Efficient Removal of Nonylphenol Isomers from Water by Use of Organo-Hydrotalcites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Sorbent
2.3. Adsorption Tests
2.4. Kinetic Tests
3. Results and Discussion
3.1. Adsorption Tests
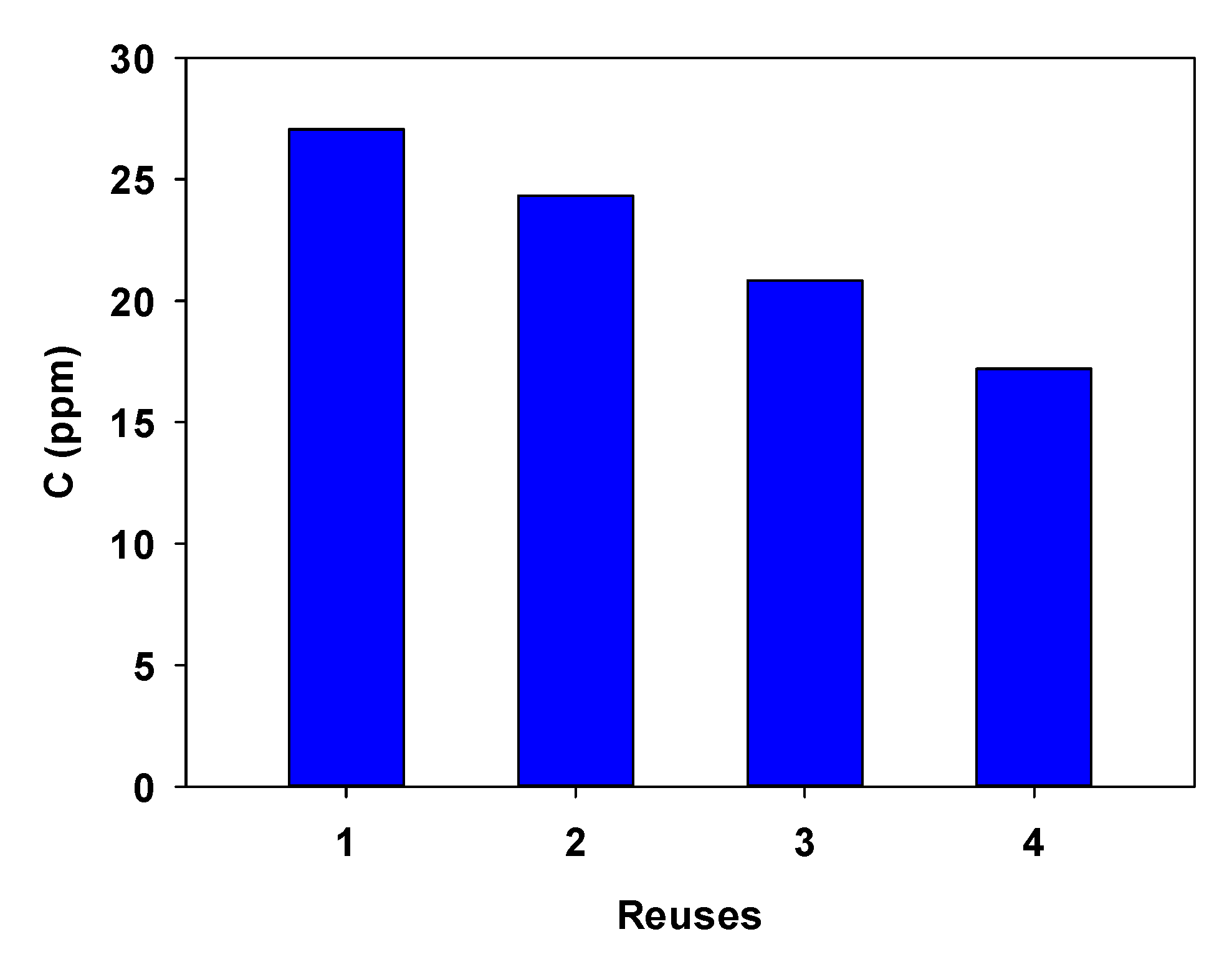
3.2. Reusability of the Adsorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruhí, A.; Acuña, V.; Barceló, D.; Huerta, B.; Mor, J.R.; Rodríguez-Mozaz, S.; Sabater, S. Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci. Total Environ. 2016, 540, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Koumaki, E.; Mamais, D.; Noutsopoulos, C. Assessment of the environmental fate of endocrine disrupting chemicals in rivers. Sci. Total Environ. 2018, 628–629, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Valbonesi, P.; Profita, M.; Vasumini, I.; Fabbri, E. Contaminants of emerging concern in drinking water: Quality assessment by combining chemical and biological analysis. Sci. Total Environ. 2021, 758, 143624. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, K. Urinary levels of 4-nonylphenol and 4-t-octylphenol in a representative sample of the Korean adult population. Int. J. Environ. Res. Public Health 2017, 14, 932. [Google Scholar] [CrossRef] [PubMed]
- Rott, E.; Kuch, B.; Lange, C.; Richter, P.; Kugele, A.; Minke, R. Removal of emerging contaminants and estrogenic activity from wastewater treatment plant effluent with UV/chlorine and UV/H2O2 advanced oxidation treatment at pilot scale. Int. J. Environ. Res. Public Health 2018, 15, 935. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G. High-energy oxidation process: An efficient alternative for wastewater organic contaminants removal. Clean Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- Martín, J.; Orta, M. del M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Removal of priority and emerging pollutants from aqueous media by adsorption onto synthetic organo-funtionalized high-charge swelling micas. Environ. Res. 2018, 164, 488–494. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Suo, Z.; Zhao, L.; Yang, H.; Xiong, X.; Pu, H.; Gan, N.; Li, H. Rapid and efficient removal of estrogenic pollutants from water by using beta- and gamma-cyclodextrin polymers. Chem. Eng. J. 2018, 344, 514–523. [Google Scholar] [CrossRef]
- Hu, S.; Xu, D.; Kong, X.; Gong, J.; Yang, Y.; Ran, Y.; Mao, J. Effect of the structure and micropore of activated and oxidized black carbon on the sorption and desorption of nonylphenol. Sci. Total Environ. 2021, 761, 144191. [Google Scholar] [CrossRef]
- Koumaki, E.; Noutsopoulos, C.; Mamais, D.; Fragkiskatos, G.; Andreadakis, A. Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. Int. J. Environ. Res. Public Health 2021, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Lester, J.N. Fate and behavior of endocrine disrupters in wastewater treatment processes. In Endocrine Disrupters in Wastewater and Sludge Treatment Processes; CRC Press: Boca Raton, FL, USA, 2002; pp. 64–68. [Google Scholar]
- Aparicio, I.; Santos, J.L.; Alonso, E. Limitation of the concentration of organic pollutants in sewage sludge for agricultural purposes: A case study in South Spain. Waste Manag. 2009, 29, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cheng, Y.; Hua, Z.; Yuan, C.; Wang, X. The effect of dissolved organic matter (DOM) on the release and distribution of endocrine-disrupting chemicals (EDCS) from sediment under hydrodynamic forces, a case study of Bisphenol a (BPA) and nonylphenol (NP). Int. J. Environ. Res. Public Health 2019, 16, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Sasai, R.; Sugiyama, D.; Takahashi, S.; Tong, Z.; Shichi, T.; Itoh, H.; Takagi, K. The removal and photodecomposition of n-nonylphenol using hydrophobic clay incorporated with copper-phthalocyanine in aqueous media. J. Photochem. Photobiol. A Chem. 2003, 155, 223–229. [Google Scholar] [CrossRef]
- Al-Ahmari, S.D.; Watson, K.; Fong, B.N.; Ruyonga, R.M.; Ali, H. Adsorption kinetics of 4-n-nonylphenol on hematite and goethite. J. Environ. Chem. Eng. 2018, 6, 4030–4036. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, H.; Jin, Z.; Jiang, Y.; Hui, C.; Xu, L.; Zhao, Y.; Du, L. Effects of Fe2O3 nanoparticles on extracellular polymeric substances and nonylphenol degradation in river sediment. Sci. Total Environ. 2021, 770, 145210. [Google Scholar] [CrossRef]
- Dai, Y.-D.; Shah, K.J.; Huang, C.P.; Kim, H.; Chiang, P.-C. Adsorption of Nonylphenol to Multi-Walled Carbon Nanotubes: Kinetics and Isotherm Study. Appl. Sci. 2018, 8, 2295. [Google Scholar] [CrossRef] [Green Version]
- You, X.; He, M.; Cao, X.; Wang, P.; Wang, J.; Li, L. Molecular dynamics simulations of removal of nonylphenol pollutants by graphene oxide: Experimental study and modelling. Appl. Surf. Sci. 2019, 475, 621–626. [Google Scholar] [CrossRef]
- Catherine, H.N.; Ou, M.H.; Manu, B.; Shih, Y.H. Adsorption mechanism of emerging and conventional phenolic compounds on graphene oxide nanoflakes in water. Sci. Total Environ. 2018, 635, 629–638. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Ren, X.; Wang, S.; Lu, L. Inorganic layered ion-exchangers for decontamination of toxic metal ions in aquatic systems. J. Mater. Chem. A 2017, 5, 19593–19606. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, K.; Lim, T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Applications of layered double hydroxide materials: Recent advances and perspective. In 50 Years of Structure and Bonding–The Anniversary Volume; Springer: Berlin/Heidelberg, Germany, 2016; pp. 65–84. [Google Scholar]
- Li, F.; Duan, X. Applications of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 193–223. ISBN 978-3-540-32495-9. [Google Scholar]
- Cosano, D.; Esquinas, C.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Use of Raman spectroscopy to assess the efficiency of MgAl mixed oxides in removing cyanide from aqueous solutions. Appl. Surf. Sci. 2016, 364, 428–433. [Google Scholar] [CrossRef]
- Kostura, B.; Radim, Š.; Plachá, D.; Kukutschová, J.; Matýsek, D. Mg–Al–CO3 hydrotalcite removal of persistent organic disruptor—Nonylphenol from aqueous solutions. Appl. Clay Sci. 2015, 114, 234–238. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, M.; Li, J.; Zhao, K.; Liu, Y. Sensitive determination of bisphenol A, 4-nonylphenol and 4-octylphenol by magnetic solid phase extraction with Fe@MgAl-LDH magnetic nanoparticles from environmental water samples. Sep. Purif. Technol. 2017, 182, 78–86. [Google Scholar] [CrossRef]
- Cosano, D.; Esquivel, D.; Romero, F.J.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Microwave-assisted synthesis of hybrid organo-layered double hydroxides containing cholate and deoxycholate. Mater. Chem. Phys. 2019, 225, 28–33. [Google Scholar] [CrossRef]
- Hameed, B.H. Equilibrium and kinetics studies of 2, 4, 6-trichlorophenol adsorption onto activated clay. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 307, 45–52. [Google Scholar] [CrossRef]
- Dinari, M.; Neamati, S. Surface modified layered double hydroxide/polyaniline nanocomposites: Synthesis, characterization and Pb2+ removal. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 589, 124438. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, M.; Liu, H.; Ouyang, S.; Ding, N.; Zhang, P. Adsorption behavior and mechanism of acid orange 7 and methylene blue on self-assembled three-dimensional MgAl layered double hydroxide: Experimental and DFT investigation. Appl. Surf. Sci. 2020, 522, 146370. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, S.; Chen, H.; He, H.; Sun, C. Adsorption behavior and mechanism of reactive brilliant red X-3B in aqueous solution over three kinds of hydrotalcite-like LDHs. Appl. Surf. Sci. 2014, 301, 329–337. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Chen, Z.; Wang, J.; Wang, S.; Hayat, T.; Wang, X. Layered double hydroxide intercalated with aromatic acid anions for the efficient capture of aniline from aqueous solution. J. Hazard. Mater. 2017, 321, 111–120. [Google Scholar] [CrossRef]
- Zheng, L.; Dang, Z.; Yi, X.; Zhang, H. Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk. J. Hazard. Mater. 2010, 176, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Dinari, M.; Shirani, M.A.; Maleki, M.H.; Tabatabaeian, R. Green cross-linked bionanocomposite of magnetic layered double hydroxide/guar gum polymer as an efficient adsorbent of Cr(VI) from aqueous solution. Carbohydr. Polym. 2020, 236, 116070. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravori, R. Pore and Solid Diffusion Models for Fixed-Bed Adsorbers. AIChE J. 1974, 20, 222–238. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]




| Solid | Mg/AlTher | Mg/Alexp a | A (nm) | C (nm) |
|---|---|---|---|---|
| Hydrotalcite | 2.5 | 2.41 | 0.305 | 9.810 |
| T (K) | Pseudo-First Order | Pseudo-Second Order | Elovich | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K1 | R2 | K2 | R2 | B | R2 | KIntra | c | ||
| 30 | 298 | 0.824 | 0.010 | 0.938 | 0.169 | 0.960 | 3.917 | 0.881 | 0.077 | 4.176 |
| 25 | 298 | 0.962 | 0.009 | 0.980 | 0.154 | 0.961 | 2.699 | 0.910 | 0.117 | 3.426 |
| 20 | 298 | 0.921 | 0.031 | 0.993 | 0.148 | 0.910 | 2.075 | 0.712 | 0.138 | 2.828 |
| 15 | 298 | 0.893 | 0.051 | 0.957 | 0.132 | 0.802 | 1.962 | 0.559 | 0.127 | 1.810 |
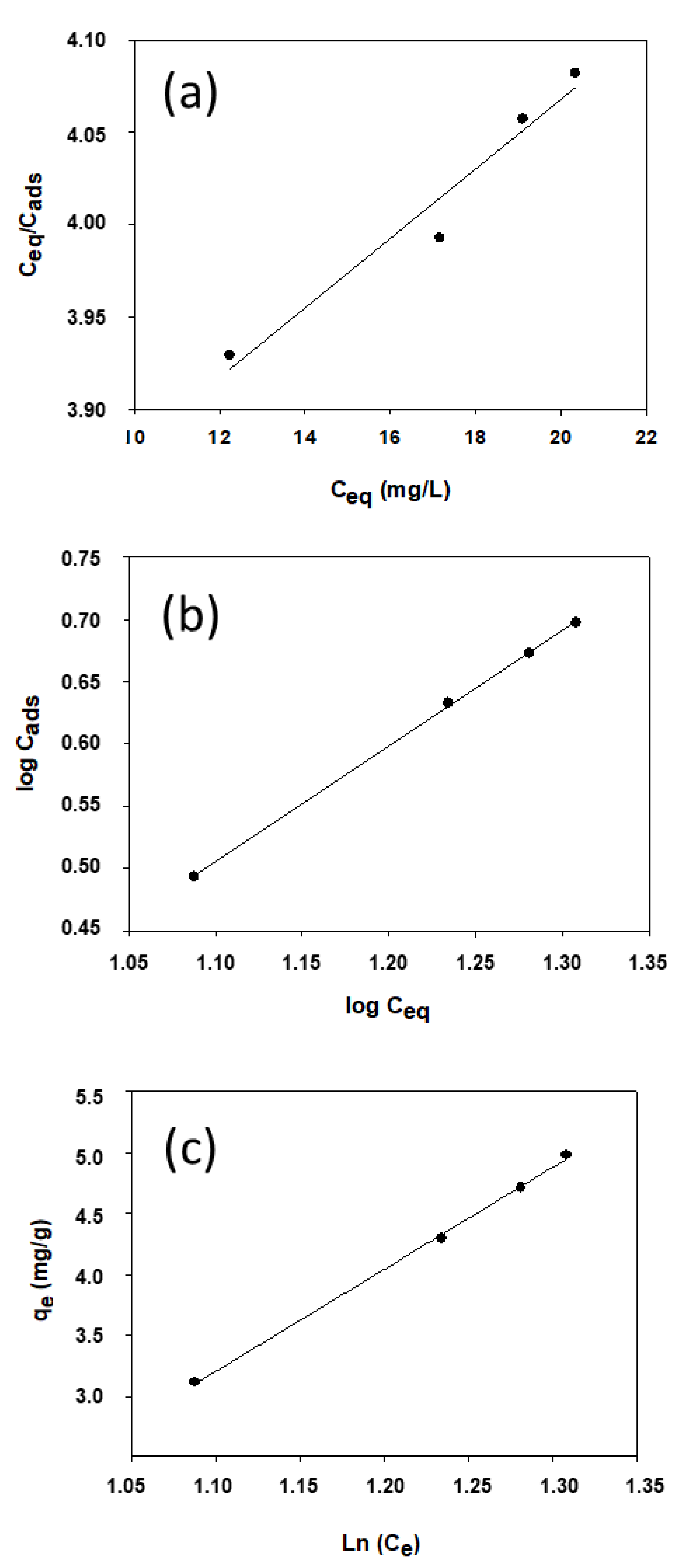
| Langmuir | Q = 53.19 | b = 5 · 10−3 | R2 = 0.956 |
| Freundlich | KF = 0.306 | n = 1.079 | R2 = 0.999 |
| Temkin | KT = 0.488 | bT = 2.919 | R2 = 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosano, D.; Esquivel, D.; Romero-Salguero, F.J.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Efficient Removal of Nonylphenol Isomers from Water by Use of Organo-Hydrotalcites. Int. J. Environ. Res. Public Health 2022, 19, 7214. https://doi.org/10.3390/ijerph19127214
Cosano D, Esquivel D, Romero-Salguero FJ, Jiménez-Sanchidrián C, Ruiz JR. Efficient Removal of Nonylphenol Isomers from Water by Use of Organo-Hydrotalcites. International Journal of Environmental Research and Public Health. 2022; 19(12):7214. https://doi.org/10.3390/ijerph19127214
Chicago/Turabian StyleCosano, Daniel, Dolores Esquivel, Francisco J. Romero-Salguero, César Jiménez-Sanchidrián, and José Rafael Ruiz. 2022. "Efficient Removal of Nonylphenol Isomers from Water by Use of Organo-Hydrotalcites" International Journal of Environmental Research and Public Health 19, no. 12: 7214. https://doi.org/10.3390/ijerph19127214






