Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change
Abstract
:1. Introduction
2. Materials and Methods
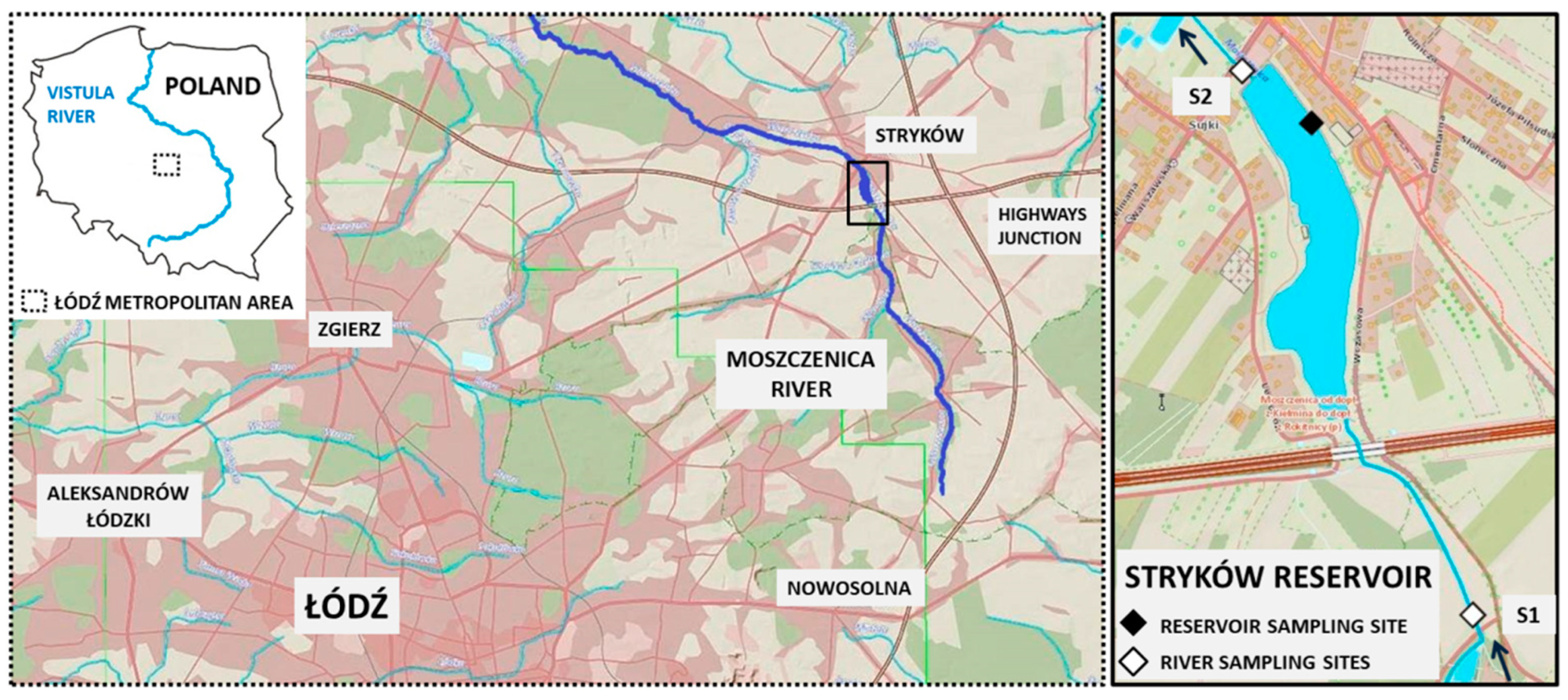
2.1. Study Area
2.2. Environmental Background
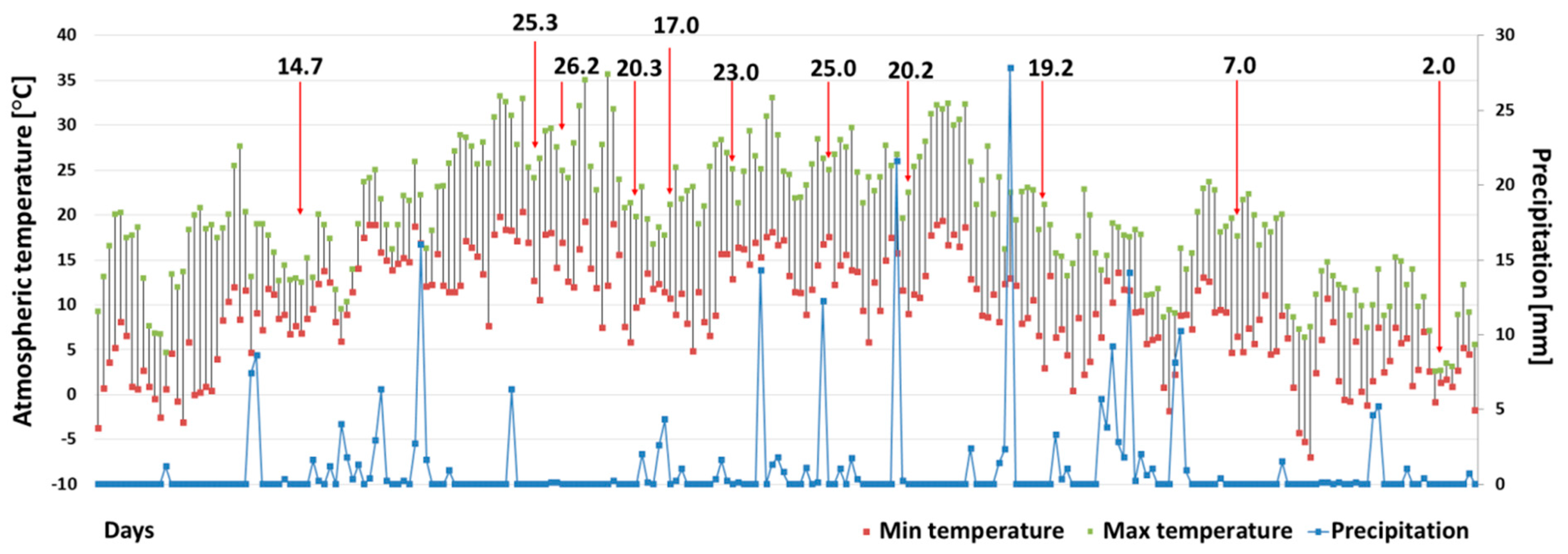
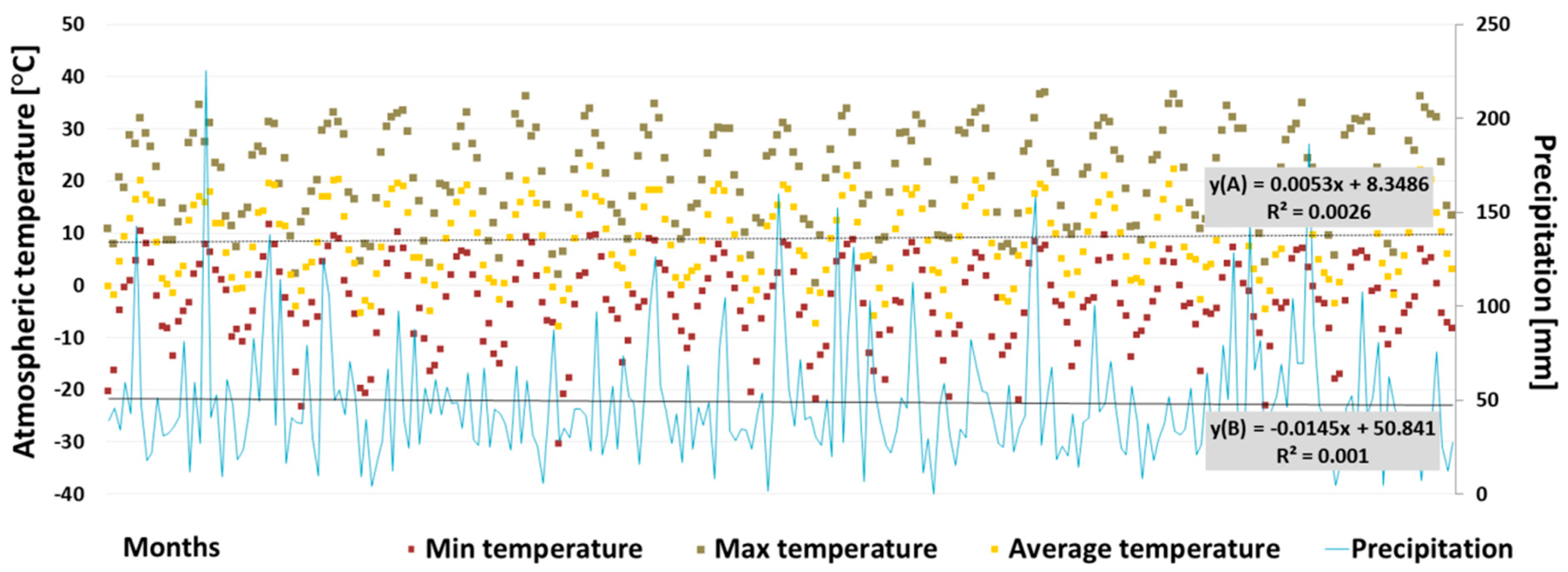
2.2.1. Climate and Hydrochemical Data Analyses
2.2.2. Ecological Status Bioassessment
2.3. Investigation of Cyanobacterial Bloom
2.4. Multivariate Data Analysis
2.5. Investigation of Cyanobacterial Toxins
3. Results
3.1. Environmental Background
3.1.1. Climate and Hydrochemical Data Analyses
3.1.2. Ecological Status Bioassessment
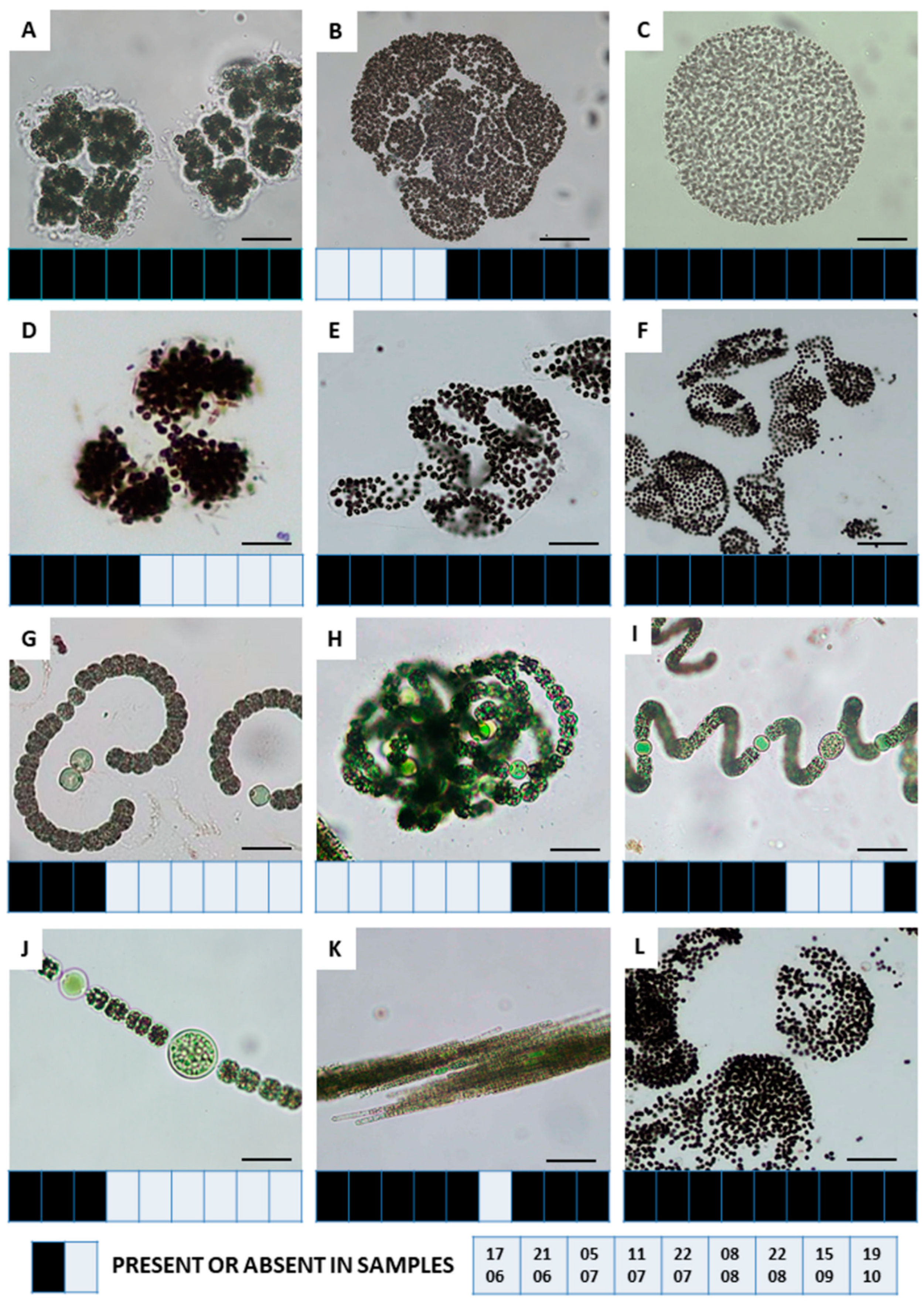
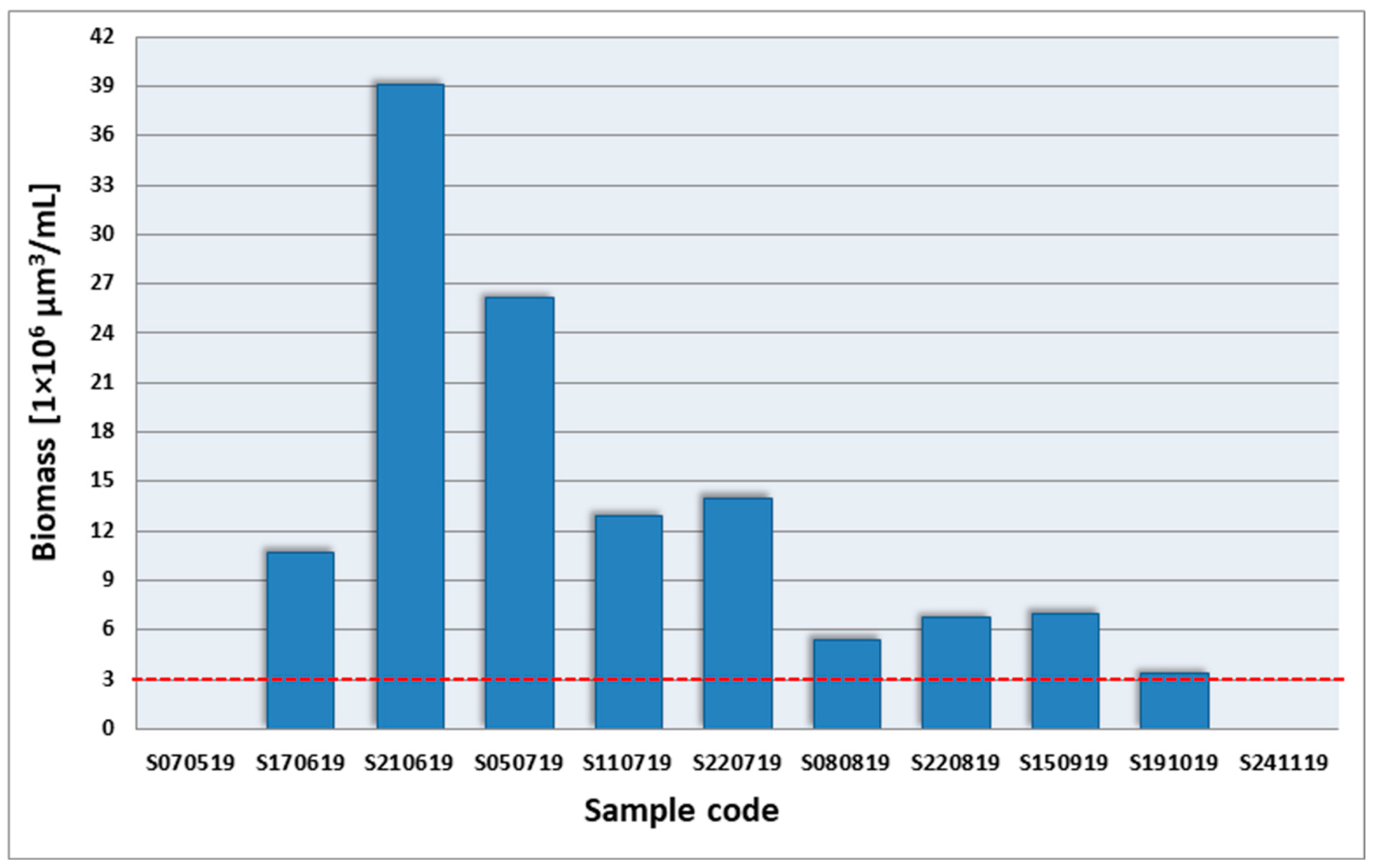
3.2. Investigation of Cyanobacterial Bloom
3.3. Multivariate Data Analysis
3.4. Investigation of Cyanobacterial Toxins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rzętała, M.A.; Rzętała, M. Rodzaj użytkowania terenu jako wskaźnik antropogenizacji zlewni i zbiorników wodnych (Type of land use as an indicator of anthropogenization of catchments and water reservoirs, in Polish). Acta Geogr. Sil. 2015, 18, 83–89. [Google Scholar]
- Pearl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Shao, N.; Yang, S.; Ren, H.; Ge, Y.; Feng, P.; Dong, B.; Zhao, Y. Predicting cyanobacteria occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, G.; Sheng, C.; Wu, J.; Li, D.; Wang, H. Improved Cyanobacteria Removal from Harmful Algae Blooms by Two-Cycle, Low-Frequency, Low-Density, and Short-Duration Ultrasonic Radiation. Water 2020, 12, 2431. [Google Scholar] [CrossRef]
- Novotny, V. The Danger of Hypertrophic Status of Water Supply Impoundments Resulting from Excessive Nutrient Loads from Agricultural and Other Sources. J. Water Sustain. 2011, 1, 1–22. [Google Scholar]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. The role of nitrogen fixation in cyanobacterial bloom toxicity in a temperate, eutrophic lake. PLoS ONE 2013, 8, e56103. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Kaliński, T.; Koper, M.; Kostrzewska-Szlakowska, I.; Kwiatkowski, J.; Mazur-Marzec, H.; Jasser, I. Predicting blooms of toxic cyanobacteria in eutrophic lakes with diverse cyanobacterial communities. Sci. Rep. 2017, 7, 8342. [Google Scholar] [CrossRef] [Green Version]
- Backer, L.C. Cyanobacterial Harmful Algal Blooms (CyanoHABs): Developing a Public Health Response. Lake Reserv. Manag. 2002, 18, 20–31. [Google Scholar] [CrossRef]
- Dokulil, M.; Teubner, K. Cyanobacterial Dominance in Lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Sharma, N.; Choudhary, K.; Rai, A. Freshwater cyanobacterial (blue-green algae) blooms: Causes, consequences and control. In Impact, Monitoring and Management of Environmental Pollution; Nemr, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Volume 4, pp. 73–95. [Google Scholar]
- Magalhães, A.; Lafayette, D.; Terencio, R. Environmental factors driving the dominance of the harmful bloom-forming cyanobacteria Microcystis and Aphanocapsa in a tropical water supply reservoir. Water Environ. Res. 2019, 91, 1466–1478. [Google Scholar] [CrossRef]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearl, W.H.; Otten, T.G. Harmfull Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water, A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; WHO CRC Press: London, UK, 2021; pp. 1–858. [Google Scholar]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Szczepocka, E.; Nowicka-Krawczyk, P.; Olszyński, R.M.; Żelazna-Wieczorek, J. Response of diatom assemblages to the disruption of the running water continuum in urban areas, and its consequences on bioassessment. PeerJ 2021, 9, e12457. [Google Scholar] [CrossRef] [PubMed]
- Reichwaldt, E.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a chaping climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Lehman, P.; Kurobe, T.; Lesmeister, S.; Baxa, D.; Tung, A.; Teh, S. Impacts of the 2014 severe drought on the Microcystis bloom in San Francisco Estuary. Harmful Algae 2017, 63, 94–108. [Google Scholar] [CrossRef]
- Costa, J.; Souza, J.; Teixeira, A.; Nabout, J.; Carneiro, F. Eutrophication in aquatic ecosystems: A scientometric study. Acta Limnol. Bras. 2018, 30, e2. [Google Scholar] [CrossRef] [Green Version]
- Górecki, M. Charakterystyka hydrologiczna i hydrochemiczna wód Moszczenicy w przekroju Gieczno (Hydrological and hydrochemical characteristics of the Moszczenica waters in the Gieczno cross-section, in Polish). Acta Univ. Lodz. Folia Geogr. Phys. 2018, 16, 15–22. [Google Scholar]
- Ziułkiewicz, M. Jakość wód powierzchniowych w strefie podmiejskiej Łodzi na przykładzie Moszczenicy (The quality of surface waters in the suburban area of Łódź on the example of Moszczenica, in Polish). Gospod. Wodna 2012, 12, 511–518. [Google Scholar]
- Jokiel, P. Zasoby Wodne Środkowej Polski na Progu XXI Wieku (Central Poland’s Water Resources at the Threshold of the 21st Century, in Polish); University of Lodz Press: Lodz, Poland, 2004; pp. 1–114. [Google Scholar]
- Żelazna-Wieczorek, J. Diatom flora in springs of Lodz Hills (Central Poland). Biodiversity, taxonomy and temporal changes of epipsammic diatom assemblages in springs affected by human impact. In Diatom Monographs; Witkowski, A., Ed.; Gantner Verlag: Ruggell, Liechtenstein, 2011; Volume 13, pp. 1–419. [Google Scholar]
- Szczepocka, E.; Nowicka-Krawczyk, P.; Kruk, A. Deceptive ecological status of urban streams and rivers—Evidence from diatom indices. Ecosphere 2018, 9, e02310. [Google Scholar] [CrossRef]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; pp. 1–942. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota I, Chroococcales. In Süβwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heyning, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Berlin/Heidelberg, Germany, 1999; Volume 19/1, pp. 1–548. [Google Scholar]
- Komárek, J.; Zapomělová, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichospermum-1. part: Coiled types. Fottea 2007, 7, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Komárek, J.; Zapomělová, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichospermum-2. part: Straight types. Fottea 2008, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Komárek, J. Cyanoprokaryota III. Heterocytous Genera. In Süβwasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; Volume 19/3, pp. 1–1130. [Google Scholar]
- Starmach, K. Plankton Roślinny Wód Słodkich (Freshwater Phytoplankton, in Polish); Panstwowe Wydawnictwo Naukowe: Warsaw, Poland, 1989; pp. 1–496. [Google Scholar]
- Wetzel, R.G. Limnology, 2nd ed.; Saunders College Publishing: Orlando, FL, USA, 1983; pp. 1–858. [Google Scholar]
- Kucała, M.; Saładyga, M.; Kaminski, A. Phytoremediation of CYN, MC-LR and ANTX-a from Water by the Submerged Macrophyte Lemna trisulca. Cells 2021, 10, 699. [Google Scholar] [CrossRef]
- Adamski, M.; Zmudzki, P.; Chrapusta, E.; Kaminski, A.; Bober, B.; Zabaglo, K.; Bialczyk, J. Characterization of cylindrospermopsin decomposition products formed under irradiation conditions. Algal Res. 2016, 18, 1–6. [Google Scholar] [CrossRef]
- Adamski, M.; Zmudzki, P.; Chrapusta, E.; Bober, B.; Kaminski, A.; Zabaglo, K.; Latkowska, E.; Bialczyk, J. Effect of pH and temperature on the stability of cylindrospermopsin. Characterization of decomposition products. Algal Res. 2016, 15, 129–134. [Google Scholar] [CrossRef]
- ISAP. Regulation of the Minister of Marine Economy and Inland Navigation of October 11, 2019 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classification of the State of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances; Dz.U.2019.2149; ISAP: Warsaw, Poland, 2019. (In Polish) [Google Scholar]
- Abbas, T.; Kajjumba, G.; Ejjada, M.; Masrura, S.; Marti, E.; Khan, E.; Jones-Lepp, T. Recent Advancements in the Removal of Cyanotoxins from Water Using Conventional and Modified Adsorbents—A Contemporary Review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Chapra, S.T.; Boehlert, B.; Fant, C.; Bierman, V.J., Jr.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate Change Impacts on Harmful Algal Blooms in U.S. Freshwaters: A Screening-Level Assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant Cells of Cyanobacteria. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 21, pp. 5–27. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Gimitrowicz-Iwan, J.; Ligęza, S.; Pranagal, J.; Smal, H.; Olenderek, H. Small Floodplain Reservoirs in the Face of Climate Change—Sink or Source of Nutrients? Water 2020, 12, 3423. [Google Scholar] [CrossRef]
- Morales-Marín, L.; Wheater, H.; Lindenschmidt, K.-E. Potential Changes of Annual-Averaged Nutrient Export in the South Saskatchewan River Basin under Climate and Land-Use Change Scenarios. Water 2018, 10, 1438. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.H. Lake-river interactions: Implications for nutrient dynamics in reservoirs. Lake Reserv. Manag. 2009, 1, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Kahru, M.; Leppänen, J.-M.; Rud, O. Cyanobacterial blooms cause heating of the sea surface. Mar. Ecol. Prog. Ser. 1993, 101, 1–7. [Google Scholar] [CrossRef]
- Rashidan, K.K.; Bird, D.F. Role of predatory bacteria in the termination of a cyanobacterial bloom. Microb. Ecol. 2001, 41, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hayes, P.K. Evidence for cyanophages active against bloom-forming freshwater cyanobacteria. Freshw. Biol. 2008, 53, 1240–1252. [Google Scholar] [CrossRef]
- Cheng, K.; van de Waal, D.B.; Niu, X.Y.; Zhao, Y.J. Combined Effects of Elevated pCO2 and Warming Facilitate Cyanophage Infections. Front. Microbiol. 2017, 8, 1096. [Google Scholar] [CrossRef] [Green Version]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Downing, J.A.; Watson, S.B.; McCauley, E. Predicting cyanobacteria dominance in lakes. Can. J. Fish. Aquat. Sci. 2001, 58, 1905–1908. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tsukada, H. Measurement of in situ specific growth rates of Microcystis (cyanobacteria) from the frequency of dividing cells. J. Phycol. 2009, 45, 1003–1009. [Google Scholar] [CrossRef]
- Kim, S.; Chung, S.; Park, H.; Cho, Y.; Lee, H. Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation. Water 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]





| Sample Code | W-Temp [°C] | pH | Diss-O2 [mg/L] | EC [μS/cm] | N-NH4 [mg/L] | N-NO3 [mg/L] | P-PO4 [mg/L] | N-NO3 /P-PO4 |
|---|---|---|---|---|---|---|---|---|
| S070519 | 14.6 | 9.1 | 15.30 | 305 | 0.02 | 0.64 | 0.02 | 32.0 |
| S170619 | 24.6 | 8.4 | 16.50 | 313 | 0.05 | 0.98 | 0.03 | 32.7 |
| S210619 | 24.6 | 9.1 | 16.97 | 296 | 0.02 | 0.01 | 0.06 | 0.2 |
| S050719 | 20.4 | 8.8 | 10.45 | 287 | 0.01 | 0.28 | 0.16 | 1.8 |
| S110719 | 17.1 | 9.1 | 13.22 | 287 | 0.02 | 0.30 | 0.43 | 0.7 |
| S220719 | 21.7 | 8.8 | 16.67 | 252 | 0.04 | 0.29 | 0.11 | 2.6 |
| S080819 | 22.3 | 9.0 | 15.73 | 234 | 0.02 | 0.38 | 0.14 | 2.7 |
| S220819 | 20.8 | 9.0 | 13.25 | 220 | 0.01 | 0.24 | 0.03 | 8.0 |
| S150919 | 19.1 | 8.5 | 13.11 | 209 | 0.01 | 0.41 | 0.01 | 41.0 |
| S191019 | 9.7 | 8.3 | 12.64 | 222 | 0.01 | 0.41 | 0.22 | 1.9 |
| S241119 | 5.0 | 7.8 | 10.47 | 227 | 0.05 | 1.26 | 0.09 | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Skrobek, I.; Ziułkiewicz, M.; Adamski, M.; Kaminski, A.; Żmudzki, P. Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. Int. J. Environ. Res. Public Health 2022, 19, 6990. https://doi.org/10.3390/ijerph19126990
Nowicka-Krawczyk P, Żelazna-Wieczorek J, Skrobek I, Ziułkiewicz M, Adamski M, Kaminski A, Żmudzki P. Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. International Journal of Environmental Research and Public Health. 2022; 19(12):6990. https://doi.org/10.3390/ijerph19126990
Chicago/Turabian StyleNowicka-Krawczyk, Paulina, Joanna Żelazna-Wieczorek, Izabela Skrobek, Maciej Ziułkiewicz, Michał Adamski, Ariel Kaminski, and Paweł Żmudzki. 2022. "Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change" International Journal of Environmental Research and Public Health 19, no. 12: 6990. https://doi.org/10.3390/ijerph19126990
APA StyleNowicka-Krawczyk, P., Żelazna-Wieczorek, J., Skrobek, I., Ziułkiewicz, M., Adamski, M., Kaminski, A., & Żmudzki, P. (2022). Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. International Journal of Environmental Research and Public Health, 19(12), 6990. https://doi.org/10.3390/ijerph19126990






