Amelioration of Coastal Salt-Affected Soils with Biochar, Acid Modified Biochar and Wood Vinegar: Enhanced Nutrient Availability and Bacterial Community Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
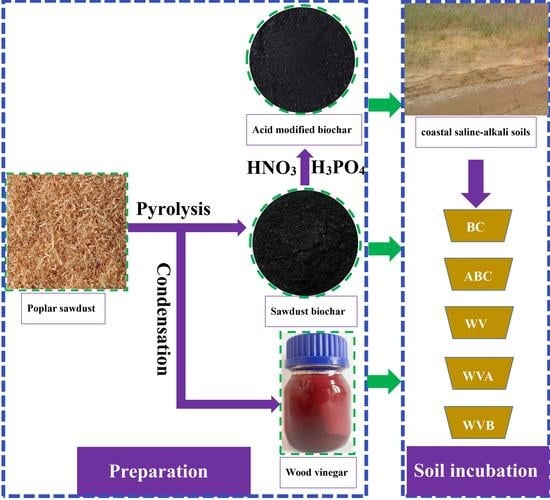
2.2. Preparation of Wood Vinegar and Biochar
2.3. Soil Incubation Experiments
2.4. Characterization
2.5. Analysis of Soil Property
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Wood Vinegar and Biochar
3.2. Improvement of Soil Properties and Nutrients
3.3. Changes in Soil Microbial Diversity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, H.; Lu, J.; Zheng, W.; Sun, J.; Li, J.; Guo, Z.; Huang, J.; Yu, S.; Yin, L.; Wang, Y.J.M.P.B. Evaluation system of coastal wetland ecological vulnerability under the synergetic influence of land and sea: A case study in the Yellow River Delta, China. Mar. Pollut. Bull. 2020, 161, 111735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; Li, Q.; Zhao, C.; Feng, Y. Influence of plants and environmental variables on the diversity of soil microbial communities in the Yellow River Delta Wetland, China. Chemosphere 2021, 274, 129967. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, G.; Bai, J.; Xia, Z.; Wang, W.; Jia, J.; Wang, X.; Liu, X.; Cui, B. Desalinization via freshwater restoration highly improved microbial diversity, co-occurrence patterns and functions in coastal wetland soils. Sci. Total Environ. 2021, 765, 142769. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2019, 298, 122468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Liu, J. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2020, 754, 142104. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Lin, Q.; Chen, X.; Li, Y.; Li, G.; Zhao, X.; Tian, Y. Synthesis, characterization and application of magnetic and acid modified biochars following alkaline pretreatment of rice and cotton straws. Sci. Total Environ. 2020, 714, 136532. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, S.; Hou, B.; Zheng, H.; Deng, W.; Liu, D.; Tang, W. Study on the preparation of wood vinegar from biomass residues by carbonization process. Bioresour. Technol. 2015, 179, 98–103. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wu, S.; Cao, Z.; Zhang, Y.; Li, H.; Jiang, F.; Lyu, J. Study on an alternative approach for the preparation of wood vinegar from the hydrothermolysis process of cotton stalk. Bioresour. Technol. 2018, 254, 231–238. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [Green Version]
- Hua, D.; Fan, Q.; Zhao, Y.; Xu, H.; Chen, L.; Li, Y. Comparison of methanogenic potential of wood vinegar with gradient loads in batch and continuous anaerobic digestion and microbial community analysis. Sci. Total Environ. 2020, 739, 139943. [Google Scholar] [CrossRef]
- Sun, H.; Feng, Y.; Ji, Y.; Shi, W.; Yang, L.; Xing, B. N2O and CH4 emissions from N-fertilized rice paddy soil can be mitigated by wood vinegar application at an appropriate rate. Atmos. Environ. 2018, 185, 153–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative study of individual and co-application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef]
- Sun, H.; Feng, Y.; Xue, L.; Mandal, S.; Wang, H.; Shi, W.; Yang, L. Responses of ammonia volatilization from rice paddy soil to application of wood vinegar alone or combined with biochar. Chemosphere 2020, 242, 125247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, Z.; Xia, D.; Jian, Z.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; You, X.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Kong, X.; Liu, Y.; Pi, J.; Li, W.; Liao, Q.; Shang, J. Low-cost magnetic herbal biochar: Characterization and application for antibiotic removal. Environ. Sci. Pollut. Res. 2017, 24, 6679–6687. [Google Scholar] [CrossRef]
- Chahinez, H.-O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Taskin, E.; Bueno, C.; Allegretta, I.; Terzano, R.; Rosa, A.H.; Loffredo, E. Multianalytical characterization of biochar and hydrochar produced from waste biomasses for environmental and agricultural applications. Chemosphere 2019, 233, 422–430. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.; Zhang, Y. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Cui, J.; Gao, M.; Wang, W.; Zhou, J.; Yang, J.; Wang, J.; Li, Y.; Jiang, C.; Peng, Y. Effects of soil amendments applied on cadmium availability, soil enzyme activity, and plant uptake in contaminated purple soil. Sci. Total Environ. 2019, 654, 1364–1371. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2020, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Zhou, C.; Heal, K.; Tigabu, M.; Xia, L.; Hu, H.; Yin, D.; Ma, X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. For. Ecol. Manag. 2020, 455, 117635. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]








| Samples | Yield % | Elemental Composition (wt%) | Atomic Ratio | pH | Ash% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C% | H% | O% | N% | H/C a | O/C a | (O + N)/C a | ||||
| BC | 36.5 | 77.3 | 5.64 | 15.3 | 1.76 | 0.87 | 0.15 | 0.17 | 8.53 ± 0.06 | 1.35 |
| ABC | 33.8 | 74.1 | 4.51 | 19.5 | 1.89 | 0.73 | 0.20 | 0.22 | 8.14 ± 0.04 | 1.10 |
| WV | 31.7 | nd | nd | nd | nd | nd | nd | nd | 4.60 ± 0.0 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Pan, X.; Kuang, S.; Chen, C.; Wang, X.; Xu, J.; Li, X.; Li, H.; Zhuang, Q.; Zhang, F.; et al. Amelioration of Coastal Salt-Affected Soils with Biochar, Acid Modified Biochar and Wood Vinegar: Enhanced Nutrient Availability and Bacterial Community Modulation. Int. J. Environ. Res. Public Health 2022, 19, 7282. https://doi.org/10.3390/ijerph19127282
Wang Z, Pan X, Kuang S, Chen C, Wang X, Xu J, Li X, Li H, Zhuang Q, Zhang F, et al. Amelioration of Coastal Salt-Affected Soils with Biochar, Acid Modified Biochar and Wood Vinegar: Enhanced Nutrient Availability and Bacterial Community Modulation. International Journal of Environmental Research and Public Health. 2022; 19(12):7282. https://doi.org/10.3390/ijerph19127282
Chicago/Turabian StyleWang, Zhangjun, Xin Pan, Shaoping Kuang, Chao Chen, Xiufen Wang, Jie Xu, Xianxin Li, Hui Li, Quanfeng Zhuang, Feng Zhang, and et al. 2022. "Amelioration of Coastal Salt-Affected Soils with Biochar, Acid Modified Biochar and Wood Vinegar: Enhanced Nutrient Availability and Bacterial Community Modulation" International Journal of Environmental Research and Public Health 19, no. 12: 7282. https://doi.org/10.3390/ijerph19127282
APA StyleWang, Z., Pan, X., Kuang, S., Chen, C., Wang, X., Xu, J., Li, X., Li, H., Zhuang, Q., Zhang, F., & Wang, X. (2022). Amelioration of Coastal Salt-Affected Soils with Biochar, Acid Modified Biochar and Wood Vinegar: Enhanced Nutrient Availability and Bacterial Community Modulation. International Journal of Environmental Research and Public Health, 19(12), 7282. https://doi.org/10.3390/ijerph19127282