Electrostatic Spray Disinfection Using Nano-Engineered Solution on Frequently Touched Surfaces in Indoor and Outdoor Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Frequently Touched Surfaces
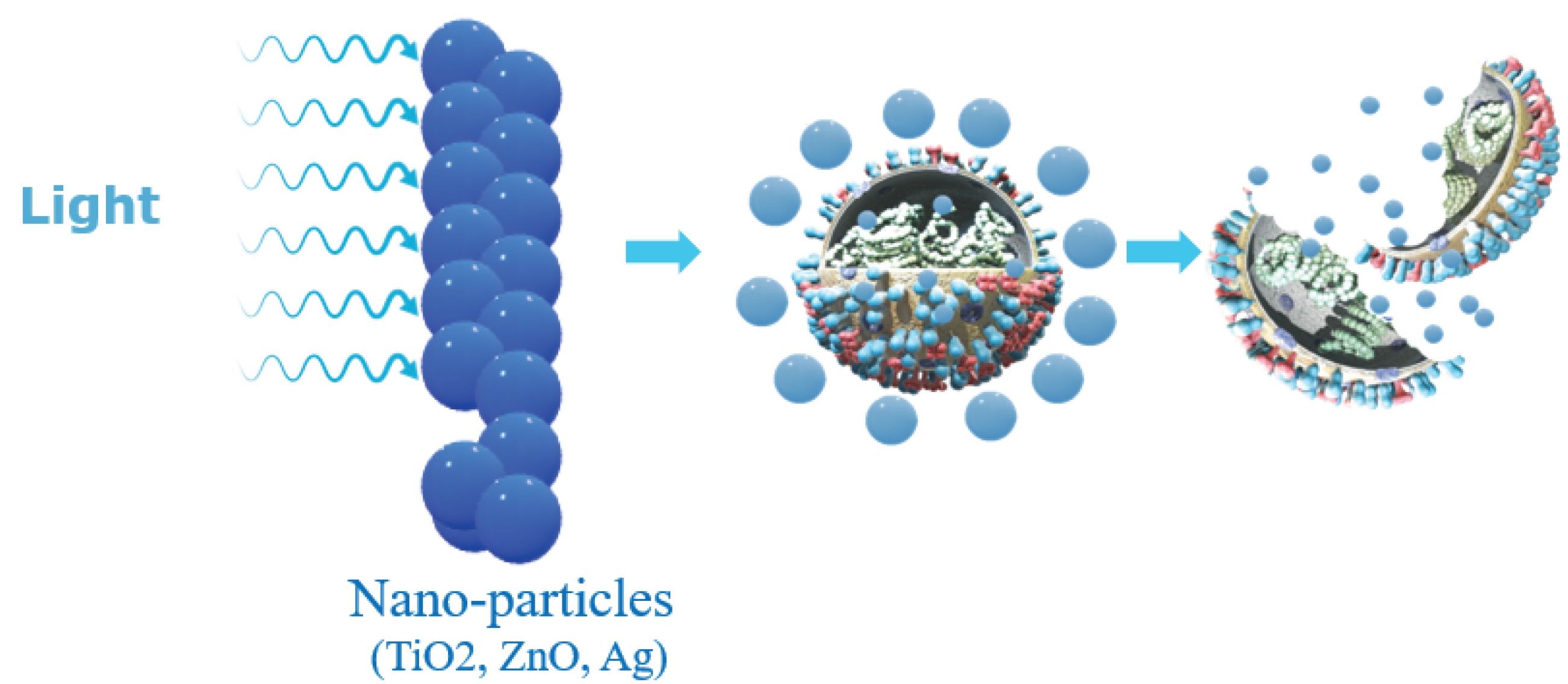
2.2. Nanoparticle-Based Disinfectant
2.3. Prototype Design
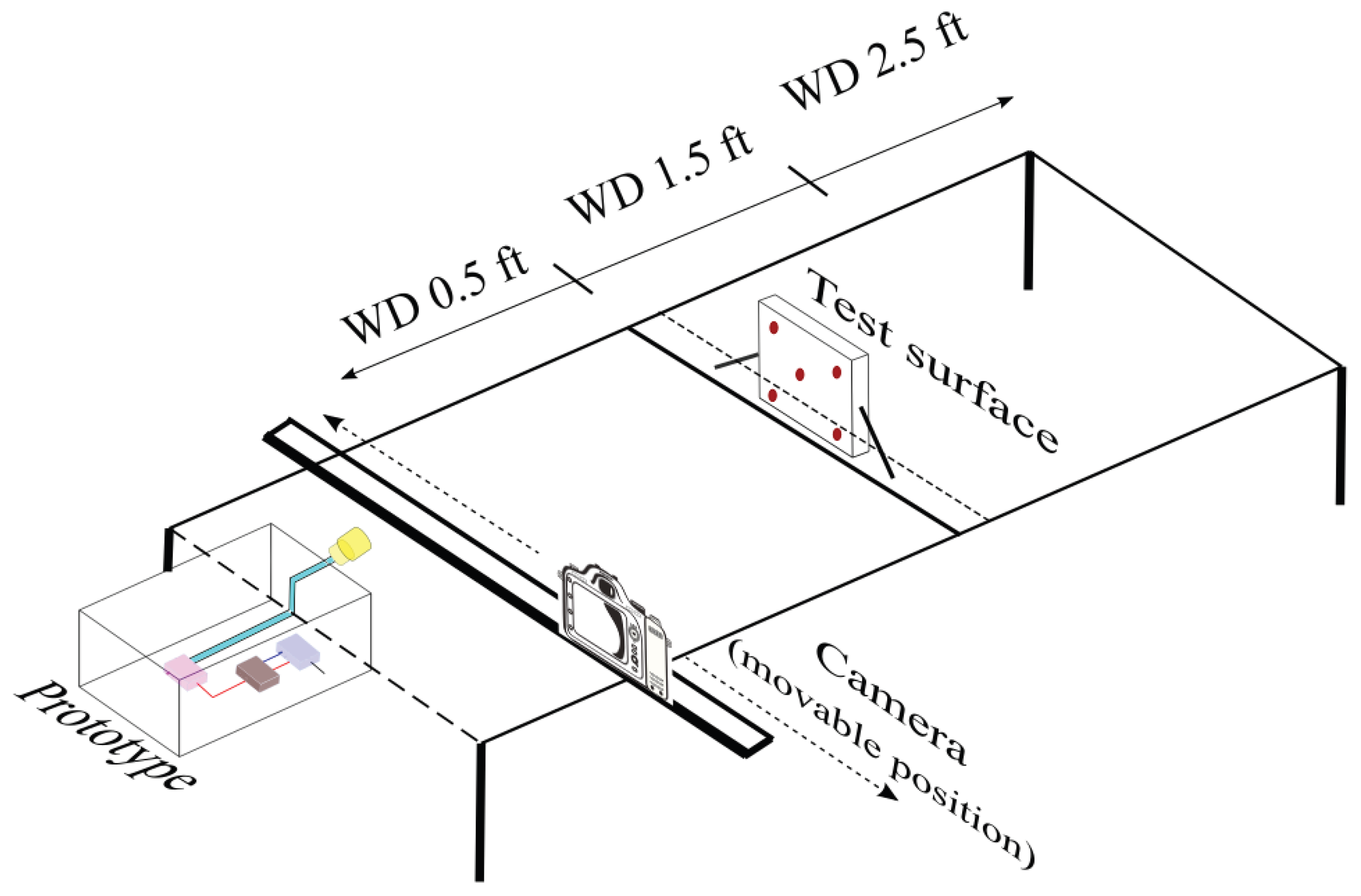
2.4. Experimental Design
2.4.1. Scanning Electron Microscopy Setup
2.4.2. Electrostatic Spray System Setup
3. Results
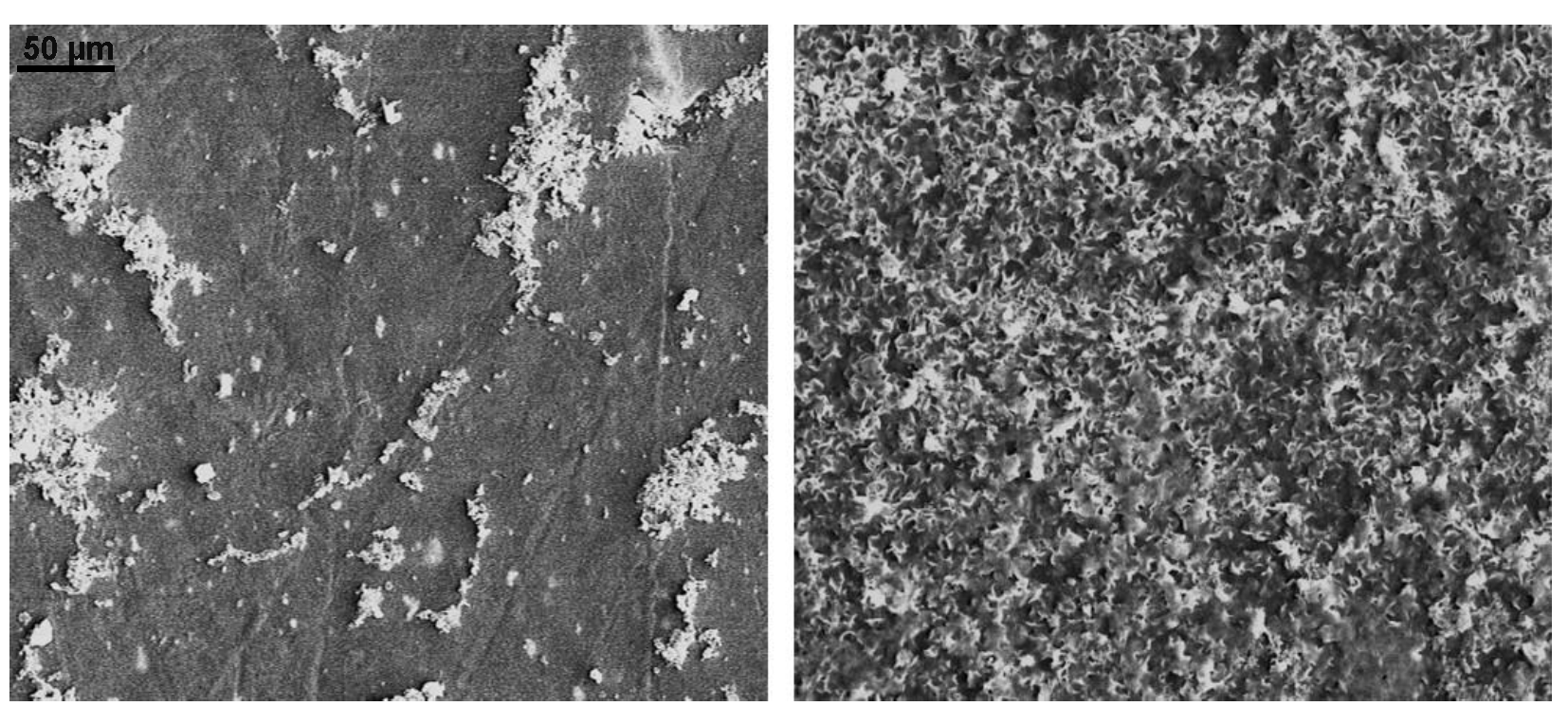
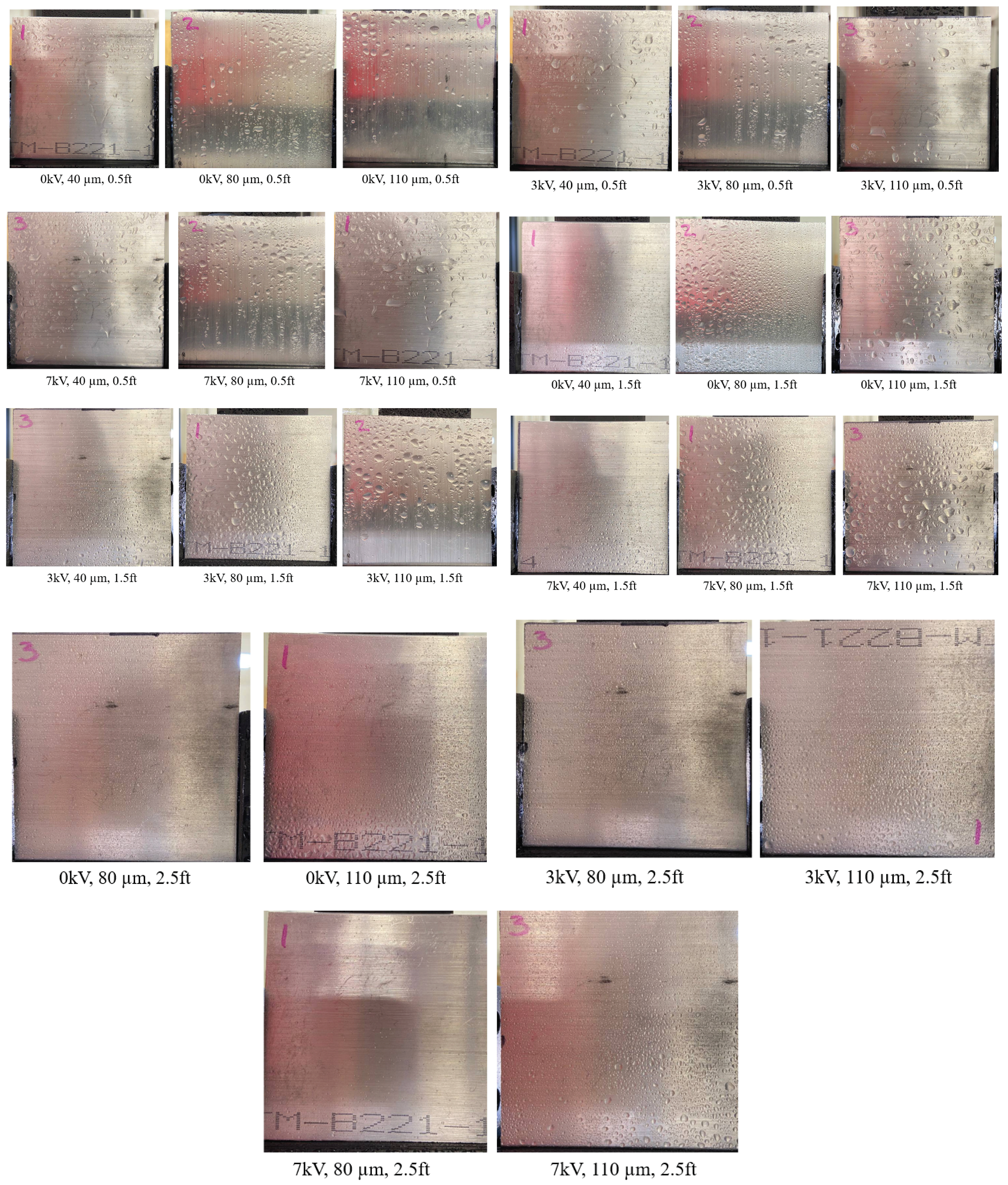
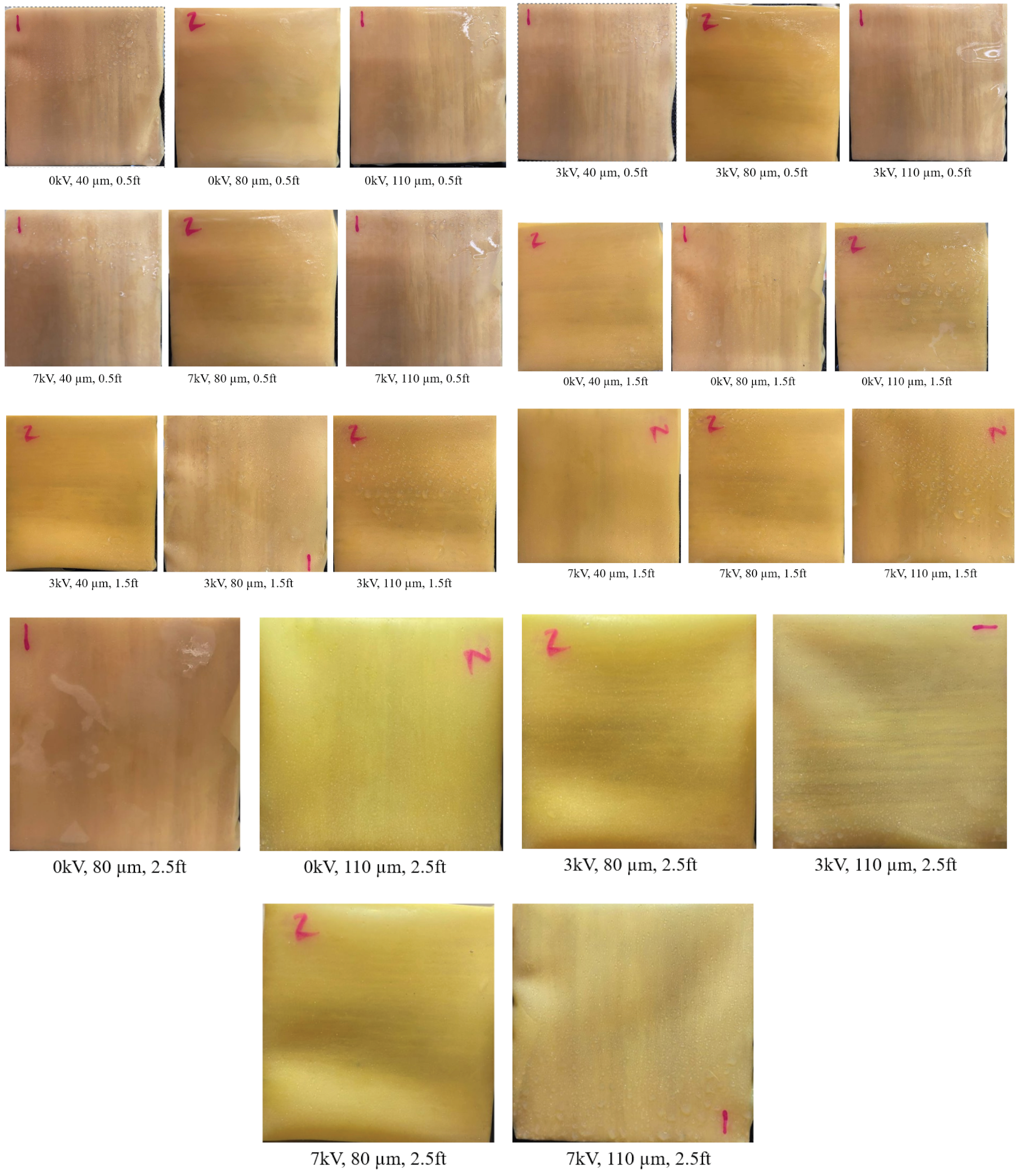
3.1. SEM Images of Electrostatic vs. Traditional Spray Deposition
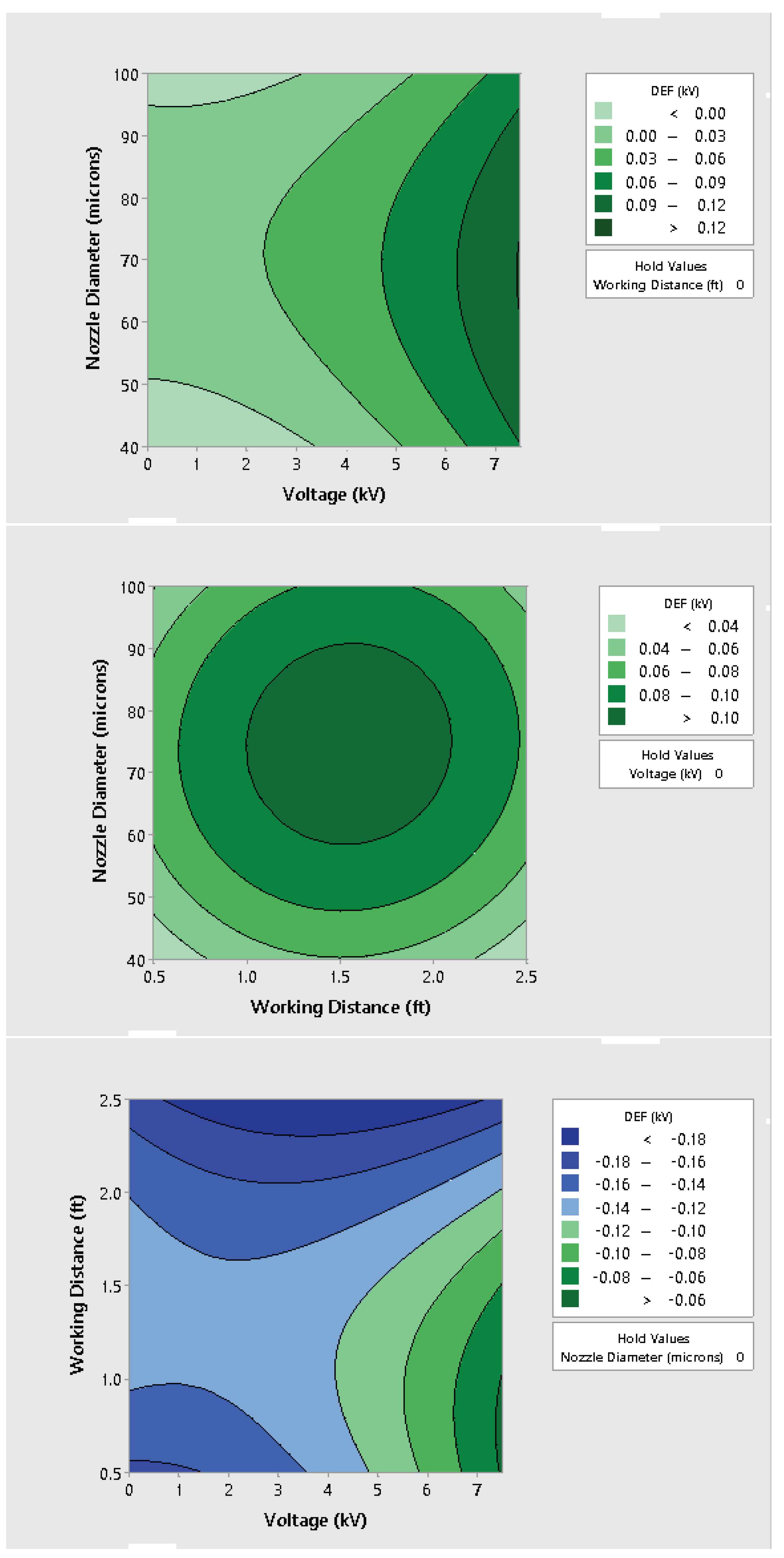
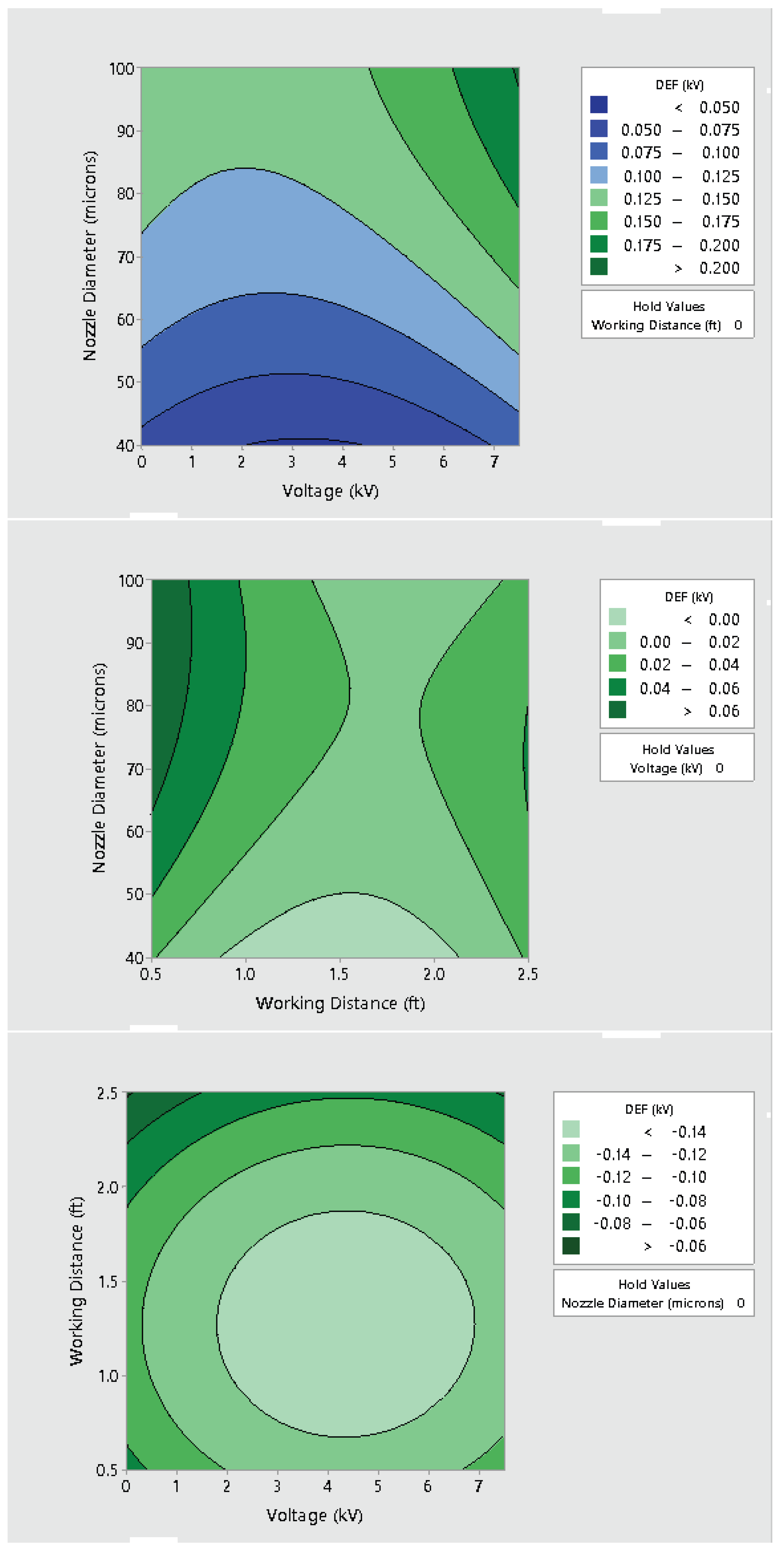
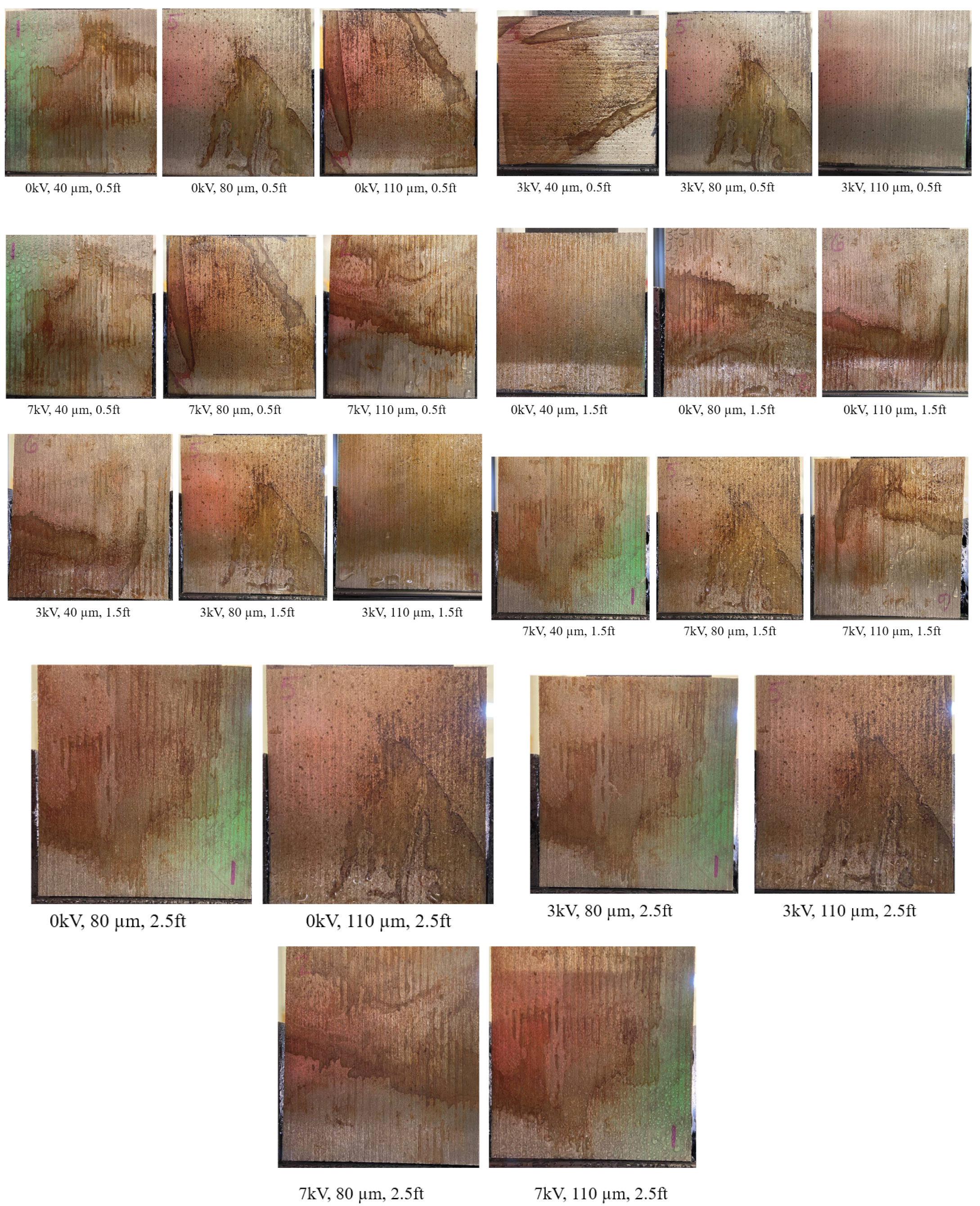
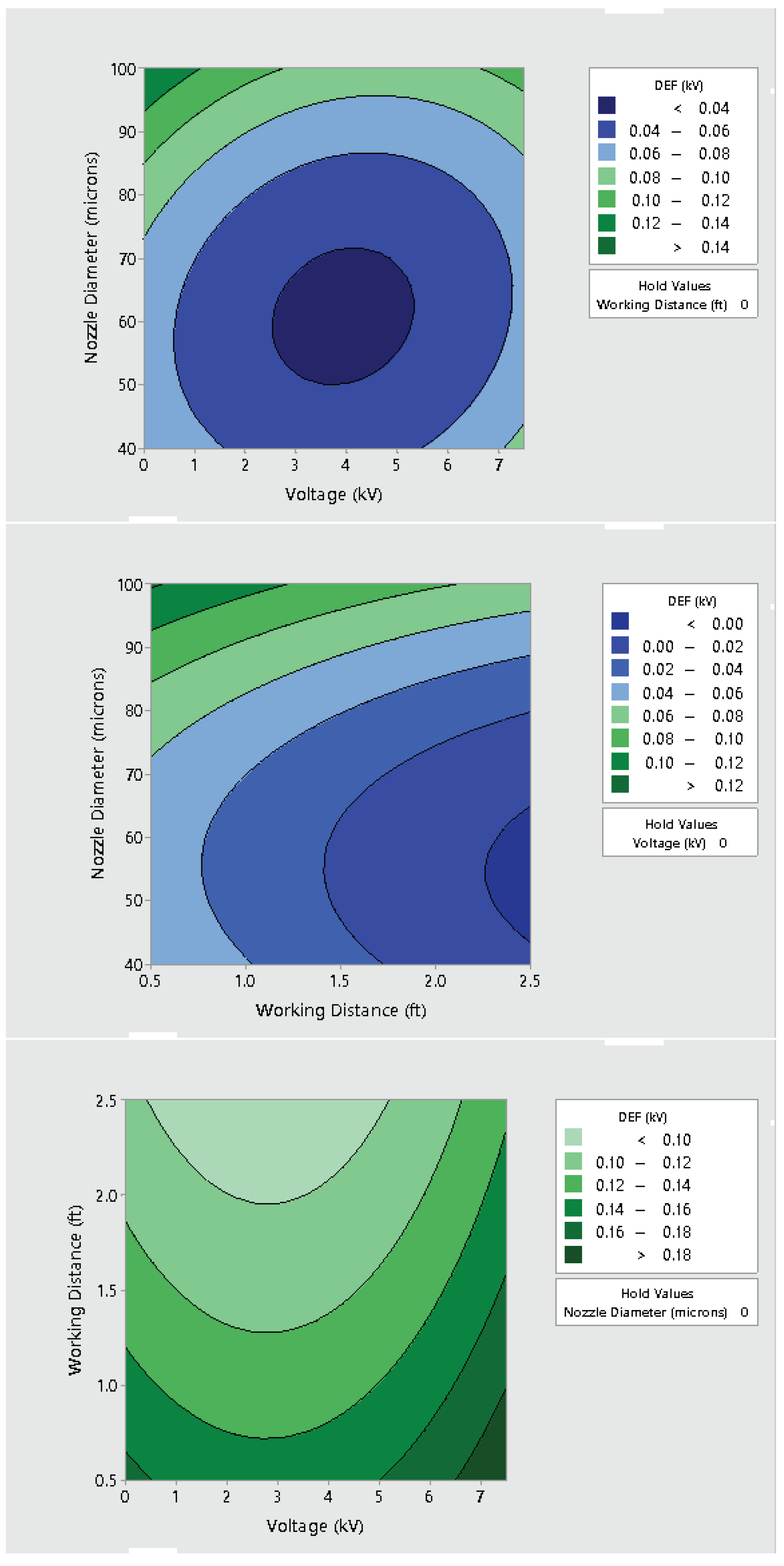
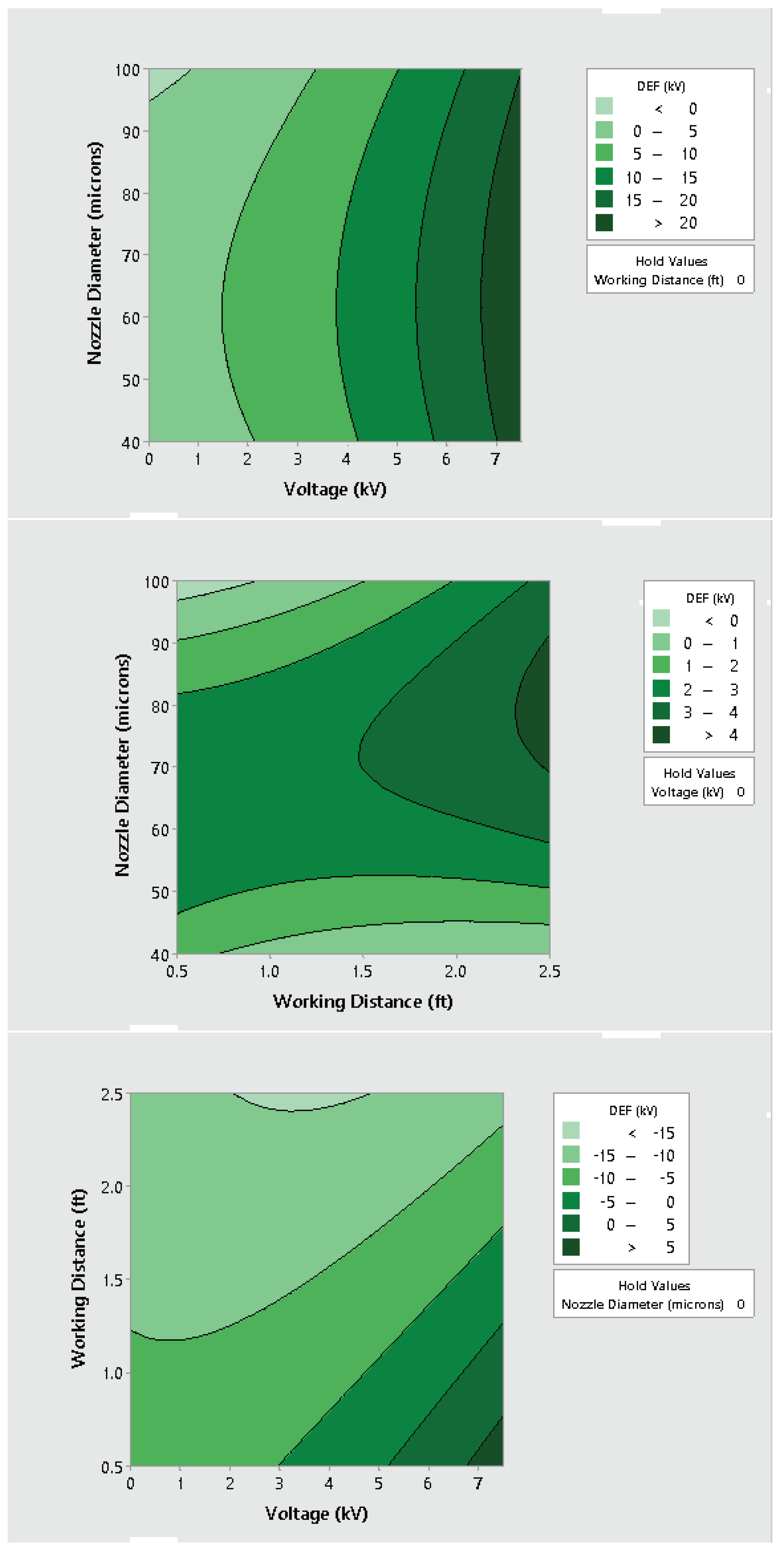
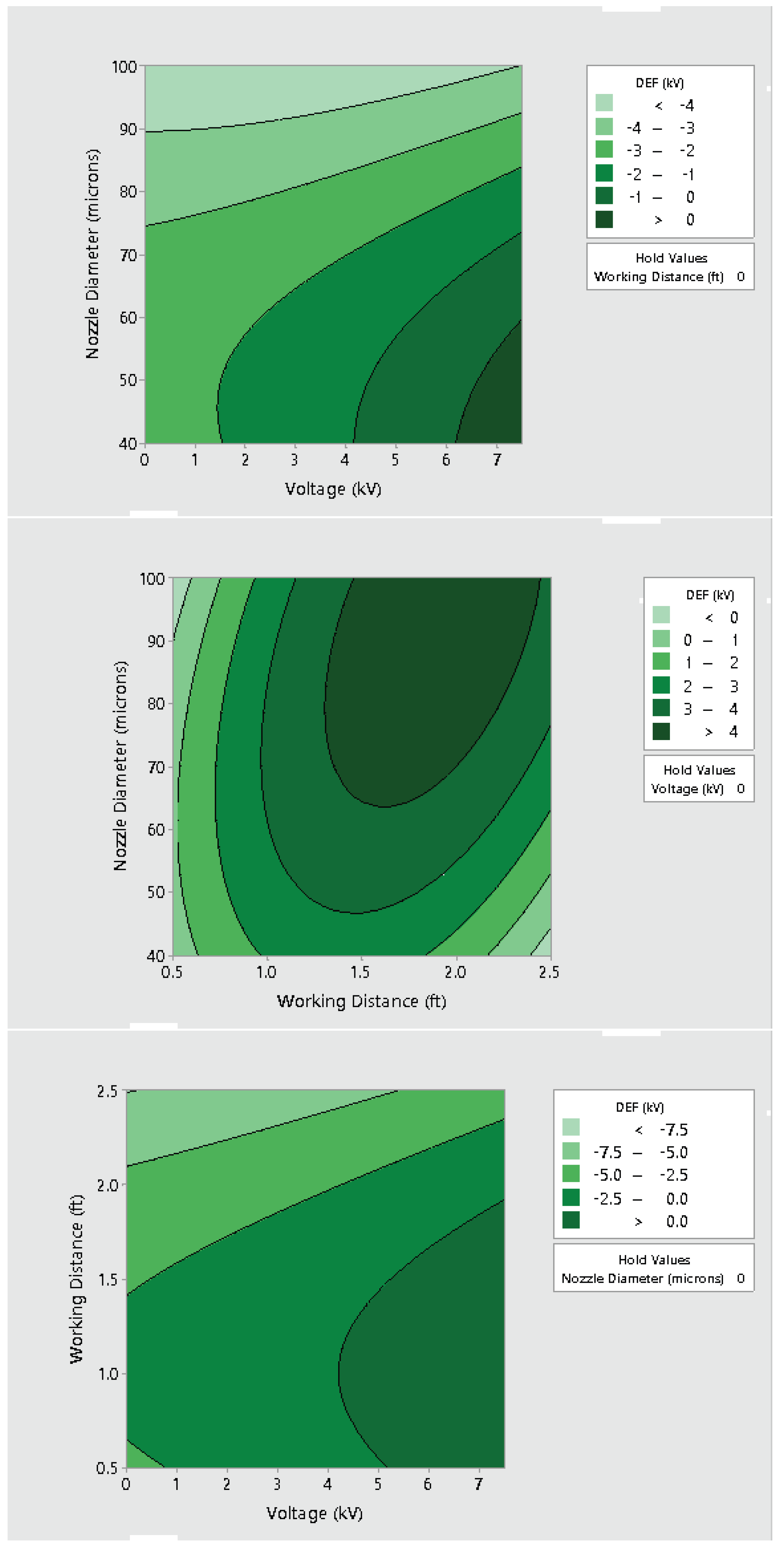
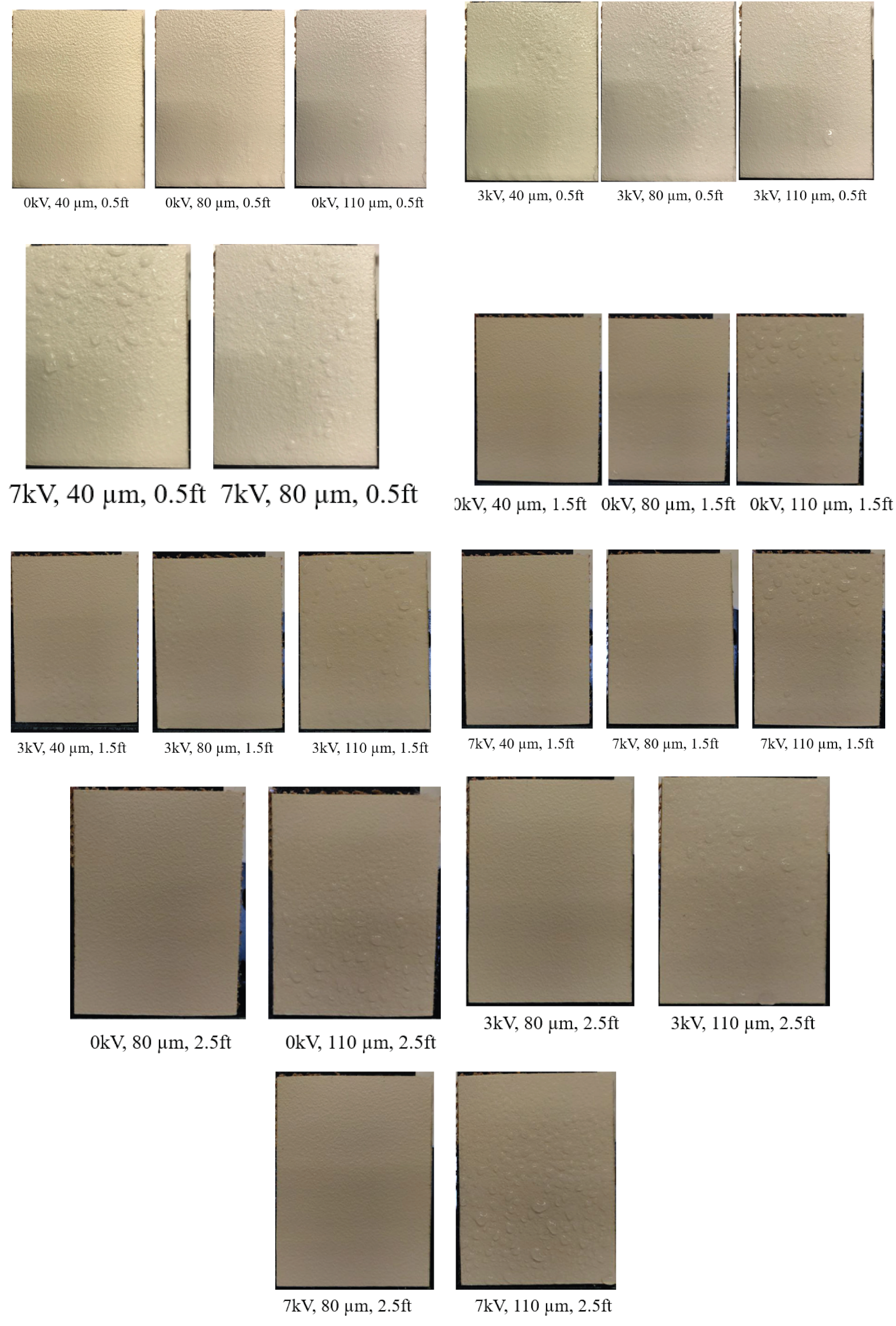
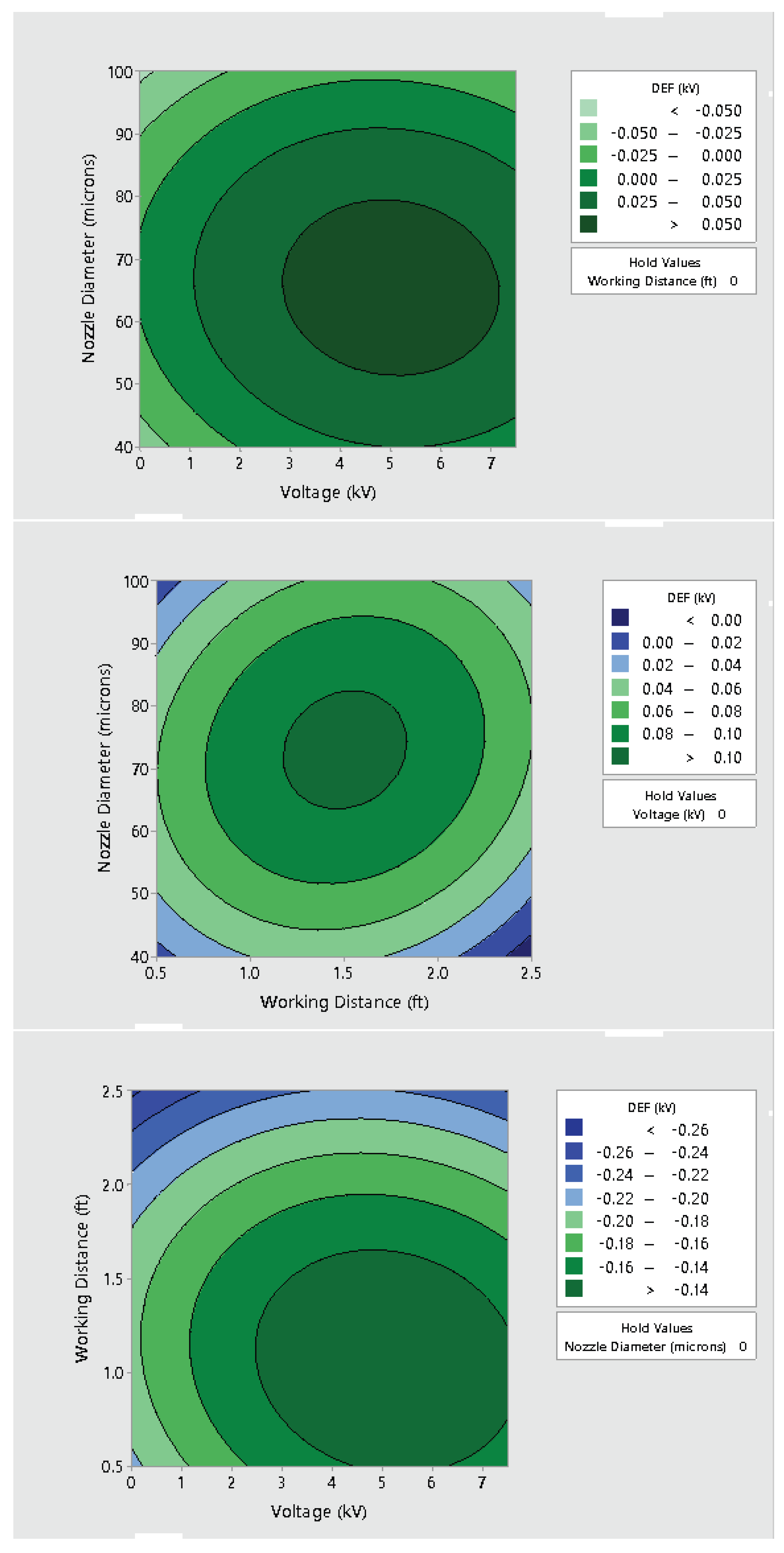
3.2. Effect of Varying Voltage, Nozzle Size and Working Distance
4. Discussion
- Using the electrostatic deposition of a nanoparticle-based solution can be a highly efficient strategy for disinfection. Previous studies have highlighted the exclusive use of both of these technologies; however, we have tried to show how the combination of both can play an important role in future disinfection strategies;
- Electrostatic spray system parameters play a crucial role in the effective spread of the disinfectant on the surfaces. The uniform spread of nanoparticles can be assured and highly optimized by tuning the controllable system settings as shown in this study;
- Each target substrate is unique due to the surface properties and environmental factors. It is very crucial to take these into account when designing a strategy for disinfection, as shown in this study. This can be very effective in ensuring proper decontamination as well as in automating the process.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



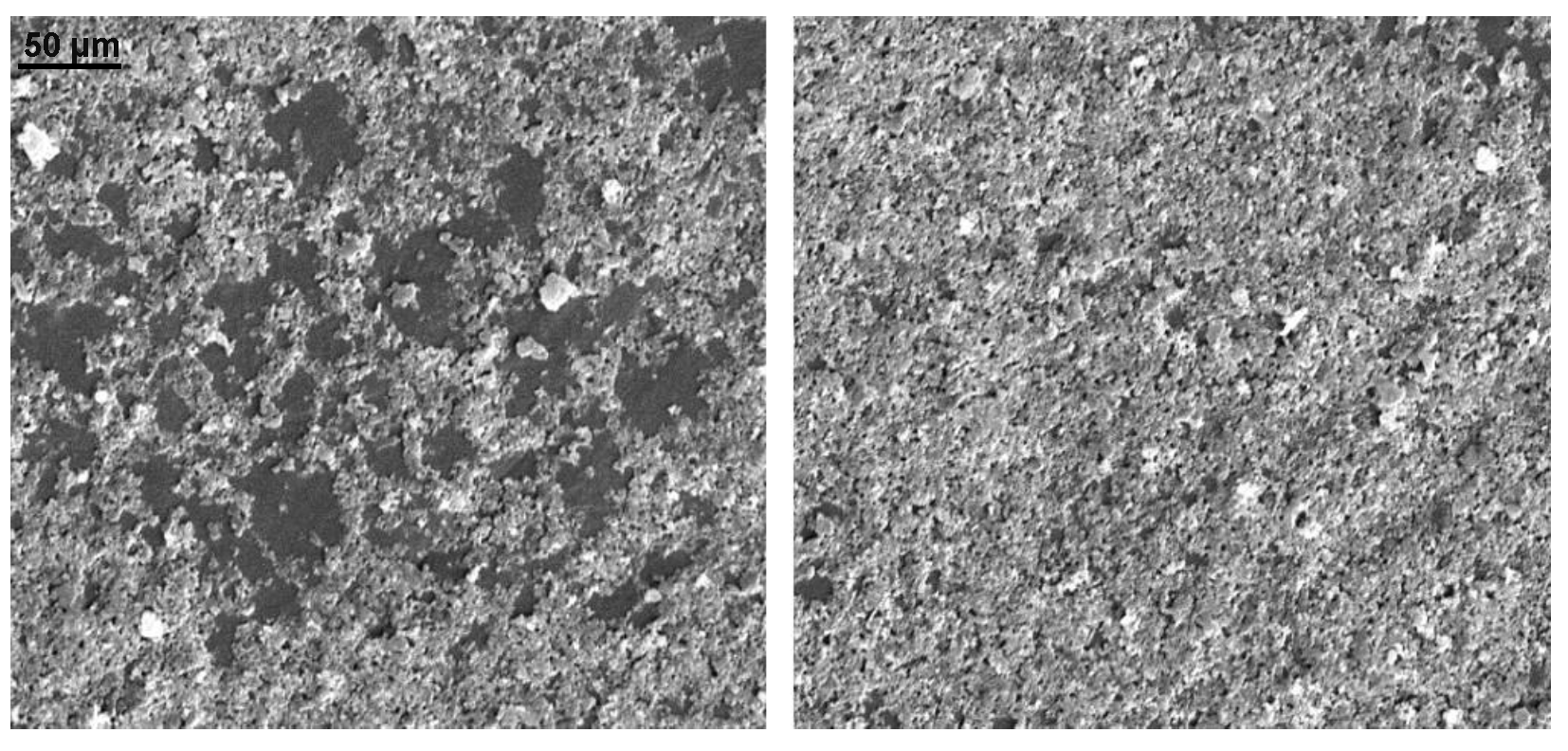
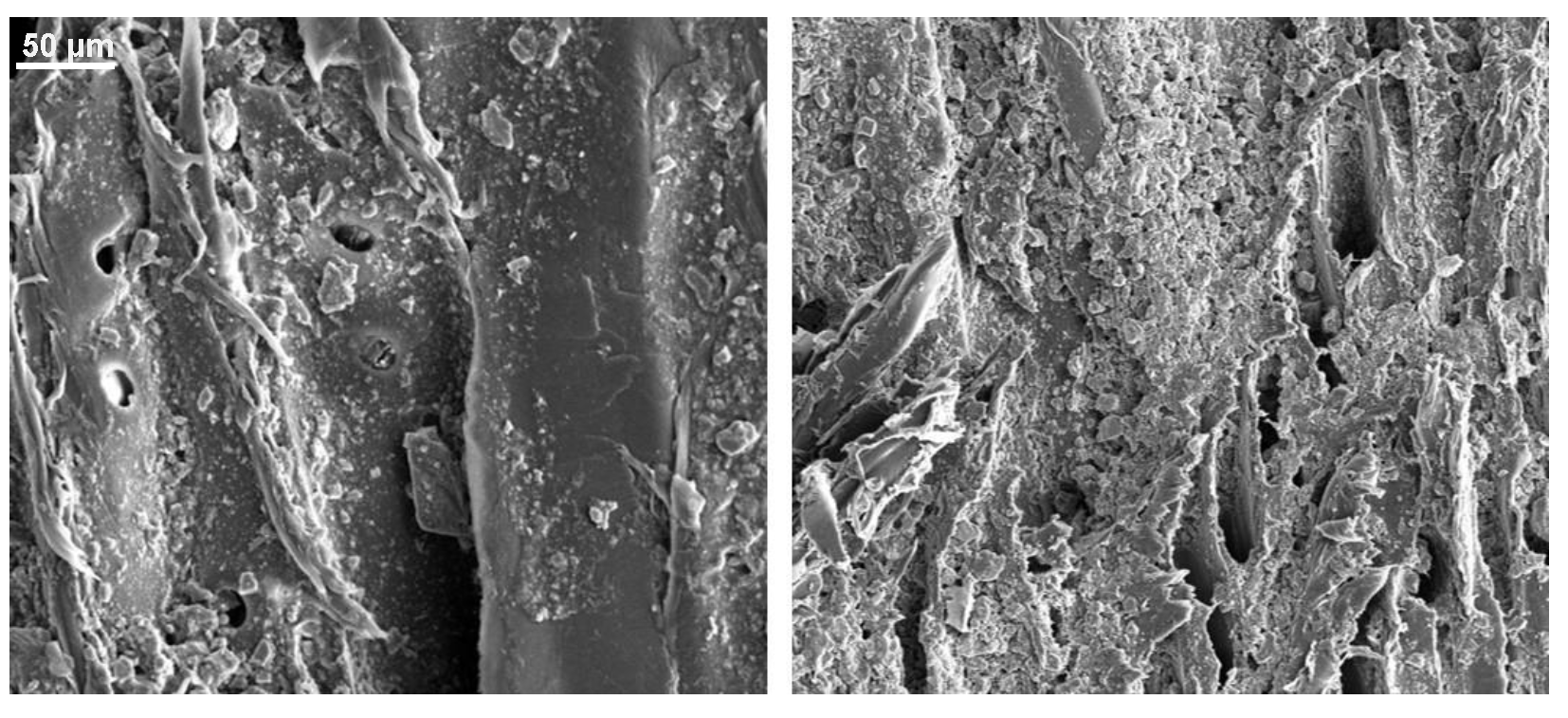
Appendix B














References
- Paul, E.; Brown, G.W.; Ridde, V. COVID-19: Time for paradigm shift in the nexus between local, national and global health. BMJ Glob. Health 2020, 5, e002622. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; Seminari, E.; Novati, S.; Asperges, E.; Biscarini, S.; Piralla, A.; Percivalle, E.; Cassaniti, I.; Baldanti, F.; Bruno, R.; et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin. Microbiol. Infect. 2020, 26, 1094.e1. [Google Scholar] [CrossRef]
- Van Damme, W.; Dahake, R.; Delamou, A.; Ingelbeen, B.; Wouters, E.; Vanham, G.; Van De Pas, R.; Dossou, J.P.; Ir, P.; Abimbola, S.; et al. The COVID-19 pandemic: Diverse contexts; different epidemics—How and why? BMJ Glob. Health 2020, 5, e003098. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Chiao, E.Y.; Amirian, E.S. Why contact tracing efforts have failed to curb coronavirus disease 2019 (COVID-19) transmission in much of the United States. Clin. Infect. Dis. 2021, 72, e415–e419. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przekwas, A.; Chen, Z. Washing hands and the face may reduce COVID-19 infection. Med. Hypotheses 2020, 144, 110261. [Google Scholar] [CrossRef]
- Rusin, P.; Maxwell, S.; Gerba, C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 2002, 93, 585–592. [Google Scholar] [CrossRef]
- Boone, S.A.; Gerba, C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Huslage, K.; Rutala, W.A.; Sickbert-Bennett, E.; Weber, D.J. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect. Control Hosp. Epidemiol. 2010, 31, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef] [Green Version]
- Pancic, F.; Carpentier, D.; Came, P. Role of infectious secretions in the transmission of rhinovirus. J. Clin. Microbiol. 1980, 12, 567–571. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Jiang, J. COVID-19 and social distancing. J. Public Health 2020, 30, 259–261. [Google Scholar] [CrossRef]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef]
- Barrett, C.; Cheung, K.L. Knowledge, socio-cognitive perceptions and the practice of hand hygiene and social distancing during the COVID-19 pandemic: A cross-sectional study of UK university students. BMC Public Health 2021, 21, 426. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef]
- Springthorpe, V.S.; Sattar, S.A. Chemical disinfection of virus-contaminated surfaces. Crit. Rev. Environ. Sci. Technol. 1990, 20, 169–229. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Vasickova, P.; Pavlik, I.; Verani, M.; Carducci, A. Issues concerning survival of viruses on surfaces. Food Environ. Virol. 2010, 2, 24–34. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.Y.; Cheng, P.K.; Lim, W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, D.; Chattopadhyay, S.; Lyon, W.G.; Wilson, J.T. Effect of surfactants on the survival and sorption of viruses. Environ. Sci. Technol. 2002, 36, 4017–4024. [Google Scholar] [CrossRef]
- Velez, K.; Healthcare, C. Evaluating Electrostatic Sprayers for Surface Disinfection. Available online: https://www.epa.gov/covid19-research/evaluating-electrostatic-sprayers-disinfectant-application (accessed on 2 June 2022).
- Cadnum, J.L.; Jencson, A.L.; Livingston, S.H.; Li, D.F.; Redmond, S.N.; Pearlmutter, B.; Wilson, B.M.; Donskey, C.J. Evaluation of an electrostatic spray disinfectant technology for rapid decontamination of portable equipment and large open areas in the era of SARS-CoV-2. Am. J. Infect. Control 2020, 48, 951–954. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Mujawar, L.H.; Schutyser, M.A.; Schroën, K.; Boom, R. Deposition of thin lipid films prepared by electrospraying. Food Bioprocess Technol. 2013, 6, 3047–3055. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Jaworek, A. Electrostatic micro-and nanoencapsulation and electroemulsification: A brief review. J. Microencapsul. 2008, 25, 443–468. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of electrode voltage, liquid flow rate, and liquid properties on spray chargeability of an air-assisted electrostatic-induction spray-charging system. J. Electrost. 2010, 68, 152–158. [Google Scholar] [CrossRef]
- Luo, C.; Loh, S.; Stride, E.; Edirisinghe, M. Electrospraying and electrospinning of chocolate suspensions. Food Bioprocess Technol. 2012, 5, 2285–2300. [Google Scholar] [CrossRef]
- Oh, H.; Kim, K.; Kim, S. Characterization of deposition patterns produced by twin-nozzle electrospray. J. Aerosol Sci. 2008, 39, 801–813. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, L.; Nyandoto, G.; Malshe, A.P. Electrostatic spray deposition of nanostructured hydroxyapatite coating for biomedical applications. J. Manuf. Sci. Eng. 2008, 130. [Google Scholar] [CrossRef]
- Hemphill, R.J. Deposition of Barium Titanate Nanoparticles by Electrostatic Spray Coating; University of Arkansas: Fayetteville, AR, USA, 2007. [Google Scholar]
- Koivisto, A.J.; Jensen, A.C.; Kling, K.I.; Kling, J.; Budtz, H.C.; Koponen, I.K.; Tuinman, I.; Hussein, T.; Jensen, K.A.; Nørgaard, A.; et al. Particle emission rates during electrostatic spray deposition of TiO2 nanoparticle-based photoactive coating. J. Hazard. Mater. 2018, 341, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, E.V.; Pereira, A.E.; De Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 125. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Sai Krishna, M.; Nalluru, S.; Sampath Kumar, N.S. Nanotechnology: An emerging approach to combat COVID-19. Emerg. Mater. 2021, 4, 119–130. [Google Scholar] [CrossRef]
- Behbudi, G. Effect of silver nanoparticles disinfectant on covid-19. Adv. Appl. NanoBio-Technol. 2021, 2, 63–67. [Google Scholar]
- Agathokleous, E.; Barceló, D.; Iavicoli, I.; Tsatsakis, A.; Calabrese, E.J. Disinfectant-induced hormesis: An unknown environmental threat of the application of disinfectants to prevent SARS-CoV-2 infection during the COVID-19 pandemic? Environ. Pollut. 2022, 292, 118429. [Google Scholar] [CrossRef]
- Robles-García, M.A.; Francisco, R.F.; Márquez-Ríos, E.; Barrera-Rodríguez, A.; Aguilar-Martínez, J.; Del toro Sánchez, C.L. Aplicaciones biomédicas, textiles y alimentarias de nanoestructuras elaboradas por electrohilado. Biotecnia 2014, 16, 44–52. [Google Scholar] [CrossRef]
- Singh, P.; Singh, D.; Sa, P.; Mohapatra, P.; Khuntia, A.; K Sahoo, S. Insights from nanotechnology in COVID-19: Prevention, detection, therapy and immunomodulation. Nanomedicine 2021, 16, 1219–1235. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Cajuba de Britto Lira Nogueira, M. Pharmaceutical nanotechnology: Which products are been designed against COVID-19? J. Nanopart. Res. 2020, 22, 276. [Google Scholar] [CrossRef]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Alsawat, M.; Al-Salmi, F.A.; Hamza, R.Z. Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—” ZnO nanoparticles have antiviral activity against (SARS-CoV-2)”. Coatings 2021, 11, 388. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.; Mullani, S.; Delekar, S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar]
- Malika, M.; Sonawane, S.S. Review on application of nanofluid/nano particle as water disinfectant. J. Indian Assoc. Environ. Manag. (JIAEM) 2019, 39, 21–24. [Google Scholar]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K.K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Wang, N.; Ferhan, A.R.; Yoon, B.K.; Jackman, J.A.; Cho, N.J.; Majima, T. Chemical design principles of next-generation antiviral surface coatings. Chem. Soc. Rev. 2021, 50, 9741–9765. [Google Scholar] [CrossRef]
- Botequim, D.; Maia, J.; Lino, M.; Lopes, L.; Simões, P.; Ilharco, L.; Ferreira, L. Nanoparticles and surfaces presenting antifungal, antibacterial and antiviral properties. Langmuir 2012, 28, 7646–7656. [Google Scholar] [CrossRef]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-based antimicrobial and antiviral surface coating strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Liu, Q.; Kordbacheh, D. Biomedical Applications of Antiviral Nanohybrid Materials Relating to the COVID-19 Pandemic and Other Viral Crises. Polymers 2021, 13, 2833. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, antiviral, and self-cleaning mats with sensing capabilities based on electrospun nanofibers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, M.; Abuhasel, K.; Khadr, M.; Hosni, F.; Alquraish, M. Surface disinfection to protect against microorganisms: Overview of traditional methods and issues of emergent nanotechnologies. Appl. Sci. 2020, 10, 6040. [Google Scholar] [CrossRef]
- Choi, H.; Chatterjee, P.; Lichtfouse, E.; Martel, J.A.; Hwang, M.; Jinadatha, C.; Sharma, V.K. Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: A review. Environ. Chem. Lett. 2021, 19, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Levitt, A.M. Preventing emerging infectious diseases: A strategy for the 21st century: Overview of the updated CDC plan. MMWR Recomm. Rep. 1998, 47, 1–14. [Google Scholar]
- Ryu, W.S. Molecular Virology of Human Pathogenic Viruses; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Carraturo, F.; Del Giudice, C.; Morelli, M.; Cerullo, V.; Libralato, G.; Galdiero, E.; Guida, M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020, 265, 115010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Chau, P.; Lee, W.; Ho, S.; Lee, D.; So, S.; Wong, S.; Tai, J.; Yuen, K. Hand-touch contact assessment of high-touch and mutual-touch surfaces among healthcare workers, patients, and visitors. J. Hosp. Infect. 2015, 90, 220–225. [Google Scholar] [CrossRef]
- Kundrapu, S.; Sunkesula, V.; Jury, L.A.; Sitzlar, B.M.; Donskey, C.J. Daily disinfection of high-touch surfaces in isolation rooms to reduce contamination of healthcare workers’ hands. Infect. Control Hosp. Epidemiol. 2012, 33, 1039–1042. [Google Scholar] [CrossRef]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Powers, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environ. Sci. Technol. Lett. 2020, 8, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Marzoli, F.; Bortolami, A.; Pezzuto, A.; Mazzetto, E.; Piro, R.; Terregino, C.; Bonfante, F.; Belluco, S. A systematic review of human coronaviruses survival on environmental surfaces. Sci. Total. Environ. 2021, 778, 146191. [Google Scholar] [CrossRef]
- Bueckert, M.; Gupta, R.; Gupta, A.; Garg, M.; Mazumder, A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: Potential for indirect transmission. Materials 2020, 13, 5211. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: Role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005, 43, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT-Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Mouritz, A.P.; Galos, J.; Linklater, D.P.; Ladani, R.B.; Kandare, E.; Crawford, R.J.; Ivanova, E.P. Towards antiviral polymer composites to combat COVID-19 transmission. Nano Sel. 2021, 2, 2061–2071. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Bonde, S.; Yadav, A.; Bhowmik, A.; Rathod, S.; Ingle, P.; Gade, A. Nanotechnology as a shield against COVID-19: Current advancement and limitations. Viruses 2021, 13, 1224. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Abolghasemi, S.; Parhizgari, N.; Moradpour, F. Effect of silver nanoparticles on common bacteria in hospital surfaces. Jundishapur J. Microbiol. 2013, 6, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar]
- Banerjee, S.; Mazumder, M.K. Adhesion of charged powders to a metal surface in the powder coating process. IEEE Trans. Ind. Appl. 1996, 32, 1243–1248. [Google Scholar] [CrossRef]
- Mittal, K.L. Particles on Surfaces: Detection, Adhesion and Removal; CRC Press: Boca Raton, FL, USA, 2006; Volume 9. [Google Scholar]
- Appah, S.; Wang, P.; Ou, M.; Gong, C.; Jia, W. Review of electrostatic system parameters, charged droplets characteristics and substrate impact behavior from pesticides spraying. Int. J. Agric. Biol. Eng. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Heu, R.; Shahbazmohamadi, S.; Yorston, J.; Capeder, P. Target material selection for sputter coating of SEM samples. Microsc. Today 2019, 27, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Ebrahimpour, K.; Nafez, A. Environmental disinfection against COVID-19 in different areas of health care facilities: A review. Rev. Environ. Health 2021, 36, 193–198. [Google Scholar] [CrossRef]
- Castaño, N.; Cordts, S.C.; Kurosu Jalil, M.; Zhang, K.S.; Koppaka, S.; Bick, A.D.; Paul, R.; Tang, S.K. Fomite transmission, physicochemical origin of virus–surface interactions, and disinfection strategies for enveloped viruses with applications to SARS-CoV-2. ACS Omega 2021, 6, 6509–6527. [Google Scholar] [CrossRef]
- Triggiano, F.; Caggiano, G.; Lopuzzo, M.; Diella, G.; Apollonio, F.; Fasano, F.; Montagna, M.T. No-Touch Automated Disinfection System Based on Hydrogen Peroxide and Ethyl Alcohol Aerosols for Use in Healthcare Environments. Int. J. Environ. Res. Public Health 2022, 19, 4868. [Google Scholar] [CrossRef]
- Kwak, D.B.; Kim, S.C.; Kuehn, T.H.; Pui, D.Y. Quantitative analysis of droplet deposition produced by an electrostatic sprayer on a classroom table by using fluorescent tracer. Build. Environ. 2021, 205, 108254. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Limongelli, L.; Nardone, M.; Carinci, F. Environmental disinfection strategies to prevent indirect transmission of SARS-CoV2 in healthcare settings. Appl. Sci. 2020, 10, 6291. [Google Scholar] [CrossRef]
- Chen, T.; O’Keeffe, J. COVID-19 in Indoor Environments—Air and Surface Disinfection Measures; National Collaborating Centre for Environmental Health: Vancouver, BC, Canada, 2020; pp. 1–25. [Google Scholar]
- Chen, T. Reducing COVID-19 Transmission through Cleaning and Disinfecting Household Surfaces; National Collaborating Centre for Environmental Health: Vancouver, BC, Canada, 2020. [Google Scholar]
- Tiwari, A.; Patnayak, D.P.; Chander, Y.; Parsad, M.; Goyal, S.M. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006, 50, 284–287. [Google Scholar] [CrossRef]
- Yang, H.; Balakuntala, M.V.; Quiñones, J.J.; Kaur, U.; Moser, A.E.; Doosttalab, A.; Esquivel-Puentes, A.; Purwar, T.; Castillo, L.; Ma, X.; et al. Occupant-centric robotic air filtration and planning for classrooms for Safer school reopening amid respiratory pandemics. Robot. Auton. Syst. 2022, 147, 103919. [Google Scholar] [CrossRef]


| Test Samples | Copper, Aluminum, Cast Iron, Stainless Steel, Acrylic, PVC, Polypropylene, Teflon, Rubber, Wood |
| Precursor Solution | Nanoxen |
| Sample Size | 1 × 1 |
| Nozzle Size | 40 m |
| Flow Rate | 1.5 g/s |
| Voltage | ON|OFF |
| Ion Generator Output | 7 kV |
| Working Distance | 1.5 feet |
| Time of Spray | 10 s |
| Independent Variables | Case Matrix | ||
|---|---|---|---|
| Spray Particle Size | 40 µm, cone spray | 80 µm, cone spray | 110 µm, fan |
| Working Distance | 0.5 feet | 1.5 feet | 2.5 feet |
| Charging Voltage | 0 kV | 3 ± 0.5 kV | 7 ± 0.5 kV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwar, T.; Dey, S.; Al-Kayyali, O.Z.A.; Zalar, A.F.; Doosttalab, A.; Castillo, L.; Castano, V.M. Electrostatic Spray Disinfection Using Nano-Engineered Solution on Frequently Touched Surfaces in Indoor and Outdoor Environments. Int. J. Environ. Res. Public Health 2022, 19, 7241. https://doi.org/10.3390/ijerph19127241
Purwar T, Dey S, Al-Kayyali OZA, Zalar AF, Doosttalab A, Castillo L, Castano VM. Electrostatic Spray Disinfection Using Nano-Engineered Solution on Frequently Touched Surfaces in Indoor and Outdoor Environments. International Journal of Environmental Research and Public Health. 2022; 19(12):7241. https://doi.org/10.3390/ijerph19127241
Chicago/Turabian StylePurwar, Tanya, Shamya Dey, Osama Zaid Ali Al-Kayyali, Aaron Floyd Zalar, Ali Doosttalab, Luciano Castillo, and Victor M. Castano. 2022. "Electrostatic Spray Disinfection Using Nano-Engineered Solution on Frequently Touched Surfaces in Indoor and Outdoor Environments" International Journal of Environmental Research and Public Health 19, no. 12: 7241. https://doi.org/10.3390/ijerph19127241
APA StylePurwar, T., Dey, S., Al-Kayyali, O. Z. A., Zalar, A. F., Doosttalab, A., Castillo, L., & Castano, V. M. (2022). Electrostatic Spray Disinfection Using Nano-Engineered Solution on Frequently Touched Surfaces in Indoor and Outdoor Environments. International Journal of Environmental Research and Public Health, 19(12), 7241. https://doi.org/10.3390/ijerph19127241