39,40] |
| Differently from what happens in infection with SARS-CoV: no significant damage to thyroid cell morphology - rare development of hypothyroidism in COVID-19 patients | [41] [41,47,48,49,66,70] |
| The association between thyroid function and COVID-19 most studied in nonmild cases | [47,50,51,52,64,65,69] |
| Hypothyroidism is not associated with complications of COVID-19 | [71] |
| Possibility of publication bias in the relationship between COVID-19 and thyroid dysfunction | [72] |
| Family | Acronym | Enzymes | Main Functions | Reference |
|---|---|---|---|---|
| Iodothyronine deiodinases | DIO | DIO1, DIO2, DIO3 | Thyroid hormone activation/inactivation (T4 in T3 conversion; T4 in rT3 and T3 in T2 conversion) | [146] |
| Glutathione peroxidases | GPx | GPx1, GPx2, Gpx3, GPx4 | Free radical scavenger Protection against inflammation H2O2 reduction and prevention of lipid peroxidation Maintenance of intracellular homeostasis and redox balance | [147] |
| Thioredoxin reductases | TrxR | TrxR1, TrxR2 | NAPH-dependent oxidoreductase activity Regulation of cell proliferation (apoptosis) | [148,149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Sabatino, L.; Coi, A.; Iervasi, G.; Vassalle, C. Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship. Int. J. Environ. Res. Public Health 2022, 19, 6912. https://doi.org/10.3390/ijerph19116912
Gorini F, Sabatino L, Coi A, Iervasi G, Vassalle C. Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship. International Journal of Environmental Research and Public Health. 2022; 19(11):6912. https://doi.org/10.3390/ijerph19116912
Chicago/Turabian StyleGorini, Francesca, Laura Sabatino, Alessio Coi, Giorgio Iervasi, and Cristina Vassalle. 2022. "Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship" International Journal of Environmental Research and Public Health 19, no. 11: 6912. https://doi.org/10.3390/ijerph19116912
APA StyleGorini, F., Sabatino, L., Coi, A., Iervasi, G., & Vassalle, C. (2022). Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship. International Journal of Environmental Research and Public Health, 19(11), 6912. https://doi.org/10.3390/ijerph19116912









