Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Collection and Sample Preparation
2.3. Instrumental Analysis
2.4. Quality Control
2.5. Statistical Analysis
3. Results and Discussion
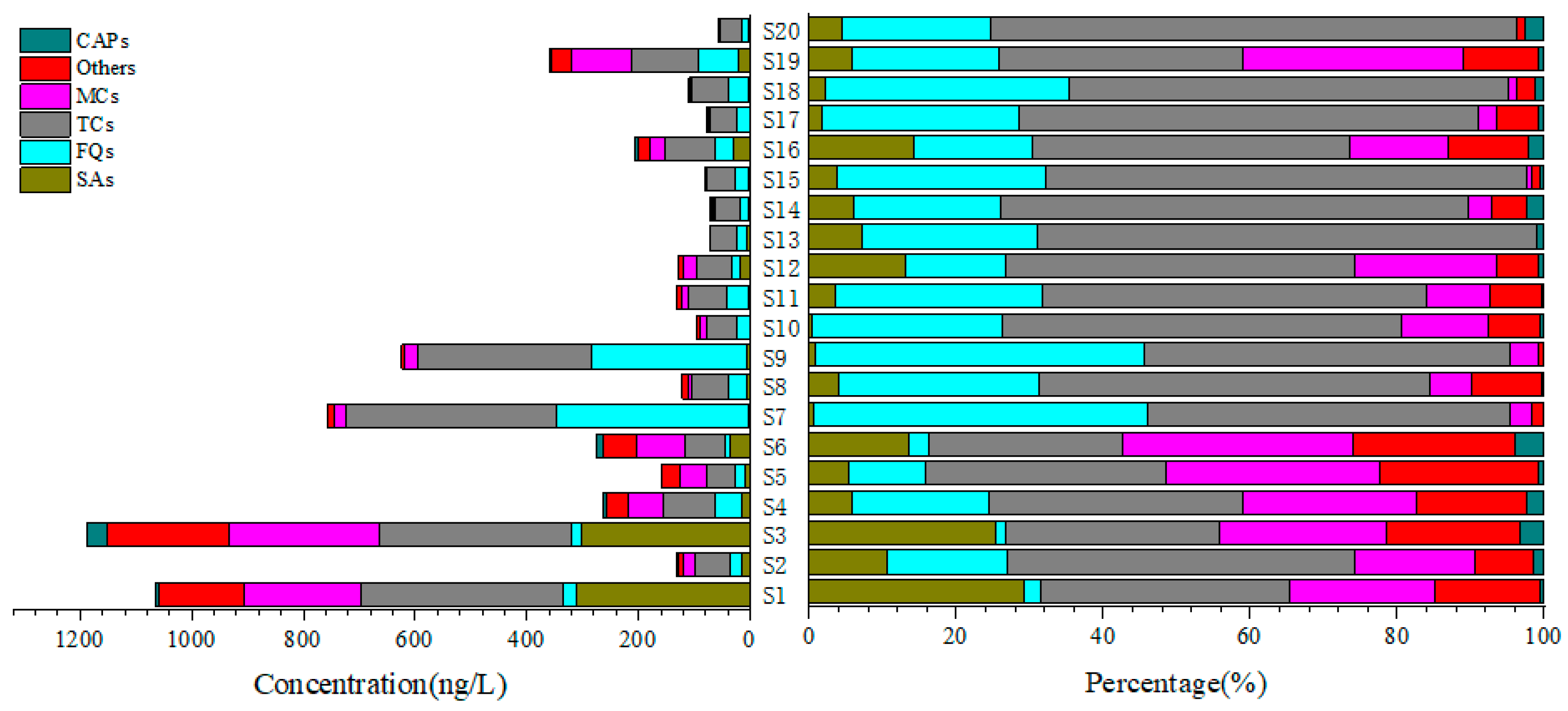
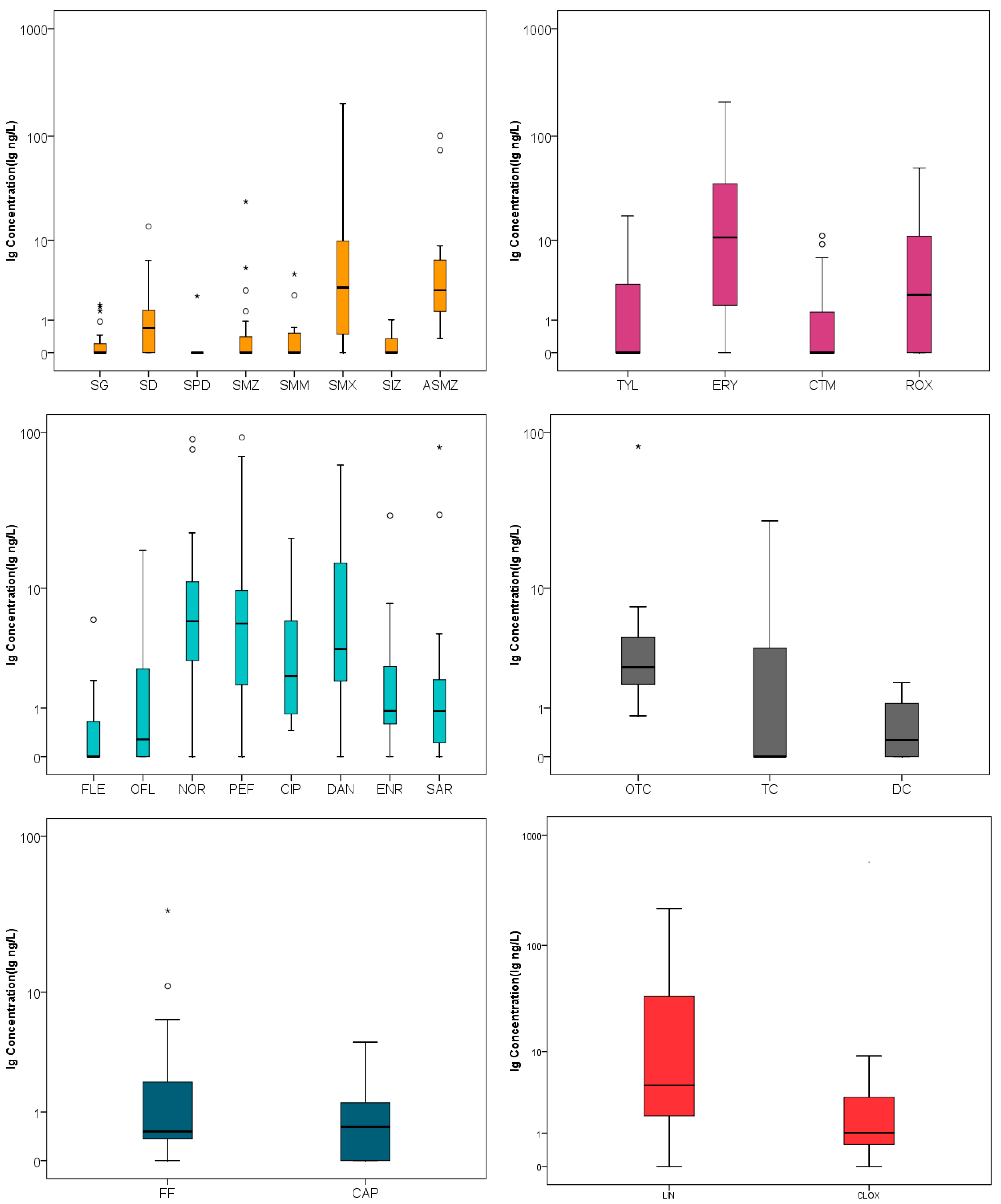
3.1. The Occurrence Characteristics of Antibiotics in the Wetland Caohai
3.2. The Cluster Analysis and Potential Sources of Antibiotics in Wetland Caohai
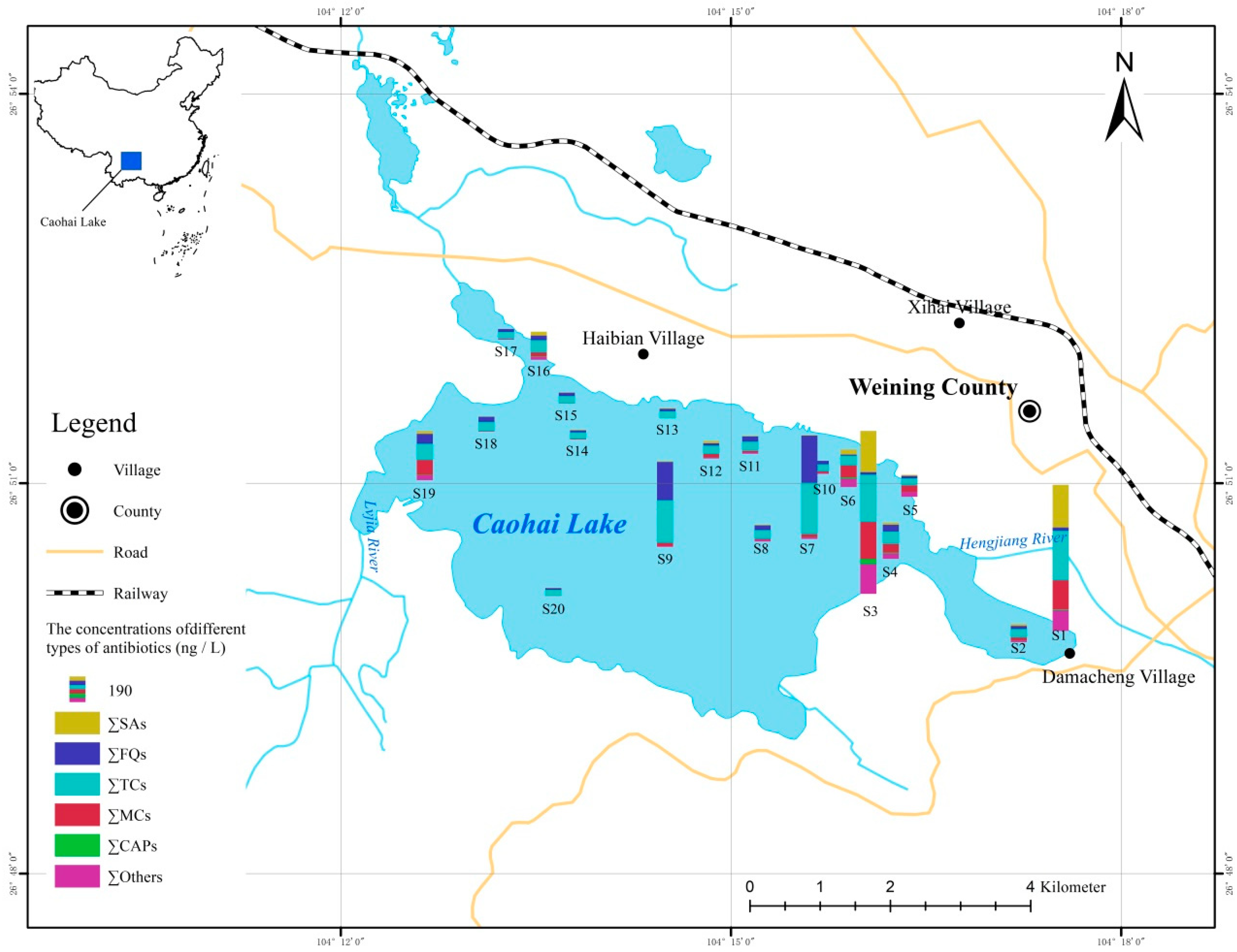
3.3. The Spatial Distribution and Attenuation Behavior of Antibiotics in Wetland Caohai
3.4. Risk Assessment of Antibiotics in Wetland Caohai
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment-A review-Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Cheng, Y.; Cao, L.; Bai, F.; Yue, S.; Xie, P.; Ma, J. Molybdenum disulfide (MoS2): A novel activator of peracetic acid for the degradation of sulfonamide antibiotics. Water Res. 2021, 201, 117291. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Dan, A.; Chen, C.X.; Zou, M.Y.; Deng, Y.Y.; Zhang, X.M.; Du, J.J.; Yang, Y. Removal efficiency, kinetic, and behavior of antibiotics from sewage treatment plant effluent in a hybrid constructed wetland and a layered biological filter. J. Environ. Manag. 2021, 288, 112435. [Google Scholar]
- Liu, K.; Zhang, D.; Xiao, X.; Cui, L.; Zhang, H. Occurrence of quinotone antibiotics and their impacts on aquatic environment in typical river-estuary system of Jiaozhou Bay, China. Ecotoxicol. Environ. Saf. 2020, 190, 109993. [Google Scholar] [CrossRef]
- Kim, S.C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Hamscher, G.; Pawelzick, H.T.; Höper, H.; Nau, H. Different behavior of tetracyclines and sulfonamides in sandy soils after repeated fertilization with liquid manure. Environ. Toxicol. Chem. 2005, 24, 861–868. [Google Scholar] [CrossRef]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Tuc Dinh, Q.; Clément, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities-A Study in Pakistan. PLoS ONE 2013, 8, 62712–62720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Zou, S.; Ling, Z.; Wang, G.; Yan, W. A Preliminary Investigation on the Occurrence and Distribution of Antibiotics in the Yellow River and its Tributaries, China. Water Environ. Res. 2009, 81, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Zhang, G.; Zou, S.C.; Li, X.D.; Liu, Y.C. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Shi, Y.; Li, W.; Liu, J.; Cai, Y. Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J. Environ. Monit. 2012, 14, 1248–1256. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhou, C.; Guo, C.; Wang, D.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 2014, 497–498, 267–273. [Google Scholar] [CrossRef]
- Ávila, C.; García-Galán, M.J.; Borrego, C.M.; Rodríguez-Mozaz, S.; García, J.; Barceló, D. New insights on the combined removal of antibiotics and ARGs in urban wastewater through the use of two configurations of vertical subsurface flow constructed wetlands. Sci. Total Environ. 2021, 755, 142554. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, W.; Pei, Y. Constructed wetland substrates: A review on development, function mechanisms, and application in contaminants removal. Chemosphere 2022, 286, 131564. [Google Scholar] [CrossRef] [PubMed]
- Kim Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar]
- Lin, S.X.; Zhang, Q.H.; Guo, Y.; Ou, Y.Y.; Lin, C.H. Pollution Characteristics and Potential Ecological Risk Assessment of Heavy Metals in Sediments of Caohai in Guizhou Province, China. Agro-Environ. Sci. 2012, 31, 2236–2241. [Google Scholar]
- Liu, F.; Mu, X.; Cui, W.; Bai-yin, B.L.F. Pollution Characteristics and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons in Surface Water of Lake Caohai, Guizhou Province. Agro-Environ. Sci. 2015, 34, 2176–2182. [Google Scholar]
- Hu, J.; Zhou, S.; Wu, P.; Qu, K. Assessment of the distribution, bioavailability and ecological risks of heavy metals in the lake water and surface sediments of the Caohai plateau wetland, China. PLoS ONE 2017, 12, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.J.; Bo, Y.W.; Zhang, Y.; Ma, S.Q.; Guo, C.S.; Zhang, L. Occurrence, distribution, and ecological risk of antibiotics in surface water in the Liaohe River Basin, China. Environ. Sci. 2017, 38, 4553–4561. [Google Scholar]
- Zhou, L.J.; Wu, Q.L.; Zhang, B.B.; Zhao, Y.G.; Zhao, B.Y. Occurrence, spatiotemporal distribution, mass balance and ecological risks of antibiotics in subtropical shallow Lake Taihu, China. Environ. Sci. Process Impacts 2016, 18, 500–513. [Google Scholar] [CrossRef]
- Watanabe, N.; Bergamaschi, B.A.; Loftin, K.A.; Meyer, M.T.; Harter, T. Use and environmental occurrence of antibiotics in freestall dairy farms with manured forage fields. Environ. Sci. Technol. 2010, 44, 6591–6600. [Google Scholar] [CrossRef]
- Luo, L.C.; Qin, B. Hydrodynamics and Its Effects on the Aquatic Ecosystem. In Lake Taihu, China; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Lei, X.; Lu, J.; Liu, Z.; Tong, Y.; Li, S. Concentration and distribution of antibiotics in water–sediment system of Bosten Lake, Xinjiang. Environ. Sci. Pollut. Res. 2015, 22, 1670–1678. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, R.; Wang, Y.; Liu, X.; Li, J.; Zhang, G. Antibiotic contamination in a typical developing city in south China: Occurrence and ecological risks in the Yongjiang River impacted by tributary discharge and anthropogenic activities. Ecotoxicol. Environ. Saf. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, J.W.; Yang, X.Y.; Sun, B.C.; Li, K.B.; Zhang, J.T. Contamination characteristic and ecological risk of antibiotics in surface water of the Weihe Guanzhong section. China Environ. Sci. 2017, 37, 2255–2262. [Google Scholar]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, H.; Du, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Occurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Wang, J.W.; Wei, H.; Yang, X.Y.; Sun, B.C.; Li, K.B.; Zhang, J.T. Occurrence and ecological risk of sulfonamide antibiotics in the surface water of the Weihe Xi’an section. Environ. Chem. 2017, 36, 2574–2583. [Google Scholar]
- Liu, X.; Lu, S.; Meng, W.; Wang, W. Occurrence, source, and ecological risk of antibiotics in Dongting Lake, China. Environ. Sci. Pollut. Res. 2018, 25, 11063–11073. [Google Scholar] [CrossRef]
- Murata, A.; Takada, H.; Mutoh, K.; Hosoda, H.; Harada, A.; Nakada, N. Nationwide monitoring of selected antibiotics: Distribution and sources of sulfonamides, trimethoprim, and macrolides in Japanese rivers. Sci. Total Environ. 2011, 409, 5305–5312. [Google Scholar] [CrossRef]
- Kurwadkar, S.T.; Adams, C.D.; Meyer, M.T.; Kolpin, D.W. Comparative mobility of sulfonamides and bromide tracer in three soils. J. Environ. Manag. 2011, 92, 1874–1881. [Google Scholar] [CrossRef]
- Pan, B.; Ning, P.; Xing, B. Part V-Sorption of pharmaceuticals and personal care products. Environ. Sci. Pollut. Res. 2009, 16, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Renew, J.E.; Smeby, K.L.; Pinkston, K.; Sedlak, D.L. Assessment of Potential Antibiotic Contaminants in. J. Contemp. Water Res. Educ. 2001, 120, 30–40. [Google Scholar]
- Gantverg, A.; Shishani, I.; Hoffman, M. Determination of chloramphenicol in animal tissues and urine: Liquid chromatography-tandem mass spectrometry versus gas chromatography-mass spectrometry. Anal. Chim. Acta 2003, 483, 125–135. [Google Scholar] [CrossRef]
- Tang, M.; Dou, X.M.; Wang, C.Y.; Tian, Z.; Yang, M.; Zhang, Y. Abundance and distribution of antibiotic resistance genes in a full-scale anaerobic-aerobic system alternately treating ribostamycin, spiramycin and paromomycin production wastewater. Environ. Geochem. Health 2017, 39, 1595–1605. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Zhao, J.L.; Yang, J.F.; Wang, L.; Yang, B.; Liu, S. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ. Pollut. 2011, 159, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Carlson, K. LC-MS2 for quantifying trace amounts of pharmaceutical compounds in soil and sediment matrices. TrAC-Trends Anal. 2005, 24, 635–644. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the haihe River basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.R.; Ma, B.H.; Zhai, X.W.; Zhang, A.Y.; Lei, C.W.; Zuo, L.; Yang, X.; Zhou, C.Y.; Wang, H.N. Florfenicol Enhances Colonization of a Salmonella enterica Serovar Enteritidis floR Mutant with Major Alterations to the Intestinal Microbiota and Metabolome in Neonatal Chickens. Appl. Environ. Microbiol. 2021, 87, 23. [Google Scholar] [CrossRef]
- Shen, J.; Qian, J.J.; Gu, J.M.; Hu, X.R. Marbofloxacin. Acta Crystallogr. Sect. E-Crystallogr. Commun. 2012, 68, o998–o999. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Basaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef]
- Li, W.; Gao, L.; Shi, Y.; Liu, J.; Cai, Y. Occurrence, distribution and risks of antibiotics in urban surface water in Beijing, China. Environ. Sci. Process Impacts 2015, 17, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef]
- Guo, R.; Wang, S.J.; Chang, S.; Wang, X.; Zhao, X.R.; Zheng, B. Distribution characteristics of antibiotics in Jiaxing drinking water source and urban river. Environ. Chem. 2016, 35, 1842–1852. [Google Scholar]
- Liu, K.; Yin, X.; Zhang, D.; Yan, D.; Cui, L.; Zhu, Z.; Wen, L. Distribution, sources, and ecological risk assessment of quinotone antibiotics in the surface sediments from Jiaozhou Bay wetland, China. Mar. Pollut. Bull. 2018, 129, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Feng, F.; Chai, Y.; Meng, X.; Sui, Q.; Chen, M.; Wei, Y.; Qi, K. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons. Sci. Total Environ. 2019, 660, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, C.H. Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite. Chemosphere 2007, 66, 1502–1512. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2014, 72, 3–27. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Song, Y.; Wang, J.; Lu, D. The Distribution Characteristics and Sources of Organic Carbon in Sediments of Caohai Lake. J. Mianyang Teach. Coll. 2017, 36, 1–9. [Google Scholar]
- Hokstad, P.; Steiro, T. Overall strategy for risk evaluation and priority setting of risk regulations. Reliab. Eng. Syst. Saf. 2006, 91, 100–111. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nagase, H.; Eguchi, K.; Hirooka, T.; Nakamura, T.; Miyamoto, K.; Hirata, K. A novel method using cyanobacteria for ecotoxicity test of veterinary antimicrobial agents. Environ. Toxicol. Chem. 2007, 26, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.; Wang, J.; Ma, Z.; Han, P.; Luan, Y.; Lu, A. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521–522, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Lützhøft, H.C.H.; Andersen, H.R.; Ingerslev, F. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 46, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Arpin-Pont, L.; Martínez-Bueno, M.J.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the marine environment: A review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Gagné, F.; Blaise, C. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Chandran, S.; Shah, H.; Diwan, V.; Tamhankar, A.J.; Stålsby Lundborg, C. Antibiotic resistance in an indian rural community: A “one-health” observational study on commensal coliform from humans, animals, and water. Int. J. Environ. Res. Public Health 2017, 14, 386. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, K.; Nagase, H.; Ozawa, M.; Endoh, Y.S.; Goto, K.; Hirata, K.; Yoshimura, H. Evaluation of antimicrobial agents for veterinary use in the ecotoxicity test using microalgae. Chemosphere 2004, 57, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bialk, B.; Stolte, S.; Arning, J.; Uebers, U.; Boschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xin, Q.; Zhu, J.M.; Cheng, J.P. The antibiotic contaminations in the main water bodies in China and the associated environmental and human health impacts. Environ. Chem. 2013, 33, 1075–1083. [Google Scholar]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Aams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, J.F.; Lv, S.H.; Chen, J.Y. Growth inhibition efect of phenicol antibiotics on Chlorellapyenoidosa. J. Jinan Univ. 2012, 33, 294–299. [Google Scholar]
- Lützhft, H.C.; Halling, S.R.B.; Rgensen, S.E. Algal Toxicity of Antibacterial Agents Applied in Danish Fish Farming. Arch. Environ. Contam. Toxicol. 1999, 36, 1–6. [Google Scholar] [CrossRef]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef]

| Study Area | SMX | NOR | PEF | DAN | SAR | OTC | ROX | ERY | LIN | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| This study | ND-201 | ND-90.5 | ND-93 | ND-62.8 | ND-80.8 | 0.80–81.8 | ND-50.3 | ND-209 | ND-216 | – |
| The Yellow River | <LOQ-56 | <LOQ-300 | – | – | – | – | <LOQ-95.0 | <LOQ-77.0 | – | [14] |
| The Liao River | ND-670 | ND-256 | – | – | – | ND-742 | ND-741 | ND-2834 | – | [26] |
| The Pearl river | <LOQ-193 | <LOQ-251 | – | – | – | – | <LOQ-169 | ND-30.6 | – | [15] |
| The Huangpu River | 2.2–765 | ND | – | – | – | 11.5–84.5 | 0.2–4.1 | – | – | [17] |
| The Hai River | ND-201 | ND-141 | – | – | ND-35.9 | – | ND-40.2 | ND-67.7 | – | [16] |
| Taihu Lake | ND-234 | ND-12.2 | ND-323 | ND-34.1 | ND-15.6 | ND-34.8 | ND-35.6 | ND-4.66 | ND-53.8 | [27] |
| Chaohu Lake | ND-171.6 | ND-70.2 | – | – | – | ND-2.90 | – | – | – | [28,18] |
| Baiyangdian Lake | ND-940 | ND-1140 | – | – | ND-28.2 | 4.28–90.3 | ND-302 | ND-121 | – | [29] |
| Bosten Lake | 1.12–13.3 | – | – | – | – | ND-20.7 | – | – | – | [30] |
| Po River | 1.83–2.39 | – | – | – | – | ND-1.82 | – | 0.28–4.62 | – | [31] |
| Pakistan part region | <LOQ-2700 | <LOQ-38 | – | – | – | 1.10–1100 | <LOQ-180 | <LOQ-310 | <LOQ-1100 | [12] |
| Seine river | ND-53.0 | ND-163 | – | – | – | – | – | – | – | [11] |
| The Yong jiang River | <LOQ-68.0 | – | – | – | – | – | ND-6.10 | ND-174 | – | [32] |
| The Wei he River | 7.60–115 | ND-39.2 | – | – | – | ND-104 | 1.57–59.5 | 23.3–59.5 | 3.63–125 | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Wang, Y.; Peng, J.; Huang, H.; Tu, X.; Zhao, H.; Zhan, N.; Rao, Z.; Zhao, G.; Yang, H. Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China. Int. J. Environ. Res. Public Health 2022, 19, 7211. https://doi.org/10.3390/ijerph19127211
Guo F, Wang Y, Peng J, Huang H, Tu X, Zhao H, Zhan N, Rao Z, Zhao G, Yang H. Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China. International Journal of Environmental Research and Public Health. 2022; 19(12):7211. https://doi.org/10.3390/ijerph19127211
Chicago/Turabian StyleGuo, Feng, Yanan Wang, Jie Peng, Hetian Huang, Xiangting Tu, Hu Zhao, Nan Zhan, Zhu Rao, Gaofeng Zhao, and Hongbo Yang. 2022. "Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China" International Journal of Environmental Research and Public Health 19, no. 12: 7211. https://doi.org/10.3390/ijerph19127211






