Development and Validation of a Novel Score for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Statistical Analysis
2.3. Risk Score Development and Internal Validation
3. Results
3.1. Baseline Characteristics
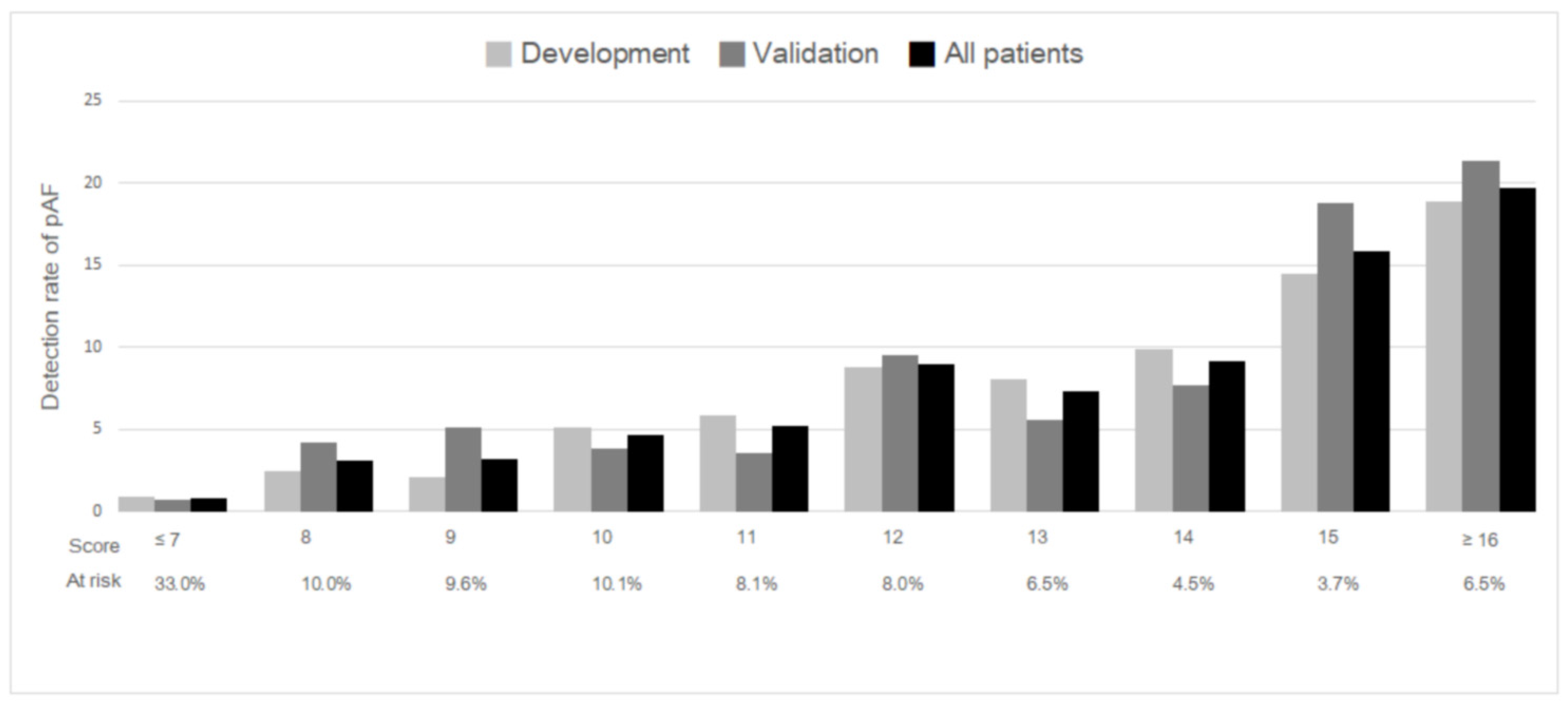
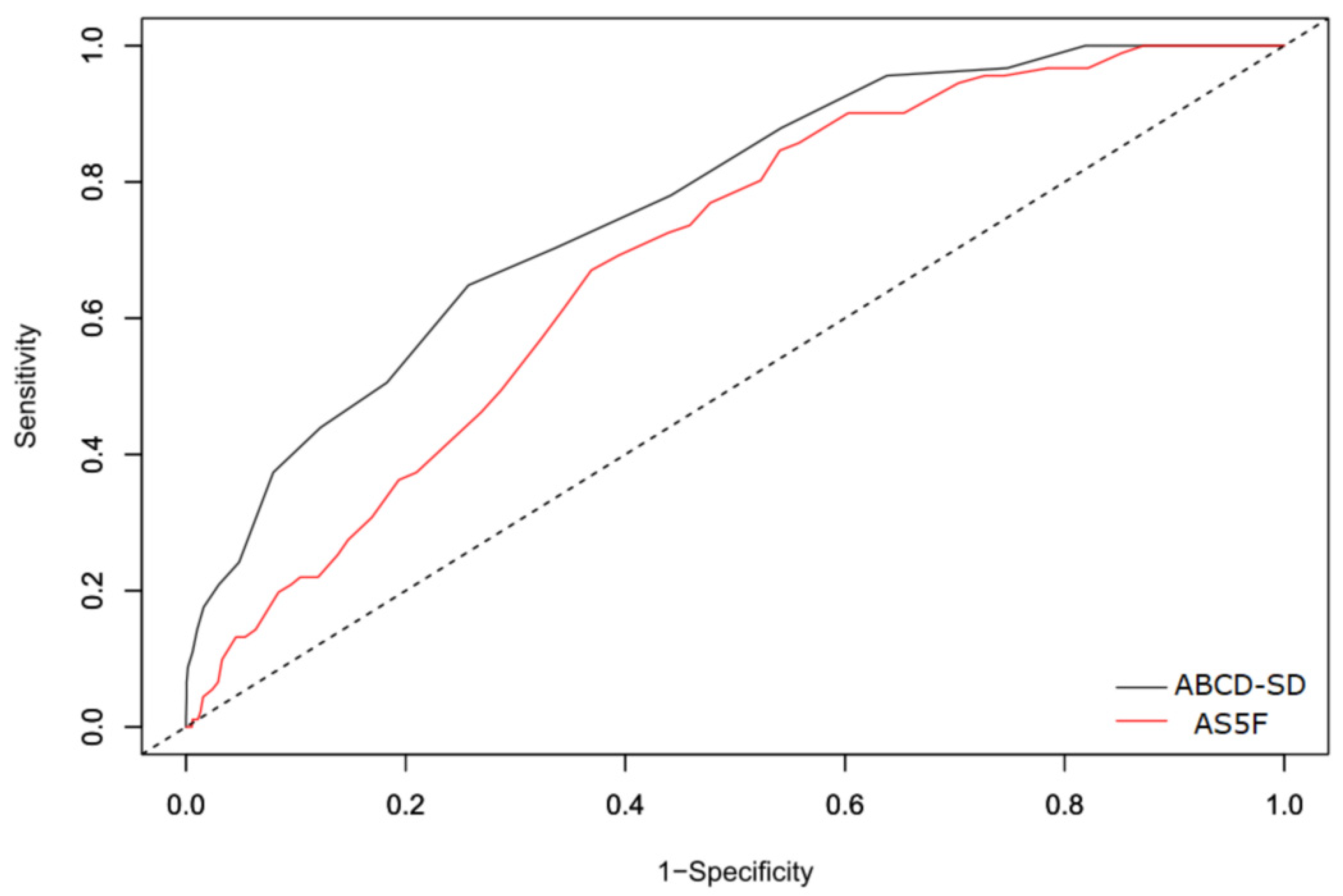
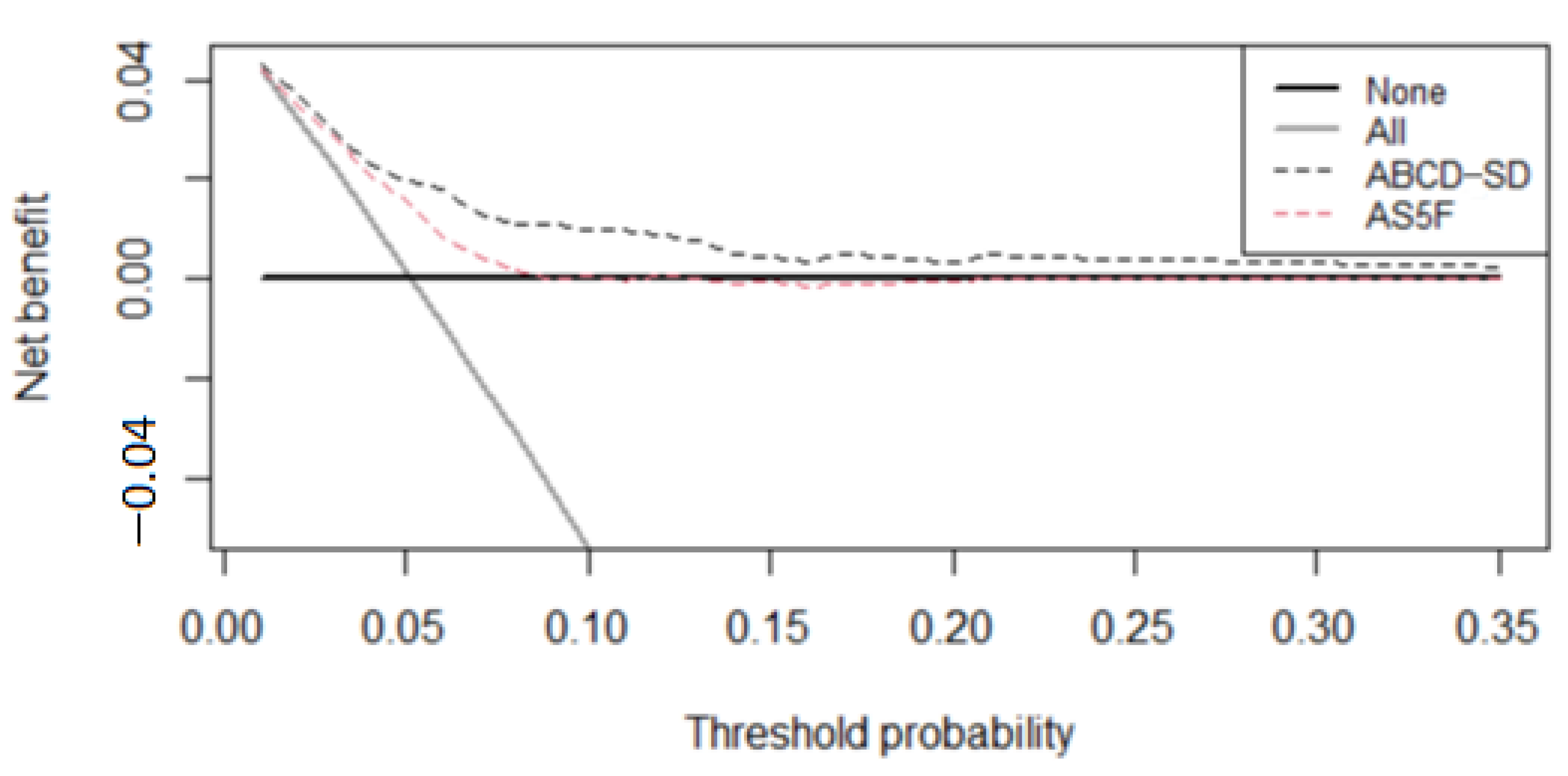
3.2. Score Development and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, A.P.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolominsky-Rabas, P.L.; Weber, M.; Gefeller, O.; Neundoerfer, B.; Heuschmann, P.U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, long-term survival in ischemic stroke subtypes: A population-based study. Stroke 2001, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, D.E.; Kahwati, L.C.; Yun, J.D.; Middleton, J.C.; Coker-Schwimmer, M.; Asher, G.N. Screening for Atrial Fibrillation with Electrocardiography: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Pajitnev, D.; Pogue, J.; Healey, J.S.; Pfeffer, M.A.; Yusuf, S.; Connolly, S.J. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: An ACTIVE W Substudy. J. Am. Coll. Cardiol. 2007, 50, 2156–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, S.; Shibazaki, K.; Kimura, K.; Sakai, K.; Aoki, J. A simple score for predicting paroxysmal atrial fibrillation in acute ischemic stroke. J. Neurol. Sci. 2013, 328, 83–86. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, M.M.; Rodrigues, A.C.T.; Alves, M.B.; Neto, M.C.; Silva, G.S. Score for atrial fibrillation detection in acute stroke and transient ischemic attack patients in a Brazilian population: The acute stroke atrial fibrillation scoring system. Clinics 2014, 69, 241–246. [Google Scholar] [CrossRef]
- Yoshioka, K.; Watanabe, K.; Zeniya, S.; Ito, Y.; Hizume, M.; Kanazawa, T.; Tomita, M.; Ishibashi, S.; Miake, H.; Tanaka, H.; et al. A Score for Predicting Paroxysmal Atrial Fibrillation in Acute Stroke Patients: iPAB Score. J. Stroke Cerebrovasc. Dis. 2015, 24, 2263–2269. [Google Scholar] [CrossRef]
- Seo, W.K.; Kang, S.H.; Jung, J.M.; Choi, J.Y.; Oh, K. Novel composite score to predict atrial Fibrillation in acute stroke patients: AF predicting score in acute stroke. Int. J. Cardiol. 2016, 209, 184–189. [Google Scholar] [CrossRef]
- Suissa, L.; Mahagne, M.H.; Lachaud, S. Score for the targeting of atrial fibrillation: A new approach to diagnosing paroxysmal atrial fibrillation. Cerebrovasc. Dis. 2011, 31, 442–447. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Lin, M.-H.; Lee, C.-P.; Yang, Y.-H.; Chen, W.-C.; Chang, G.-H.; Tsai, Y.-T.; Chen, P.-C.; Tsai, Y.-H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-T.; Hsieh, C.-Y.; Tsai, T.-T.; Wang, Y.-C.; Sung, S.-F. Performance of ICD-10-CM Diagnosis Codes for Identifying Acute Ischemic Stroke in a National Health Insurance Claims Database. Clin. Epidemiol. 2020, 12, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culebras, A.; Messe, S.R. Summary of evidence-based guideline update: Prevention of stroke in nonvalvular atrial fibrillation: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 83, 1220. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar]
- Wein, T.; Lindsay, M.P.; Côté, R.; Foley, N.; Berlingieri, J.; Bhogal, S.; Bourgoin, A.; Buck, B.; Cox, J.; Davidson, D.; et al. Canadian stroke best practice recommendations: Secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int. J. Stroke 2018, 13, 420–443. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [Green Version]
- Fuster, V.; Rydén, L.E.; Asinger, R.W.; Cannom, D.S.; Crijns, H.J.; Frye, R.L.; Halperin, J.L.; Kay, G.N.; Klein, W.W.; Lévy, S.; et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Eur. Heart J. 2001, 22, 1852–1923. [Google Scholar]
- Sung, S.-F.; Hsieh, C.-Y.; Lin, H.-J.; Chen, Y.-W.; Yang, Y.-H.K.; Li, C.-Y. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int. J. Cardiol. 2016, 215, 277–282. [Google Scholar] [CrossRef]
- Sung, S.-F.; Hsieh, C.-Y.; Lin, H.-J.; Chen, Y.-W.; Chen, C.-H.; Yang, Y.-H.K.; Hu, Y.-H. Validity of a stroke severity index for administrative claims data research: A retrospective cohort study. BMC Health Serv. Res. 2016, 16, 509. [Google Scholar] [CrossRef] [Green Version]
- Uphaus, T.; Weber-Krüger, M.; Grond, M.; Toenges, G.; Jahn-Eimermacher, A.; Jauss, M.; Kirchhof, P.; Wachter, R.; Gröschel, K. Development and validation of a score to detect paroxysmal atrial fibrillation after stroke. Neurology 2019, 92, e115–e124. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Lee, C.-H.; Sung, S.-F. Development of a novel score to predict newly diagnosed atrial fibrillation after ischemic stroke: The CHASE-LESS score. Atherosclerosis 2020, 295, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y. Patients with Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhao, M.; Sun, Y.; Hou, Z.; Wang, C.; Yun, C.; Li, Y.; Li, Z.; Wang, M.; Wu, S.; et al. Frequency of Visit-to-Visit Variability of Resting Heart Rate and the Risk of New-Onset Atrial Fibrillation in the General Population. Am. J. Cardiol. 2021, 155, 45–51. [Google Scholar] [CrossRef]
- Moons, K.G.; Harrell, F.E.; Steyerberg, E.W. Should scoring rules be based on odds ratios or regression coefficients? J. Clin. Epidemiol. 2002, 55, 1054–1055. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-Y.; Kao, H.-M.; Sung, K.-L.; Sposato, L.A.; Sung, S.-F.; Lin, S.-J. Validation of Risk Scores for Predicting Atrial Fibrillation Detected After Stroke Based on an Electronic Medical Record Algorithm: A Registry-Claims-Electronic Medical Record Linked Data Study. Front. Cardiovasc. Med. 2022, 9, 888240. [Google Scholar] [CrossRef]
- Li, Y.G.; Pastori, D.; Farcomeni, A.; Yang, P.S.; Jang, E.; Joung, B.; Wang, Y.-T.; Guo, Y.-T.; Lip, G.Y.H. A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External Application in 451,199 Korean Subjects. Chest 2019, 155, 510–518. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.-J.S.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J.G.M. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Kwong, C.; Ling, A.Y.; Crawford, M.H.; Zhao, S.X.; Shah, N.H. A Clinical Score for Predicting Atrial Fibrillation in Patients with Cryptogenic Stroke or Transient Ischemic Attack. Cardiology 2017, 138, 133–140. [Google Scholar] [CrossRef]
- Ashburner, J.M.; Wang, X.; Li, X.; Khurshid, S.; Ko, D.; Lipsanopoulos, A.T.; Lee, P.R.; Carmichael, T.; Turner, A.C.; Jackson, C.; et al. Re-CHARGE-AF: Recalibration of the CHARGE-AF Model for Atrial Fibrillation Risk Prediction in Patients with Acute Stroke. J. Am. Heart Assoc. 2021, 10, e022363. [Google Scholar] [CrossRef] [PubMed]
- Ruigómez, A.; Johansson, S.; Wallander, M.-A.; Rodríguez, L.A.G. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc. Disord. 2005, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, R.B.; Sullivan, L.M.; Levy, D.; Pencina, M.J.; Massaro, J.M.; D’Agostino, R.B.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.K.; Norby, F.L.; Whitsel, E.A.; Soliman, E.Z.; Chen, L.Y.; Loehr, L.R.; Fuster, V.; Heiss, G.; Coresh, J.; Alonso, A. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-U. J. Am. Coll. Cardiol. 2017, 69, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Erdener, S.E. Seeking predictors for paroxysmal atrial fibrillation in stroke with an online clinical database. North. Clin. Istanb. 2020, 7, 378–385. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lee, C.H.; Wu, D.P.; Sung, S.F. Prediction of new-onset atrial fibrillation after first-ever ischemic stroke: A comparison of CHADS2, CHA2DS2-VASc and HATCH scores and the added value of stroke severity. Atherosclerosis 2018, 272, 73–79. [Google Scholar] [CrossRef]
- Freedman, B.; Potpara, T.S.; Lip, G.Y. Stroke prevention in atrial fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanabe, N.; Yagihara, N.; Watanabe, T.; Aizawa, Y.; Kodama, M. Association between lipid profile and risk of atrial fibrillation. Circ. J. 2011, 75, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Lopez, F.L.; Lutsey, P.L.; Arking, D.E.; Pankow, J.S.; Agarwal, S.K.; Chen, L.Y.; Alonso, A. Blood lipid levels, lipid-lowering medications, the incidence of atrial fibrillation: The atherosclerosis risk in communities study. Circ. Arrhythmia Electrophysiol. 2012, 5, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Mora, S.; Akinkuolie, A.O.; Sandhu, R.K.; Conen, D.; Albert, C.M. Paradoxical Association of Lipoprotein Measures with Incident Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gao, L.; Wang, Z.; Guan, B.; Guan, X.; Wang, B.; Han, X.; Xiao, X.; Bin Waleed, K.; Chandran, C.; et al. Lipid profile and incidence of atrial fibrillation: A prospective cohort study in China. Clin. Cardiol. 2018, 41, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtzinis, G.; Kahan, T.; Boström, K.B.; Schiöler, L.; Wallin, L.C.; Hjerpe, P.; Hasselström, J.; Manhem, K. Relation Between Lipid Profile and New-Onset Atrial Fibrillation in Patients with Systemic Hypertension (From the Swedish Primary Care Cardiovascular Database [SPCCD]). Am. J. Cardiol. 2018, 122, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Choi, E.; Han, K.; Oh, S. Low Lipid Levels and High Variability are Associated with the Risk of New-Onset Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e012771. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Havekes, L.M.; Rensen, P.C. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 2005, 11, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lundbæk, J.A.; Birn, P.; Hansen, A.J.; Søgaard, R.; Nielsen, C.; Girshman, J.; Bruno, M.J.; Tape, S.E.; Egebjerg, J.; Greathouse, D.V.; et al. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 2004, 123, 599–621. [Google Scholar] [CrossRef] [Green Version]
- Balse, E.; El-Haou, S.; Dillanian, G.; Dauphin, A.; Eldstrom, J.; Fedida, D.; Coulombe, A.; Hatem, S.N. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 14681–14686. [Google Scholar] [CrossRef] [Green Version]
- Bastiaanse, E.; Höld, K.M.; Van Der Laarse, A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 1997, 33, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, M.; Muñoz-Venturelli, P.; Billot, L.; Wang, X.; Song, L.; Arima, H.; Lavados, P.M.; Hackett, M.L.; Olavarría, V.V.; Brunser, A.; et al. Low blood pressure and adverse outcomes in acute stroke: HeadPoST study explanations. J. Hypertens. 2021, 39, 273–279. [Google Scholar] [CrossRef]
- Ouyang, M.; Muñoz-Venturelli, P.; Billot, L.; Wang, X.; Song, L.; Arima, H.; Lavados, P.M.; Hackett, M.L.; Olavarría, V.V.; Brunser, A.; et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: Data from the Austrian Stroke registry. Eur. Heart J. 2004, 25, 1734–1740. [Google Scholar]
- Temu, T.M.; Lane, K.A.; Shen, C.; Ng’Ang’A, L.; Akwanalo, C.O.; Chen, P.-S.; Emonyi, W.; Heckbert, S.R.; Koech, M.M.; Manji, I.; et al. Clinical characteristics and 12-month outcomes of patients with valvular and non-valvular atrial fibrillation in Kenya. PLoS ONE 2017, 12, e0185204. [Google Scholar] [CrossRef] [Green Version]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Vargas, E.R.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- Giruparajah, M.; Bosch, J.; Vanassche, T.; Mattina, K.; Connolly, S.J.; Pater, C.; Hart, R.G. Global survey of the diagnostic evaluation and management of cryptogenic ischemic stroke. Int. J. Stroke 2015, 10, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Schaer, B.; Zellweger, M.; Cron, T.; Kaiser, C.; Osswald, S. Value of routine holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke 2004, 35, e68–e70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suissa, L.; Lachaud, S.; Mahagne, M.H. Optimal timing and duration of continuous electrocardiographic monitoring for detecting atrial fibrillation in stroke patients. J. Stroke Cerebrovasc. Dis. 2013, 22, 991–995. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Haeusler, K.G.; Healey, J.S.; Freedman, B.; Boriani, G.; Brachmann, J.; Brandes, A.; Bustamante, A.; Casadei, B.; Crijns, H.J.G.M.; et al. Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation 2019, 140, 1834–1850. [Google Scholar] [CrossRef]
- Baturova, M.A.; Sheldon, S.H.; Carlson, J.; Brady, P.A.; Lin, G.; Rabinstein, A.A.; Friedman, P.A.; Platonov, P.G. Electrocardiographic and Echocardiographic predictors of paroxysmal atrial fibrillation detected after ischemic stroke. BMC Cardiovasc. Disord. 2016, 16, 209. [Google Scholar] [CrossRef] [Green Version]
- Shiroto, H.; Tomita, H.; Hagii, J.; Metoki, N.; Fujita, A.; Kamada, T.; Takahashi, K.; Saito, S.; Sasaki, S.; Hitomi, H.; et al. Impact of Atrial Natriuretic Peptide Value for Predicting Paroxysmal Atrial Fibrillation in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 772–778. [Google Scholar] [CrossRef]
| Acute Ischemic Stroke | p Value | ||||
|---|---|---|---|---|---|
| Total (N = 6033) | Without AF (N = 5016) | With pAF (N = 274) | With Sustained AF (N = 743) | ||
| Age, years | 67 (57–76) | 65 (55–75) | 74 (65–81) * | 74 (65–81) ** | <0.001 |
| Male | 3921 (65.0) | 3342 (66.6) | 157 (57.3) * | 422 (56.8) ** | <0.001 |
| eNIHSS | 4 (4–9) | 4 (4–7) | 4 (4−11) * | 4 (4–11) ** | <0.001 |
| eNIHSS ≤ 5 | 4009 (66.5) | 3511 (70.0) | 153 (55.8) | 345 (46.4) | |
| eNIHSS 6–13 | 1151 (19.1) | 929 (18.5) | 58 (21.2) | 164 (22.1) | |
| eNIHSS > 13 | 873 (14.5) | 576 (11.5) | 63 (23.0) | 234 (31.5) | |
| Hypertension | 4391 (72.8) | 3663 (73.0) | 206 (75.2) | 522 (70.3) | 0.188 |
| Diabetes mellitus | 2169 (36.0) | 1874 (37.4) | 83 (30.3) | 212 (28.5) ** | <0.001 |
| Dyslipidemia | 2956 (49.0) | 2595 (51.7) | 92 (33.6) * | 269 (36.2) ** | <0.001 |
| Congestive heart failure | 334 (5.5) | 212 (4.2) | 27 (9.9) * | 95 (12.8) ** | <0.001 |
| Coronary artery disease | 578 (9.6) | 445 (8.9) | 40 (14.6) * | 93 (12.5) ** | < 0.001 |
| Current smoker | 1839 (30.5) | 1631 (32.5) | 65 (23.7) * | 143 (19.2) ** | < 0.001 |
| Prior stroke or TIA | 1280 (21.2) | 1058 (21.1) | 53 (19.3) | 169 (22.7) | 0.436 |
| Total cholesterol, mmol/L | 4.53 (3.91–5.23) | 4.58 (3.96–5.31) | 4.30 (3.76–4.92) * | 4.30 (3.68–4.92) ** | <0.001 |
| Triglyceride, mmol/L | 1.24 (0.89–1.79) | 1.31 (0.94–1.86) | 1.04 (0.79–1.54) * | 0.96 (0.70–1.32) ** | <0.001 |
| Creatinine, μmol/L | 84.86 (68.95–107.85) | 83.98 (68.95–106.96) | 89.28 (72.49–113.15) | 88.40 (70.72–112.27) ** | 0.002 |
| ALT, U/L | 21 (16–29) | 21 (16–29) | 20 (15–27) | 20 (15–28) ** | 0.006 |
| Mean SBP, mmHg | 148.3 (135.4–162.8) | 149.4 (136.4–164.1) | 144.6 (132.5–158.5) * | 142.1 (132.6–155.8) ** | <0.001 |
| Mean DBP, mmHg | 83.5 (76.8–91.1) | 84.0 (77.1–91.8) | 79.8 (73.9–87.2) * | 81.8 (75.5–89.2) ** | <0.001 |
| Mean HR, bpm | 73.4 (66.4–80.3) | 72.7 (65.9–79.4) | 73.8 (66.2–81.1) | 77.8 (70.2–87.8) ** | <0.001 |
| SD of HR, bpm | 6.9 (5.1–9.3) | 6.6 (5.0–8.6) | 8.6 (6.2–12.4) * | 9.5 (7.2–12.4) ** | <0.001 |
| CV of HR | 0.09 (0.07–0.12) | 0.09 (0.07–0.12) | 0.12 (0.09–0.16) * | 0.12 (0.09–0.15) ** | <0.001 |
| β-Coefficient | OR | 95% CI | p Value | Points | |
|---|---|---|---|---|---|
| Age (per 10 years) | 0.522 | 1.69 | 1.48–1.92 | <0.001 | 2 |
| Coronary artery disease | 0.576 | 1.78 | 1.16–2.72 | 0.008 | 2 |
| Dyslipidemia | −0.513 | 0.60 | 0.43–0.83 | 0.002 | −2 |
| SD of heart rate (per 3 bpm) | 0.439 | 1.55 | 1.38–1.75 | <0.001 | 2 |
| Mean SBP (per 20 mmHg) | −0.240 | 0.79 | 0.67–0.93 | 0.005 | −1 |
| Risk Score | Risk Category | N (%) | Detection Rate of pAF |
|---|---|---|---|
| ≤7 | Low | 1757 (33.2) | 0.8% |
| 8–14 | Medium | 2992 (56.6) | 5.5% |
| ≥15 | High | 541 (10.2) | 18.3% |
| Overall | 5290 (100) | 5.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-D.; Kuo, Y.-W.; Lee, C.-P.; Huang, Y.-C.; Lee, M.; Lee, T.-H. Development and Validation of a Novel Score for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. Int. J. Environ. Res. Public Health 2022, 19, 7277. https://doi.org/10.3390/ijerph19127277
Lee J-D, Kuo Y-W, Lee C-P, Huang Y-C, Lee M, Lee T-H. Development and Validation of a Novel Score for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. International Journal of Environmental Research and Public Health. 2022; 19(12):7277. https://doi.org/10.3390/ijerph19127277
Chicago/Turabian StyleLee, Jiann-Der, Ya-Wen Kuo, Chuan-Pin Lee, Yen-Chu Huang, Meng Lee, and Tsong-Hai Lee. 2022. "Development and Validation of a Novel Score for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke" International Journal of Environmental Research and Public Health 19, no. 12: 7277. https://doi.org/10.3390/ijerph19127277
APA StyleLee, J.-D., Kuo, Y.-W., Lee, C.-P., Huang, Y.-C., Lee, M., & Lee, T.-H. (2022). Development and Validation of a Novel Score for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. International Journal of Environmental Research and Public Health, 19(12), 7277. https://doi.org/10.3390/ijerph19127277






