Solid Anorganic Particles and Chronic Rhinosinusitis: A Histopathology Study
Abstract
:1. Introduction
1.1. Definition and Difference between Fine and Ultrafine (Micro- and Nanosized) Particles
1.2. Possible Role of Solid Anorganic Particles in Chronic Rhinosinusitis
1.3. Clinical Research of MPs and NPs and the Aim of the Study
2. Materials and Methods
- -
- Age over 18 years;
- -
- Clinical diagnosis of CRS according to EPOS2012 criteria (the criteria are identical to criteria named in EPOS2020);
- -
- Endoscopically verified hypertrophy of inferior turbinates;
- -
- Insufficient response to conservative therapy (nasal corticosteroid spray administration for 6 months or longer), as subjectively assessed by the patient;
- -
- No history of turbinate surgery and/or sinonasal tumor;
- -
- Immunocompetency and aptitude for surgery under general anaesthesia;
- -
- Written informed consent obtained.
- -
- Age over 18 years.
- -
- No history of chronic inflammatory disease of sinonasal mucosa (i.e., CRS, allergic fungal rhinosinusitis, allergic rhinitis) or other significant pathologies and surgical procedures in sinonasal region during their life (for example sinonasal tumors);
- -
- No endoscopic signs of inferior turbinate hypertrophy.
2.1. Sample Acquisition and Preparation
2.2. Raman Microspectroscopy
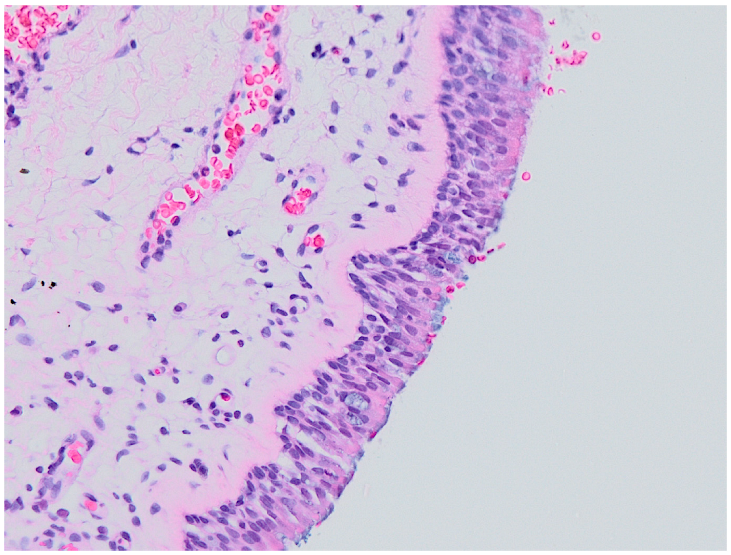
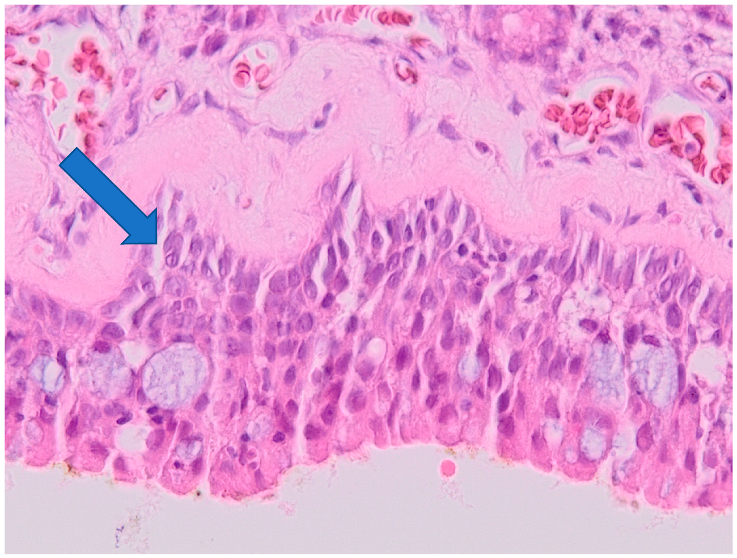
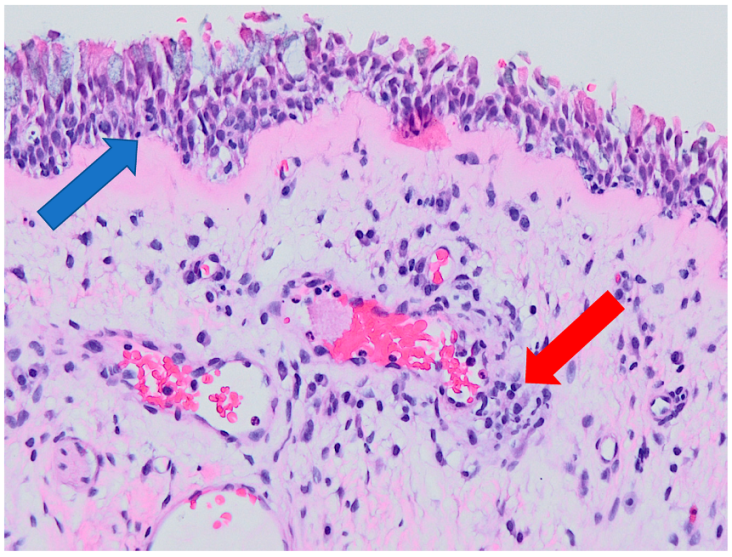
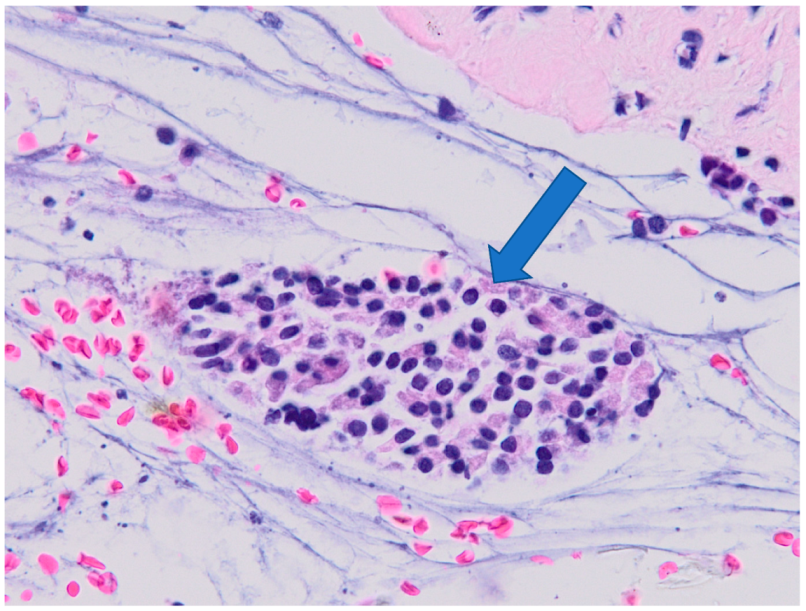
2.3. Histological Examination
- 0
- Normal histology (no inflammation);
- 1
- Epithelial hyperplasia;
- 2
- Epithelial hyperplasia with mild signs of inflammation;
- 3
- Chronic inflammation.
2.4. Correlation of Detected Compounds, Histology and Smoking and Occupational History
3. Results
3.1. Detected Compounds
3.2. Histology
3.3. Correlation of Histology and Detected Compounds
3.4. Correlation of Smoking Status and Detected Compounds
3.5. Correlation of Occupational History and Detected Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ríos, A.L.; Tejeda-Benítez, L.P.; Bustillo-Lecompte, C.F. Sources, characteristics, toxicity, and control of ultrafine particles: An overview. Geosci. Front. 2022, 13, 101147. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef] [PubMed]
- HSE. Health Effects of Particles Produced for Nanotechnologies; HSE Books EH75/6; HSE: Sudbury, UK, 2004; Volume EH75/6.
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products of Nanotechnologies. Adopted by the SCENIHR during the 10th Plenary Meeting of 10 March 2006 after Public Consultation Dostupné z. n.d. Available online: http://EcEuropaEu/Health/Ph_risk/Committees/04_scenihr/Docs/Scenihr_o_003bPdf (accessed on 12 December 2021).
- Dvorackova, J.; Bielnikova, H.; Kukutschova, J.; Peikertova, P.; Filip, P.; Zelenik, K.; Kominek, P.; Uvirova, M.; Pradna, J.; Cermakova, Z.; et al. Detection of nano- and micro-sized particles in routine biopsy material—Pilot study. Biomed. Pap. 2015, 159, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro-Riviere, N.A.; Lang, C.T. Nanotoxicology: Characterization, Dosing and Health Effects; Informa Healthcare Inc.: Wilmington, DE, USA, 2007. [Google Scholar]
- Zeleník, K.; Kukutschová, J.; Dvořáčková, J.; Bielniková, H.; Peikertová, P.; Čábalová, L.; Komínek, P. Possible role of nano-sized particles in chronic tonsillitis and tonsillar carcinoma: A pilot study. Eur. Arch. Otorhinolaryngol. 2013, 270, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, A.; Rao, P.J.; Selvam, G.; Murthy, P.B.; Reddy, P.N. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol. Lett. 2011, 205, 105–115. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. Vitr. 2011, 25, 657–25663. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Lunts, A.; Kreyling, W.; Cox, C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health Part A 2002, 65, 1531–1543. [Google Scholar] [CrossRef]
- Čabanová, K.; Motyka, O.; Čábalová, L.; Hrabovská, K.; Bielniková, H.; Kuzníková, Ľ.; Dvořáčková, J.; Zeleník, K.; Komínek, P.; Kukutschová, J. Metal particles in mucus and hypertrophic tissue of the inferior nasal turbinates from the human upper respiratory tract. Environ. Sci. Pollut. Res. 2020, 27, 28146–28154. [Google Scholar] [CrossRef]
- Al-Rawi, M.; Diabaté, S.; Weiss, C. Uptake and intracellular localization of submicron and nano-sized SiO2 particles in HeLa cells. Arch. Toxicol. 2011, 85, 813–826. [Google Scholar] [CrossRef]
- Bogdan, J.; Jackowska-Tracz, A.; Zarzyńska, J.; Pławińska-Czarnak, J. Chances and limitations of nanosized titanium dioxide practical application in view of its physicochemical properties. Nanoscale Res. Lett. 2015, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čabanová, K.; Motyka, O.; Bielniková, H.; Čábalová, L.; Handlos, P.; Zabiegaj, D.; Zeleník, K.; Dvořáčková, J.; Komínek, P.; Heviánková, S.; et al. Identification of the phase composition of solid microparticles in the nasal mucosa of patients with chronic hypertrophic rhinitis using Raman microspectroscopy. Sci. Rep. 2021, 11, 18989. [Google Scholar] [CrossRef] [PubMed]
- Čábalová, L.; Čabanová, K.; Bielniková, H.; Kukutschová, J.; Dvořáčková, J.; Dědková, K.; Zeleník, K.; Komínek, P. Micro- and Nanosized Particles in Nasal Mucosa: A Pilot Study. BioMed Res. Int. 2015, 2015, 505986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalati, P.F.; Keshavarzian, E.; Abouali, O.; Faramarzi, A.; Tu, J.; Shakibafard, A. Numerical analysis of micro- and nano-particle deposition in a realistic human upper airway. Comput. Biol. Med. 2012, 42, 39–49. [Google Scholar] [CrossRef]
- Se, C.M.; Inthavong, K.; Tu, J. Unsteady Particle Deposition in a Human Nasal Cavity during Inhalation. J. Comput. Multiph. Flows 2010, 2, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Inthavong, K.; Wen, J.; Tu, J.; Xue, C. Comparison of micron- and nanoparticle deposition patterns in a realistic human nasal cavity. Respir. Physiol. Neurobiol. 2009, 166, 142–151. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Leland, E.M.; Zhang, Z.; Kelly, K.M.; Ramanathan, M. Role of Environmental Air Pollution in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2021, 21, 42. [Google Scholar] [CrossRef]
- Khalmuratova, R.; Park, J.-W.; Shin, H.-W. Immune Cell Responses and Mucosal Barrier Disruptions in Chronic Rhinosinusitis. Immune Netw. 2017, 17, 60. [Google Scholar] [CrossRef] [Green Version]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine 2014, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Čabanová, K.; Peikertová, P.; Bielniková, H.; Barošová, H.; Motyka, O.; Čábalová, L.; Dvořáčková, J.; Komínek, P.; Kukutschová, J. Raman microspectroscopy as a useful tool for nanopathology. J. Raman Spectrosc. 2017, 48, 357–362. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Element Res. 2016, 172, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Yang, Z.; Huang, R.; Chen, J.; Wang, R.; Lin, Y. Toxicity of Carbon Nanotubes. Curr. Drug Metab. 2013, 14, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Khalid, I.; Khalid, T.J.; Jennings, J.H. A welder with pneumosiderosis: A case report. Cases J. 2009, 2, 6639. [Google Scholar] [CrossRef] [Green Version]
- Kukutschová, J.; Roubíček, V.; Mašláň, M.; Jančík, D.; Slovák, V.; Malachová, K.; Pavlíčková, Z.; Filip, P. Wear performance and wear debris of semimetallic automotive brake materials. Wear 2010, 268, 86–93. [Google Scholar] [CrossRef]
- Schleimer, R.P. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 331–357. [Google Scholar] [CrossRef] [Green Version]
- Alekseenko, S.I.; Skalny, A.V.; Ajsuvakova, O.P.; Skalnaya, M.G.; Notova, S.V.; Tinkov, A.A. Mucociliary transport as a link between chronic rhinosinusitis and trace element dysbalance. Med. Hypotheses 2019, 127, 5–10. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Öst, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Katainen, A. Smoking and workers’ autonomy: A qualitative study on smoking practices in manual work. Health 2012, 16, 134–150. [Google Scholar] [CrossRef] [PubMed]

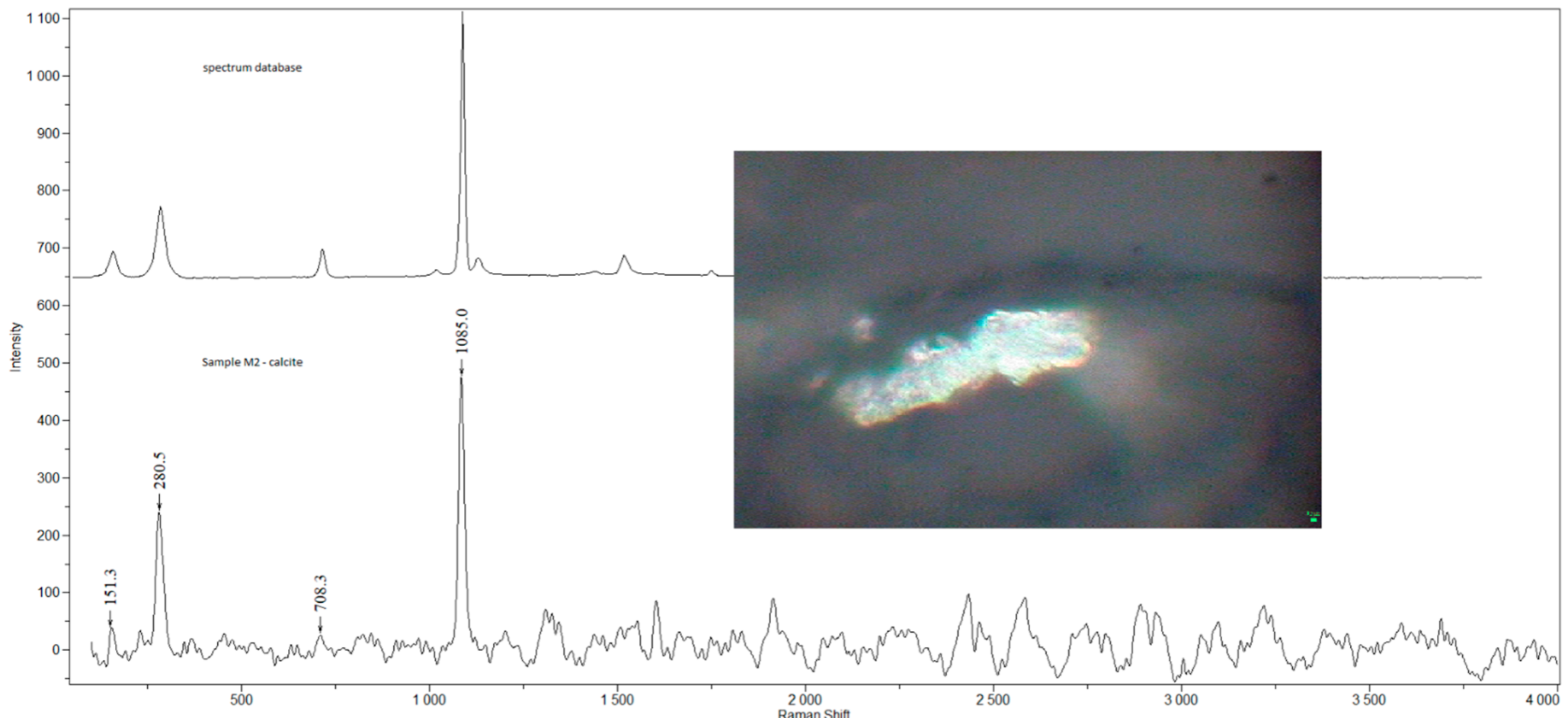
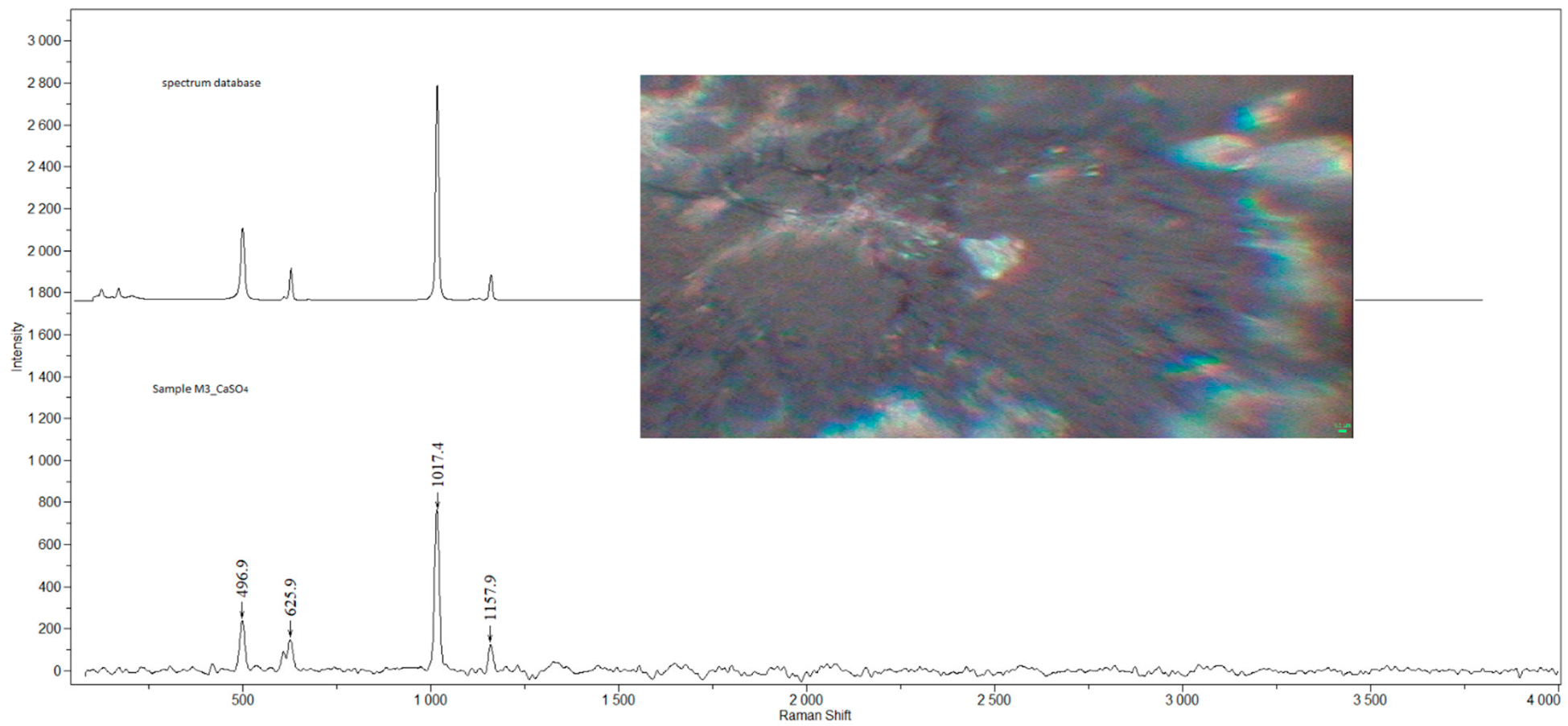
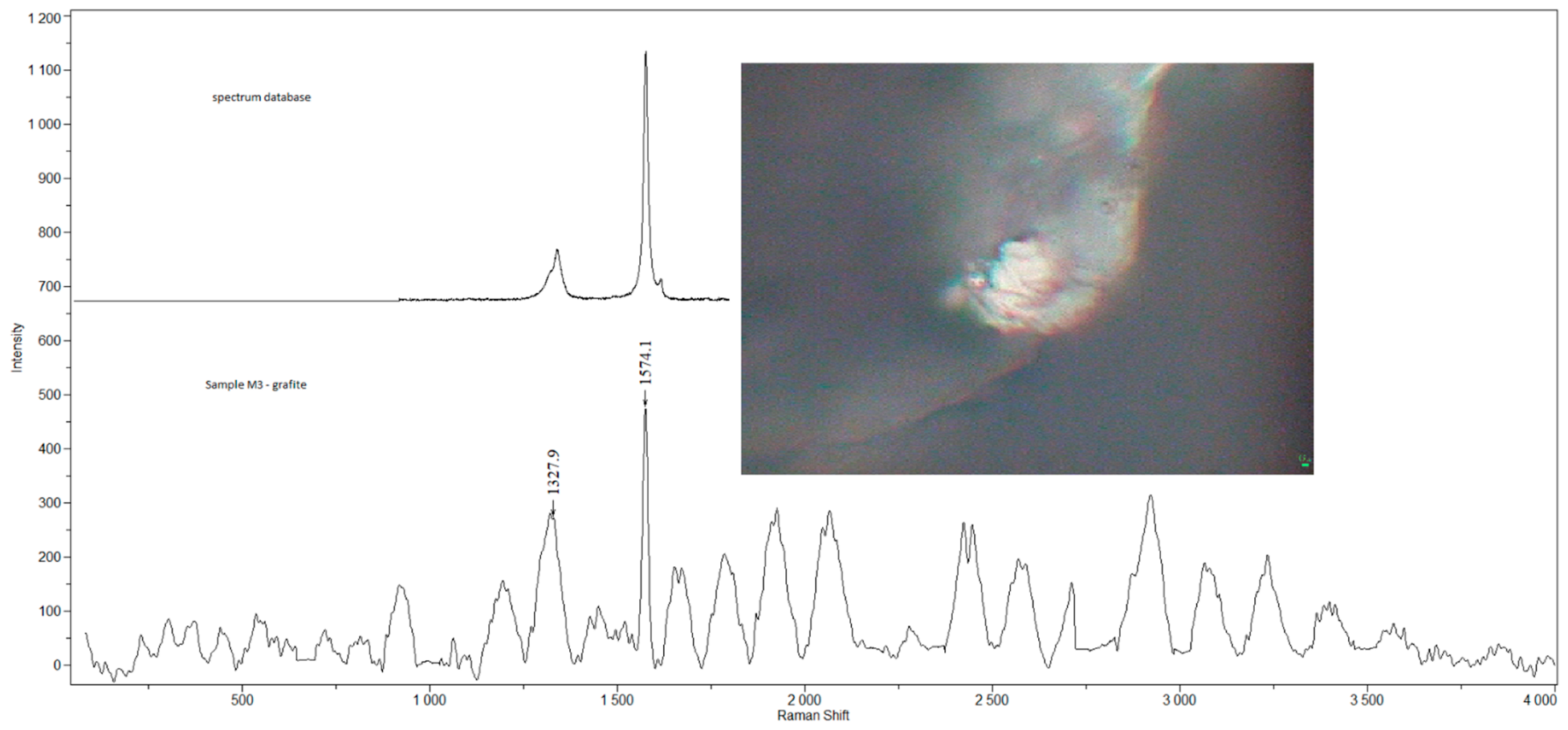
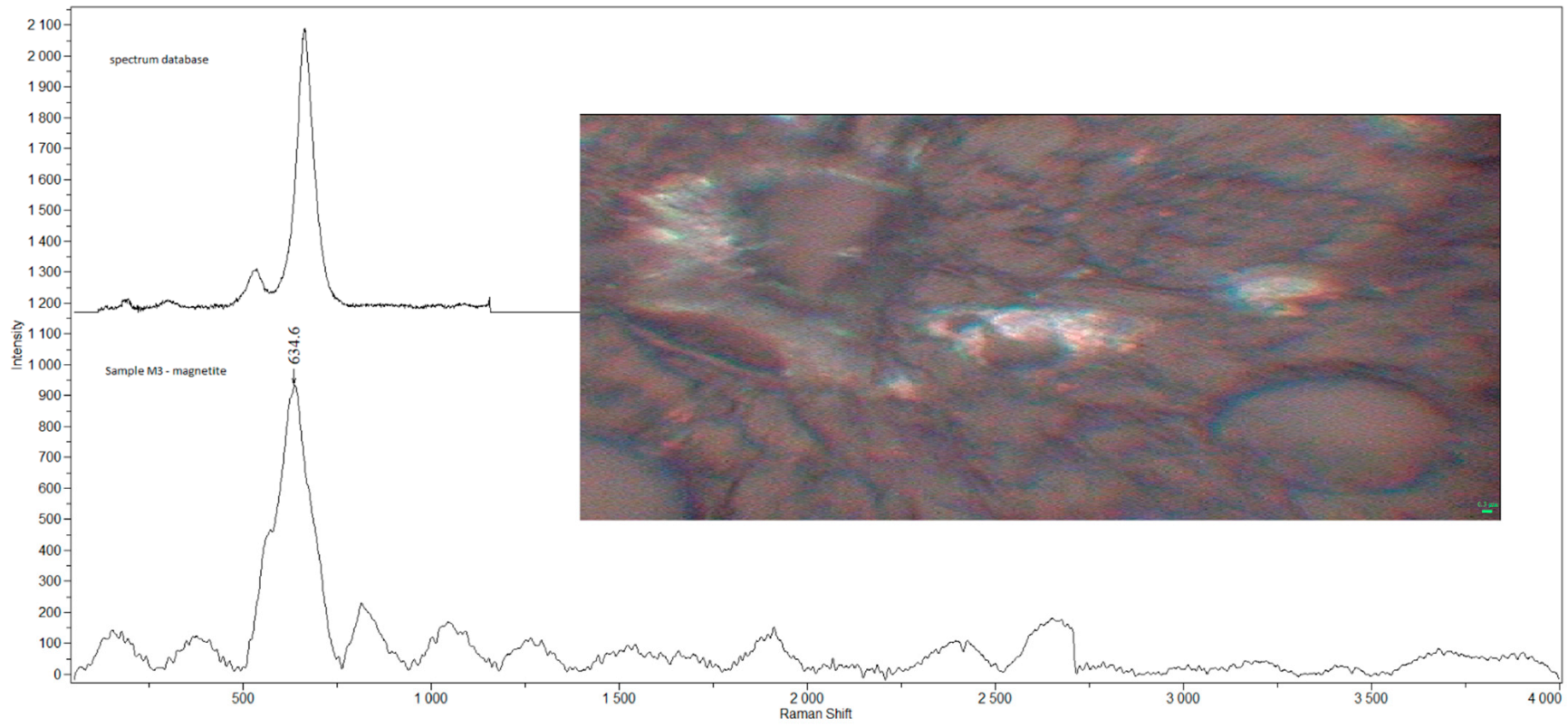
| Sample | Sex | Age | Smoking Status | Occupation | Detected Compounds | Histology (Severity of Inflammation) |
|---|---|---|---|---|---|---|
| M11 | m | 42 | Y | builder (M) | GR | 0 |
| M32 | m | 31 | N | administrator (O) | AC, Al comp., ankerite | 0 |
| M1 | m | 78 | N | welder (M) | AC, ankerite, CaCO3, GR, TiO2-A | 1 |
| M2 | m | 38 | N | programmer (O) | CaCO3, Fe3O4, TiO2-A, TiO2-R | 1 |
| M3 | m | 38 | N | welder (M) | AC, CaSO4, Fe3O4, GR, TiO2-A | 1 |
| M4 | m | 65 | Y | policeman (M) | AC, ankerite, GR, TiO2-A, TiO2-R | 1 |
| M14 | m | 32 | N | policeman (M) | AC, Fe2O3, GR, TiO2-A | 1 |
| M16 | m | 44 | Y | driver (M) | CaCO3, GR, TiO2-A, TiO2-R | 1 |
| M17 | f | 42 | N | warehouse keeper (M) | ankerite, BaSO4, TiO2-A | 1 |
| M18 | m | 34 | Y | carrier (M) | AC, CaCO3, GR, SiO2, TiO2-A | 1 |
| M20 | m | 35 | Y | tinsmith (M) | ankerite, CaCO3, GR, SiO2 | 1 |
| M33 | m | 43 | N | machinist (M) | AC, GR, TiO2-A | 1 |
| M35 | f | 65 | N | manager (O) | CaCO3, TiO2-R | 1 |
| M40 | m | 54 | N | bailiff (O) | - | 1 |
| M8 | m | 44 | Y | labourer (M) | CaCO3, GR | 2 |
| M10 | m | 37 | N | manager (O) | GR, TiO2-A | 2 |
| M21 | m | 31 | N | labourer (M) | ankerite, GR | 2 |
| M2 | m | 28 | N | rolling mill operator (M) | TiO2-A | 2 |
| M25 | m | 44 | N | executive director (O) | AC, Si comp., TiO2-A, TiO2-R | 2 |
| M28 | m | 42 | Y | policeman (M) | AC, ankerite, BaSO4, GR | 2 |
| M29 | m | 25 | Y | unemployed (O) | AC, GR, TiO2-A | 2 |
| M30 | m | 39 | Y | welder (M) | AC, Al comp., (Ca, Mg)CO3 | 2 |
| M5 | f | 26 | N | student (O) | Fe3O4, GR | 3 |
| M6 | f | 44 | N | labourer (M) | AC, CaCO3 | 3 |
| M7 | m | 28 | N | student (O) | GR | 3 |
| M9 | f | 58 | N | artist (O) | GR, TiO2-A | 3 |
| M12 | f | 45 | N | manager (O) | GR, TiO2-A | 3 |
| M13 | m | 40 | N | clerk (O) | Fe2O3, TiO2-A | 3 |
| M15 | f | 53 | N | shop assistant (M) | ankerite, GR, TiO2-A | 3 |
| M19 | m | 48 | N | waiter (M) | ankerite, CaCO3, Fe2O3, SiO2, TiO2-A | 3 |
| M23 | m | 28 | N | operator (M) | TiO2-A | 3 |
| M24 | m | 54 | N | policeman (M) | AC, GR | 3 |
| M26 | m | 42 | N | train dispatcher (M) | AC, CaCO3, GR | 3 |
| M27 | m | 25 | N | student (O) | AC | 3 |
| M31 | f | 20 | N | student (O) | GR, TiO2-A, TiO2-R | 3 |
| M34 | f | 41 | N | seamstress (M) | GR, TiO2-A | 3 |
| M36 | m | 36 | N | IT technician (O) | GR, TiO2-A | 3 |
| M37 | m | 44 | N | production supervisor (M) | - | 3 |
| M38 | m | 34 | N | electrotechnician (M) | - | 3 |
| M39 | m | 55 | N | businessman (O) | - | 3 |
| Sample | Sex | Age | Smoking Status | Occupation | Detected Compounds |
|---|---|---|---|---|---|
| R1 | m | 71 | N | M | - |
| R5 | m | 57 | Y | M | - |
| R6 | m | 77 | N | M | TiO2 |
| R7 | f | 78 | N | O | AC |
| R8 | m | 44 | N | M | - |
| R9 | m | 67 | Y | M | - |
| R10 | m | 37 | Y | M | - |
| R11 | f | 87 | N | O | - |
| R12 | f | 84 | N | O | |
| R13 | f | 84 | N | O | - |
| Detected Compound | Number of Samples/40 | Percent of Samples |
|---|---|---|
| Graphite TiO2 amorphous carbon | 24 | 60.0% |
| 23 | 57.5% | |
| 15 | 37.5% | |
| CaCO3 Ca(Fe, Mg, Mn)(CO3)2 | 10 | 25.0% |
| 9 | 22.5 % | |
| iron compounds | 6 | 15.0% |
| Severity of Inflammation | Number of Samples/40 | Percent of Samples |
|---|---|---|
| 0 (no inflammation) 1 (epithelial hyperplasia) 2 (mild signs of inflammation) | 2 | 5.0% |
| 13 | 32.5% | |
| 7 | 17.5% | |
| 3 (chronic inflammation) | 18 | 45.0% |
| Detected Compound | Group A/22 Samples | Group B/18 Samples | ||
|---|---|---|---|---|
| Number of Samples | Percent of Samples | Number of Samples | Percent of Samples | |
| graphite | 14 | 63.0% | 9 | 50.0% |
| TiO2 | 14 | 63.0% | 9 | 50.0% |
| amorphous carbon | 7 | 31.8% | 4 | 22.2% |
| CaCO3 | 7 | 31.8% | 3 | 16.7% |
| Ca(Fe, Mg, Mn)(CO3)2 | 7 | 31.8% | 2 | 11.1% |
| iron compounds | 3 | 13.6% | 3 | 16.7% |
| Detected Compound | Smokers/9 Samples | Non-Smokers/31 Samples | ||
|---|---|---|---|---|
| Number of Samples | Percent of Samples | Number of Samples | Percent of Samples | |
| graphite | 8 | 88.9% | 16 | 51.6% |
| TiO2 | 4 | 44.4% | 19 | 61.3% |
| amorphous carbon | 5 | 55.6% | 10 | 32.3% |
| CaCO3 | 4 | 44.4% | 6 | 19.4% |
| Ca(Fe, Mg, Mn)(CO3)2 | 3 | 33.3% | 6 | 19.4% |
| iron compounds | 0 | 0.0% | 6 | 19.4% |
| Detected Compound | Manual Workers/22 Samples | Office Workers/18 Samples | ||
|---|---|---|---|---|
| Number of Samples | Percent of Samples | Number of Samples | Percent of Samples | |
| graphite | 17 | 77.3% | 7 | 38.9% |
| TiO2 | 13 | 59.1% | 10 | 55.5% |
| amorphous carbon | 11 | 50.0% | 4 | 22.2% |
| CaCO3 | 8 | 36.4% | 2 | 11.1% |
| Ca(Fe, Mg, Mn)(CO3)2 | 8 | 36.4% | 1 | 5.6% |
| iron compounds | 3 | 14.6% | 2 | 11.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čábalová, L.; Čabanová, K.; Bielniková, H.; Kukutschová, J.; Dvořáčková, J.; Zeleník, K.; Komínek, P. Solid Anorganic Particles and Chronic Rhinosinusitis: A Histopathology Study. Int. J. Environ. Res. Public Health 2022, 19, 7269. https://doi.org/10.3390/ijerph19127269
Čábalová L, Čabanová K, Bielniková H, Kukutschová J, Dvořáčková J, Zeleník K, Komínek P. Solid Anorganic Particles and Chronic Rhinosinusitis: A Histopathology Study. International Journal of Environmental Research and Public Health. 2022; 19(12):7269. https://doi.org/10.3390/ijerph19127269
Chicago/Turabian StyleČábalová, Lenka, Kristina Čabanová, Hana Bielniková, Jana Kukutschová, Jana Dvořáčková, Karol Zeleník, and Pavel Komínek. 2022. "Solid Anorganic Particles and Chronic Rhinosinusitis: A Histopathology Study" International Journal of Environmental Research and Public Health 19, no. 12: 7269. https://doi.org/10.3390/ijerph19127269






