The Significance of Adaptation and Coping with Disease among Patients with Diagnosed Gynaecological Cancer in the Context of Disease Acceptance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Research Instruments
2.2.1. Mental Adaptation to Cancer Scale (Mini-Mac)
2.2.2. Acceptance of Illness Scale (AIS)
2.2.3. Coping and Adaptation Processing Scale (CAPS)
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Adaptation to Cancer Scale (Mini-Mac)
3.3. Acceptance of Illness Scale (AIS)
3.4. Coping and Adaptation Processing Scale (CAPS)
3.5. Acceptance of the Disease and Adaptation to Cancer, and Mental Adaptation
4. Discussion
5. Conclusions
- The studied group of women diagnosed with gynaecological cancer displayed a moderate level of disease acceptance.
- Constructive adaptive mechanisms were the most prevalent among the studied women.
- The location of cancer had no bearing on the women’s adaptation to the disease and coping mechanisms.
- Acceptance of the disease and an active attitude of the patients determined their adaptation to gynaecological cancer.
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahar, Y.A.; Kastelein, A.W.; Klaassen, I.; Jansen, C.H.J.R.; Latul, Y.P.; Vittori, M.; Biri, A.; Kahraman, K.; Griffioen, A.W.; Amant, F.; et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188446. [Google Scholar] [CrossRef]
- Available online: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/ (accessed on 20 January 2022).
- Yvette, C.; Terrie, B.S.; Pharm, R.P. Gynecologic Cancers: What Every Woman Schould Know. US Pharm. 2017, 42, 18–23. [Google Scholar]
- Available online: https://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=968#WOMEN (accessed on 1 January 2020).
- Available online: https://epid.coi.waw.pl/krn/ (accessed on 1 January 2020).
- Braun, M.M.; Overbeek-Wager, E.A.; Robert, J.; Grumbo, R.G. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar] [PubMed]
- Religioni, U.; Czerw, A.; Budzik, M.P.; Deptała, A.; Badowska-Kozakiewicz, A.M. Assessment of pain, acceptance of the disease, adaptation to life and strategies of coping with the disease in patients with endometrial cancer. Eur. J. Gynecol. Oncol. 2020, 6, 1016–1022. [Google Scholar] [CrossRef]
- Cogliano, V.J. International Agency for Research on Cancer (IARC). Toxicol. Pathol. 2016, 34, 405–406. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Cornelison, R.; Llaneza, D.C.; Landen, C.N. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef] [Green Version]
- Erickson, B.K.; Conner, M.G.; Landen, C.N. The role of the fallopian tube in the origin of ovarian cancer. Am. J. Obstet. Gynecol. 2013, 209, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Harries, M.; Kaye, S.B. Recent advances in the treatment of epithelial ovarian cancer. Expert Opin. Investig. Drugs 2001, 10, 1715–1724. [Google Scholar] [CrossRef]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef]
- Basta, A.; Bidziński, M.; Bieńkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J.; et al. Zalecenia Polskiego Towarzystwa Ginekologii Onkologicznej dotyczące diagnostyki i leczenia raka jajnika. Curr. Gynecol. Oncol. 2017, 15, 5–23. [Google Scholar] [CrossRef]
- Rogala, D.; Mazur, A.; Maślińska, M.; Koper, K.; Staniszewska, M. Psychological adaptation to cancer and strategies for coping with pain in patients with cervical cancer. Arch. Perinat. Med. 2016, 22, 1–7. [Google Scholar]
- Muszalik, M.; Repka, I.; Puto, G.; Kowal-Skałka, J.; Kędziora-Kornatowska, K. Assessment of Functional Status and Quality of Life of Elderly Patients Undergoing Radiotherapy and Radiotherapy Combined with Chemotherapy—A Cross-Sectional Study. Clin. Interv. Aging 2021, 16, 9–18. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping. Wyd. 8; Springer Publishing Company: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Heszen, I. Psychologia Stresu; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 2021. [Google Scholar]
- Sygit-Kowalkowska, E. Radzenie sobie ze stresem jako zachowanie zdrowotne człowieka–perspektywa psychologiczna. Hygeia Public Health 2014, 49, 202–208. [Google Scholar]
- Parker, J.D.A.; Endler, N.S. Coping with copingassessment: A criticalreviev. Eur. J. Pers. 1992, 6, 321–344. [Google Scholar] [CrossRef]
- Czerw, A.; Religioni, U.; Deptała, A. Assessment of pain, acceptance of illness, adjustment to life with cancer and coping strategies in breast cancer patients. Breast Cancer 2016, 23, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Kozak, G. Different strategies of managing neoplasia in the course of chosen cancers. Anest. Ratow. 2012, 6, 162–170. [Google Scholar]
- Religioni, U.; Czerw, A.; Deptała, A. Acceptance of cancer in patients diagnosed with lung, breast, colorectal and prostate carcinoma. Iran. J. Public Health 2015, 44, 1135–1142. [Google Scholar]
- Juczyński, Z. NPPPZ Narzędzia Pomiaru w Promocji i Psychologii Zdrowia, 2nd ed.; Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego: Warszawa, Poland, 2012. [Google Scholar]
- Pettingale, K.W. Doping and cancer prognosis. J. Psychosom. Res. 1984, 28, 363–364. [Google Scholar] [CrossRef]
- Bussel, V.; Naus, M. A longitudinal investigation of coping and posttraumatic growth in breast cancer survivors. J. Psychosoc. Oncol. 2010, 28, 61–78. [Google Scholar] [CrossRef]
- Roy, C. Coping and Adaptation Processing Scale (CAPS) Long Form (47-items) informations for Users. In CAPS User Manual4-13–15; Roy, C., William, F., Eds.; Connell School of Nursing Boston College: Boston, MA, USA, 2015; pp. 2–6. [Google Scholar]
- Syriala, K.L.; Jensen, M.P.; Mendoza, M.E.; Yi, J.C.; Fisher, H.M.; Keefe, F.J. Psychological and behavioral approaches to cancer pain management. J. Clin. Oncol. 2014, 32, 1703–1711. [Google Scholar] [CrossRef] [Green Version]
- Lõhmussaar, K.; Boretto, M.; Clevers, H. Human-Derived Model Systems in Gynecological Cancer Research. Trends Cancer 2020, 6, 1031–1043. [Google Scholar] [CrossRef]
- Malicka, I.; Szczepańska, J.; Anioł, K.; Rymaszewska, J.; Woźniewski, M. Zaburzenia nastroju i strategie przystosowania do choroby u kobiet leczonych operacyjnie z powodu nowotworu piersi i narządów rodnych. Współcz. Onkol. 2009, 13, 41–46. [Google Scholar]
- Rogala, D.; Mazur, A.; Maślińska, M.; Krawczak, M. Przystosowanie do choroby nowotworowej u pacjentek z rakiem szyjki macicy. Piel. Pol. 2016, 2, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Kupcewicz, E.; Olewińska, J.; Pikus, H.; Jóźwik, M. Mental adaptation to cancer in women with gynaecological cancer. J. Pre-Clin. Clin. Res. 2017, 2, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Kupcewicz, E.; Olewińska, J.; Pikus, H.; Jóźwik, M. Differentiating factors of mental adaptation to disease in women treated for gynaecological cancer. J. Educ. Health Sport 2017, 7, 40–56. [Google Scholar]
- Religioni, U.; Czerw, A.; Budzik, M.P.; Deptała, A.; Badowska-Kozakiewicz, A.M. Assessment of Pain, Acceptance of the Disease, Adaptation to Life and Strategies for Coping with the Disease among Patients with Ovarian Cancer. Iran. J. Public Health 2021, 50, 833–834. [Google Scholar] [CrossRef]
- Cipora, E.; Konieczny, M.; Sobieszczański, J. Acceptance of illness by women with breast cancer. Ann. Agric. Environ. Med. 2018, 25, 167–171. [Google Scholar] [CrossRef]
- Czerw, A.; Religioni, U.; Szymański, F.; Nieradko-Heluszko, A.; Mękal, D.; Kowalczuk, A.; Merks, P.; Borowska, M.; Bogdan, M.; Pajewska, M. Normalization of the Mini-MAC (Mental Adjustment to Cancer) Questionnaire among Cancer Patients. Int. J. Environ. Res. Public Health 2021, 18, 12603. [Google Scholar] [CrossRef]
- de Groot, J.M.; Mah, K.; Fyles, A.; Winton, S.; Greenwood, S.; DePetrillo, D.; Devins, G.M. Do single and partnered women with gynecologic cancer differ in types and intensities of illness- and treatment-related psychosocial concerns? A pilot study. J. Psychosom. Res. 2007, 63, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kulpa, M.; Owczarek, K.; Stypula-Ciuba, B. The impact of mental adjustment to cancer to the quality of life. Med. Paliat. 2013, 5, 106. [Google Scholar]
- Rozema, H.; Vollink, T.; Lechner, L. The role of illness representations in coping and health of patients treated for breast cancer. Psycho-Oncology 2009, 18, 849–857. [Google Scholar] [CrossRef]
- Ashley, L.; Marti, J.; Jones, H.; Velikova, G.; Wright, P. Illness perceptions within 6 months of cancer diagnosis are an independent prospective predictor of health-related quality of life 15 months post-diagnosis. Psycho-Oncology 2015, 24, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Hopman, P.; Rijken, M. Illness perceptions of cancer patients: Relationships with illness characteristics and coping. Psycho-Oncology 2015, 24, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, K.; Purushotham, A.D.; McLatchie, E.; George, W.D.; Murray, G.D. A 1-year prospective study of individual variation in distress, and illness perceptions, after treatment for breast cancer. J. Psychosom. Res. 2005, 58, 335–342. [Google Scholar] [CrossRef]
- de Rooij, B.H.; Ezendam, N.P.M.; Nicolaije, K.A.H.; Lodder, P.; Vos, M.C.; Pijnenborg, J.M.A.; Boll, D.; Kruitwagen, R.F.P.M.; van de Poll-Franse, L.V. Survivorship care plans have a negative impact on long-term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: The ROGY care trial. Qual. Life Res. 2018, 27, 1533–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska-Więczkowska, H.; Żychlińska, E. Akceptacja choroby nowotworowej i jej związek z jakością życia osób starszych objętych opieką paliatywną stacjonarną i domową. Med. Rodz. 2015, 4, 151–156. [Google Scholar]



| Education (n, % *) | |||||
|---|---|---|---|---|---|
| Cancer Type | Higher | Primary | Secondary | Vocational | p |
| Ovarian Cancer | 6 (26.1) | 3 (13.0) | 11 (47.8) | 3 (13.0) | 0.4631 |
| Uterine Cancer | 9 (15.5) | 8 (13.8) | 25 (43.1) | 16 (27.6) | |
| Marital Status (n) | |||||
| Cancer Type | Divorced | Married | Single | Widowed | 0.287 |
| Ovarian Cancer | 1 (4.3) | 8 (34.8) | 5 (21.7) | 9 (39.10) | |
| Uterine Cancer | 8 (13.8) | 15 (25.9) | 6 (10.3) | 29 (50.0) | |
| Place of Residence (n) | |||||
| Cancer Type | City >100 K | City 10–100 K | City <10 K | Village | 0.972 |
| Ovarian Cancer | 7 (30.4) | 7 (30.4) | 3 (13.0) | 6 (26.1) | |
| Uterine Cancer | 17 (29.3) | 16 (27.6) | 10 (17.2) | 15 (25.9) | |
| Employment Status (n) | |||||
| Cancer Type | Pension | Unemployed | Retirement pension | Employed | 0.419 |
| Ovarian Cancer | 1 (4.3) | 2 (8.7) | 9 (39.10) | 11 (47.8) | |
| Uterine Cancer | 3 (5.2) | 3 (5.2) | 34 (58.6) | 18 (31.0) | |
| Variable | Whole Group (n = 81) | Ovarian Cancer (n = 23) | Uterine Cancer (n = 58) | p |
|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | ||
| Anxiety Preoccupation | 18.26 ± 5.09 | 18.87 ± 5.54 | 18.02 ± 4.93 | 0.50 |
| Helplessness/Hopelessness | 13.31 ± 4.37 | 13.35 ± 5.22 | 13.29 ± 4.04 | 0.96 |
| Fighting Spirit | 22.99 ± 3.53 | 23.13 ± 3.32 | 22.93 ± 3.64 | 0.82 |
| Positive Re-evaluation | 22.12 ± 3.25 | 22.96 ± 2.64 | 21.79 ± 3.43 | 0.15 |
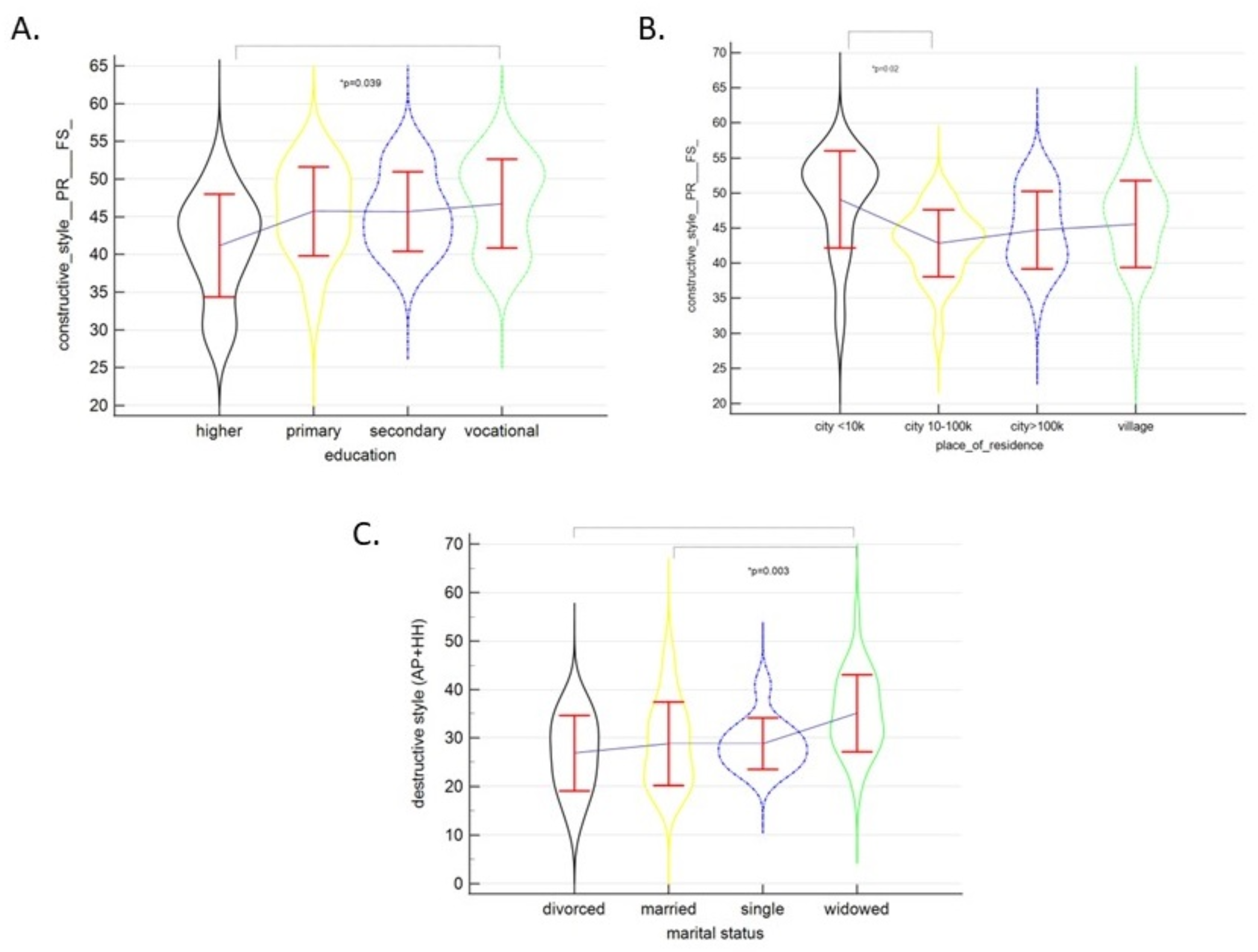
| Constructive Style (PR + FS) | 45.11 ± 6.01 | 46.09 ± 4.77 | 44.72 ± 6.43 | 0.36 |
| Destructive Style (AP + HH) | 31.57 ± 8.41 | 32.22 ± 9.59 | 31.31 ± 7.98 | 0.66 |
| Variable | Parameter | Whole Group (n = 81) | Ovarian Cancer (n = 23) | Uterine Cancer (n = 58) |
|---|---|---|---|---|
| Constructive Style | r | 0.13 | 0.25 | 0.15 |
| p | 0.24 | 0.24 | 0.28 | |
| Destructive Style | r | 0.20 | 0.02 | 0.34 |
| p | 0.07 | 0.94 | 0.01 |
| Variable | Constructive Style | p | ||
|---|---|---|---|---|
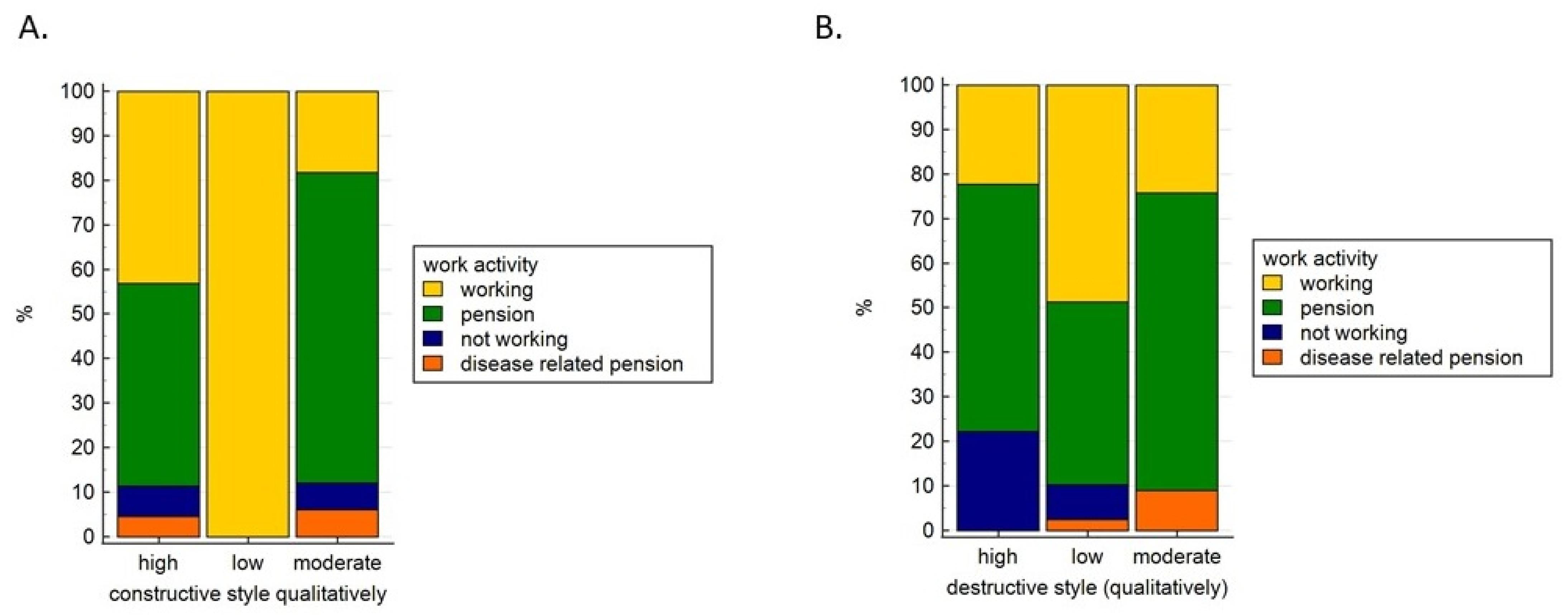
| Cancer Type (n, %) | High | Low | Moderate | |
| Ovarian Cancer | 15 (65.2) | 0 (0.0) | 8 (23.8) | 0.28 |
| Uterine Cancer | 29 (50.0) | 4 (6.9) | 25 (43.1) | |
| Destructive Style | ||||
| Cancer Type | High | Low | Moderate | |
| Ovarian Cancer | 4 (17.4) | 12 (52.2) | 7 (30.4) | 0.35 |
| Uterine Cancer | 5 (8.6) | 27 (46.6) | 26 (44.8) | |
| Place of Residence | n (%) | M ± SD | p |
|---|---|---|---|
| City > 100 K | 24 (29.6) | 24.71 ± 9.35 | 0.54 |
| City 10–100 K | 23 (28.4) | 27.43 ± 9.80 | |
| City < 10 K | 13 (16.0) | 26.08 ± 9.47 | |
| Village | 21 (25.9) | 28.38 ± 6.70 | |
| Marital Status | |||
| Divorced | 9 (11.1) | 29.67 ± 8.50 | 0.12 |
| Married | 23 (28.4) | 29.57 ± 8.63 | |
| Single | 11 (13.6) | 25.18 ± 9.23 | |
| Widowed | 38 (46.9) | 24.61 ± 8.60 | |
| Employment Status | |||
| Pension | 4 (4.9) | 27.00 ± 6.78 | 0.87 |
| Unemployed | 5 (6.2) | 24.60 ± 9.61 | |
| Retirement pension | 43 (53.1) | 26.21 ± 9.18 | |
| Employed | 29 (35.8) | 27.62 ± 8.79 | |
| Education | |||
| Higher | 15 (18.5) | 27.20 ± 7.67 | 0.881 |
| Primary | 11 (13.6) | 27.00 ± 7.84 | |
| Secondary | 36 (44.4) | 25.78 ± 9.90 | |
| Vocational | 19 (23.5) | 27.68 ± 8.65 | |
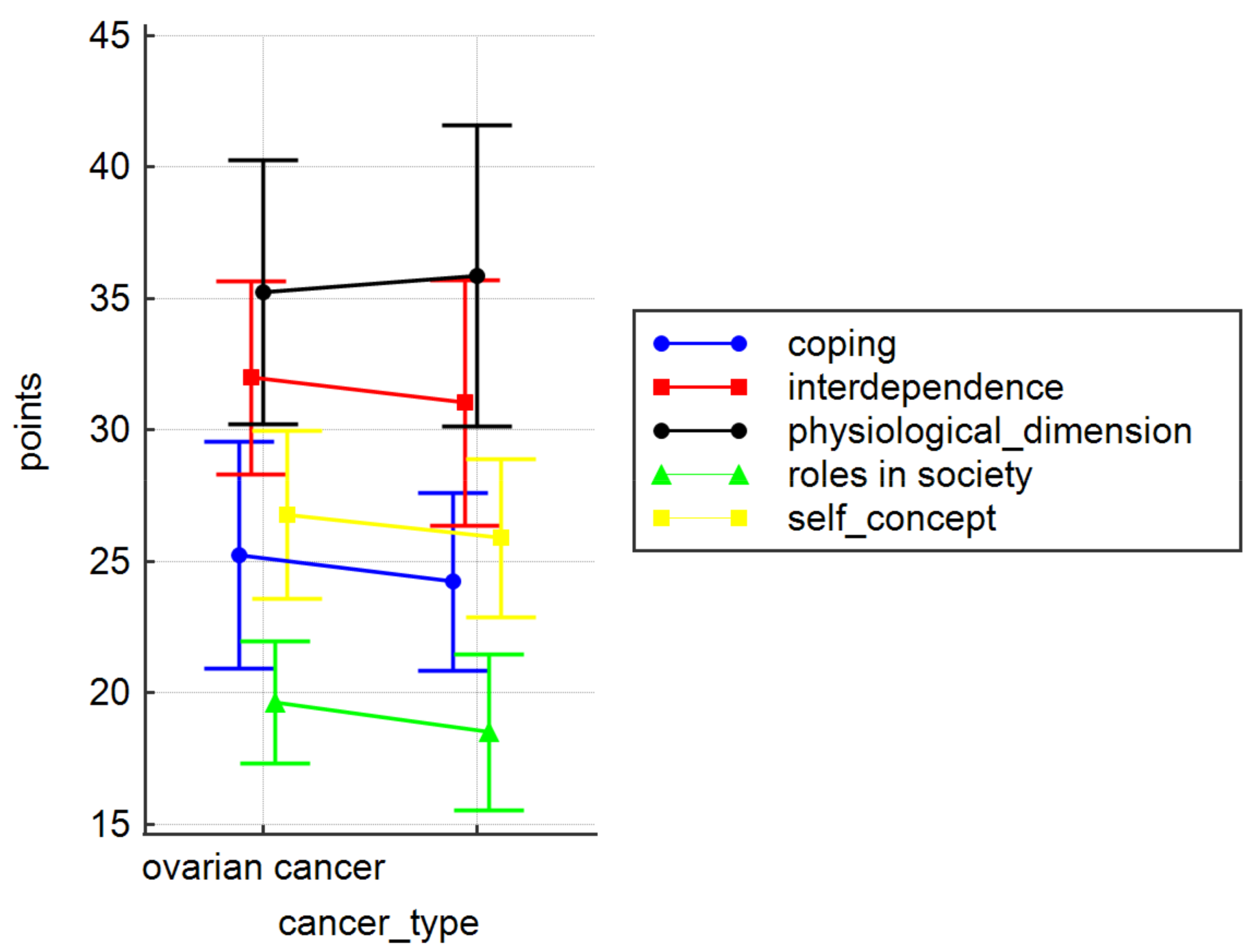
| Variable | Physical Dimension | Self-Concept | Roles in Society | ||||
|---|---|---|---|---|---|---|---|
| Place of Residence | n | M ± SD | p | M ± SD | p | M ± SD | p |
| City > 100 K | 24 (29.6) | 36.58 ± 4.32 | 0.03 | 25.96 ± 2.27 | 0.17 | 18.79 ± 2.45 | 0.89 |
| City 10–100 K | 23 (28.4) | 33.43 ± 5.67 | 26.13 ± 3.29 | 18.78 ± 3.07 | |||
| City < 10 K | 13 (16.0) | 38.85 ± 7.21 | 27.77 ± 3.19 | 19.38 ± 3.33 | |||
| Village | 21 (25.9) | 35.19 ± 4.43 | 25.38 ± 3.37 | 18.62 ± 2.80 | |||
| Marital Status | |||||||
| Divorced | 9 (11.1) | 33.00 ± 7.18 | 0.27 | 27.22 ± 2.82 | 0.6 | 19.44 ± 2.24 | 0.66 |
| Married | 23 (28.4) | 35.43 ± 4.17 | 26.43 ± 3.59 | 18.35 ± 2.67 | |||
| Single | 11 (13.6) | 34.73 ± 4.94 | 25.91 ± 2.59 | 18.45 ± 2.73 | |||
| Widowed | 38 (46.9) | 36.76 ± 5.85 | 25.79 ± 2.95 | 19.11 ± 3.10 | |||
| Employment Status | |||||||
| Pension | 4 (4.9) | 33.75 ± 9.54 | 0.49 | 25.75 ± 4.03 | 0.89 | 17.50 ± 5.00 | 0.68 |
| Unemployed | 5 (6.2) | 38.20 ± 2.17 | 25.20 ± 1.92 | 18.00 ± 1.87 | |||
| Retirement pension | 43 (53.1) | 36.14 ± 6.17 | 26.28 ± 3.22 | 19.02 ± 3.14 | |||
| Employed | 29 (35.8) | 34.86 ± 4.06 | 26.17 ± 2.99 | 18.90 ± 2.13 | |||
| Education | |||||||
| Higher | 15 (18.5) | 33.93 ± 2.91 | 0.09 | 26.33 ± 2.72 | 0.86 | 19.53 ± 2.26 | 0.29 |
| Primary | 11 (13.6) | 38.73 ± 5.87 | 26.64 ± 3.56 | 19.91 ± 1.81 | |||
| Secondary | 36 (44.4) | 34.89 ± 5.66 | 25.83 ± 2.40 | 18.31 ± 2.85 | |||
| Vocational | 19 (23.5) | 36.84 ± 5.99 | 26.32 ± 4.19 | 18.68 ± 3.51 | |||
| Variable | Coping | Interdependence | CAPS total | ||||
| Place of Residence | |||||||
| City > 100 K | 24 (29.6) | 24.50 ± 2.50 | 0.36 | 30.63 ± 3.61 | 0.22 | 136.46 ± 9.71 | 0.24 |
| City 10–100 K | 23 (28.4) | 24.87 ± 4.59 | 32.04 ± 4.60 | 135.26 ± 15.53 | |||
| City < 10 K | 13 (16.0) | 25.69 ± 4.55 | 32.08 ± 6.06 | 143.77 ± 21.46 | |||
| Village | 21 (25.9) | 23.48 ± 2.99 | 30.86 ± 3.93 | 133.52 ± 13.01 | |||
| Marital Status | |||||||
| Divorced | 9 (11.1) | 25.33 ± 3.71 | 0.82 | 33.56 ± 4.33 | 0.27 | 138.56 ± 11.75 | 0.78 |
| Married | 23 (28.4) | 24.00 ± 3.84 | 30.61 ± 4.53 | 134.83 ± 15.77 | |||
| Single | 11 (13.6) | 24.73 ± 3.69 | 30.09 ± 4.59 | 133.91 ± 15.04 | |||
| Widowed | 38 (46.9) | 24.61 ± 3.66 | 31.58 ± 4.23 | 137.84 ± 14.90 | |||
| Employment Status | |||||||
| Pension | 4 (4.9) | 24.25 ± 5.68 | 0.35 | 30.25 ± 7.50 | 0.69 | 131.50 ± 20.04 | 0.89 |
| Unemployed | 5 (6.2) | 24.20 ± 2.17 | 31.60 ± 3.78 | 137.20 ± 8.04 | |||
| Retirement pension | 43 (53.1) | 23.93 ± 3.99 | 30.88 ± 4.56 | 136.26 ± 16.91 | |||
| Employed | 29 (35.8) | 25.52 ± 2.98 | 32.07 ± 3.87 | 137.52 ± 11.47 | |||
| Education | |||||||
| Higher | 15 (18.5) | 25.47 ± 2.70 | 0.62 | 32.27 ± 3.37 | 0.16 | 137.53 ± 10.20 | 0.24 |
| Primary | 11 (13.6) | 24.64 ± 2.69 | 32.45 ± 3.08 | 142.36 ± 12.75 | |||
| Secondary | 36 (44.4) | 24.00 ± 4.05 | 30.08 ± 4.27 | 133.11 ± 14.38 | |||
| Vocational | 19 (23.5) | 24.74 ± 4.12 | 32.26 ± 5.57 | 138.84 ± 18.36 | |||
| Variable | Parameter | Whole Group (n = 81) | Ovarian Cancer (n = 23) | Uterine Cancer (n = 58) |
|---|---|---|---|---|
| CAPS Total Score | r | 0.02 | 0.01 | 0.02 |
| p | 0.84 | 0.95 | 0.91 | |
| Interdependence | r | 0.15 | 0.09 | 0.16 |
| p | 0.18 | 0.69 | 0.22 | |
| Physiological Dimension | r | −0.29 | −0.26 | −0.29 |
| p | 0.01 | 0.23 | 0.03 | |
| Roles in Society | r | 0.08 | −0.19 | 0.14 |
| p | 0.49 | 0.39 | 0.29 | |
| Self-concept | r | 0.18 | 0.22 | 0.15 |
| p | 0.11 | 0.31 | 0.27 | |
| Coping | r | 0.13 | 0.21 | 0.08 |
| p | 0.25 | 0.34 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieder-Huszla, S.; Owsianowska, J.; Chudecka-Głaz, A.; Branecka-Woźniak, D.; Jurczak, A. The Significance of Adaptation and Coping with Disease among Patients with Diagnosed Gynaecological Cancer in the Context of Disease Acceptance. Int. J. Environ. Res. Public Health 2022, 19, 7218. https://doi.org/10.3390/ijerph19127218
Wieder-Huszla S, Owsianowska J, Chudecka-Głaz A, Branecka-Woźniak D, Jurczak A. The Significance of Adaptation and Coping with Disease among Patients with Diagnosed Gynaecological Cancer in the Context of Disease Acceptance. International Journal of Environmental Research and Public Health. 2022; 19(12):7218. https://doi.org/10.3390/ijerph19127218
Chicago/Turabian StyleWieder-Huszla, Sylwia, Joanna Owsianowska, Anita Chudecka-Głaz, Dorota Branecka-Woźniak, and Anna Jurczak. 2022. "The Significance of Adaptation and Coping with Disease among Patients with Diagnosed Gynaecological Cancer in the Context of Disease Acceptance" International Journal of Environmental Research and Public Health 19, no. 12: 7218. https://doi.org/10.3390/ijerph19127218







