Abstract
Despite the negative influence of cannabis use on the development and prognosis of first-episode psychosis (FEP), there is little evidence on effective specific interventions for cannabis use cessation in FEP. The aim of this study was to compare the efficacy of a specific cognitive behavioral therapy (CBT) for cannabis cessation (CBT-CC) with treatment as usual (TAU) in FEP cannabis users. In this single-blind, 1-year randomized controlled trial, 65 participants were randomly assigned to CBT-CC or TAU. The primary outcome was the reduction in cannabis use severity. The CBT-CC group had a greater decrease in cannabis use severity and positive psychotic symptoms over time, and a greater improvement in functioning at post-treatment than TAU. The treatment response was also faster in the CBT-CC group, reducing cannabis use, anxiety, positive and general psychotic symptoms, and improving functioning earlier than TAU in the follow-up. Moreover, patients who stopped and/or reduced cannabis use during the follow-up, decreased psychotic symptoms and increased awareness of disease compared to those who continued using cannabis. Early intervention based on a specific CBT for cannabis cessation, may be effective in reducing cannabis use severity, in addition to improving clinical and functional outcomes of FEP cannabis users.
1. Introduction
Cannabis is the most commonly used substance in first-episode psychosis patients (FEP) with a cannabis use rate of 64% among these patients, of which 30% have a cannabis use disorder [1]. Meanwhile, the prevalence of problematic cannabis use among the young in the general population is between 1.5 and 2.9% [2]. There is evidence about cannabis use as a risk factor in the development and evolution of psychosis [3]. Specifically, cannabis use is associated with an earlier psychosis onset [4,5,6,7] and increased risk of transition in individuals at clinical high risk of psychosis [7]. The early age of onset of cannabis use and the severity and frequency of use was also associated with an increased risk of developing psychosis [4,7,8,9,10], indicating a dose-dependent response relationship [9,11]. Moreover, the relationship between cannabis use and psychosis may be modulated by sex [12] and genetic factors, increasing the risk in individuals with genetic vulnerability [7,13,14].
Cannabis use also has a negative impact on the clinical and functional outcomes of FEP patients. Its consumption has been associated with poor adherence to psychological and pharmacological treatment [15,16,17], increased severity of psychotic symptoms [11,18,19], and a greater risk of relapse and hospitalizations [20,21,22] in these patients. Moreover, patients with FEP who use cannabis have a poorer functional outcome at follow-up [18,19,23,24,25,26]. Cannabis use cessation, conversely, has been related to an improvement in clinical and functional outcomes and a lower risk of relapse in FEP [18,20,21]. Cannabis use, therefore, should be a priority objective in the treatment of early psychosis in order to reduce cannabis consumption and improve outcomes in FEP. However, despite the negative influence of cannabis use on the development and prognosis of the disease, there is little evidence with regard to effective specific interventions to reduce cannabis in FEP.
The randomized controlled trial (RCT) studies that have assessed the effectiveness of psychosocial interventions to reduce cannabis use in FEP included interventions based on motivational intervention [27] or combined interventions based on motivational intervention and cognitive behavior therapy [28,29,30,31]. These studies found no benefits from the interventions compared with treatment as usual condition (TAU) in terms of reducing cannabis, except in the study conducted by Bonsack et al. [27], although this benefit was not sustained at follow-up. There was also no improvement in clinical and functional outcomes; only in one study did patient quality of life improve at post-treatment [30]. Generally, the results of these studies failed to draw definite conclusions and clearly indicate whether the interventions were effective in terms of reducing cannabis use, including the tendencies towards reductions in the amount but not the frequency of cannabis use [27,29,31]. A recent RCT conducted by Cather et al. [32], assessed the efficacy of an integrated program composed of family psychoeducation and individual resiliency training for substance use in FEP patients and found no reduction in substance use at follow-up compared to TAU, suggesting that modifications to the program are needed, with special emphasis on earlier intervention [33].
Since there is clear evidence about the association between cannabis use and worse clinical and functional outcomes of FEP and since RCTs have not found an improvement in outcomes for these patients, further research is needed to determine the most effective strategies for addressing cannabis use in FEP patients. For this reason, we designed a psychotherapy for FEP cannabis users based not only on the cannabis use reduction but also on psychosis prevention, with a dual approach.
The main aim of this article was to compare the efficacy of a specific cognitive behavioral therapy (CBT) program for cannabis cessation with standard treatment in patients with FEP who are cannabis users. The specific objectives of the study were:
- To assess whether a specific CBT program for cannabis cessation is associated with a greater reduction in the use of cannabis than standard treatment at post-treatment and in the follow-up.
- To assess whether this type of program for cannabis cessation is associated with better outcomes of the psychotic disorder (i.e., reduction in symptoms and improvement in psychosocial functioning) than standard treatment at post-treatment and in the follow-up.
- To analyze the relation between cannabis abstinence and clinical and functional outcomes of patients.
2. Materials and Methods
2.1. Design
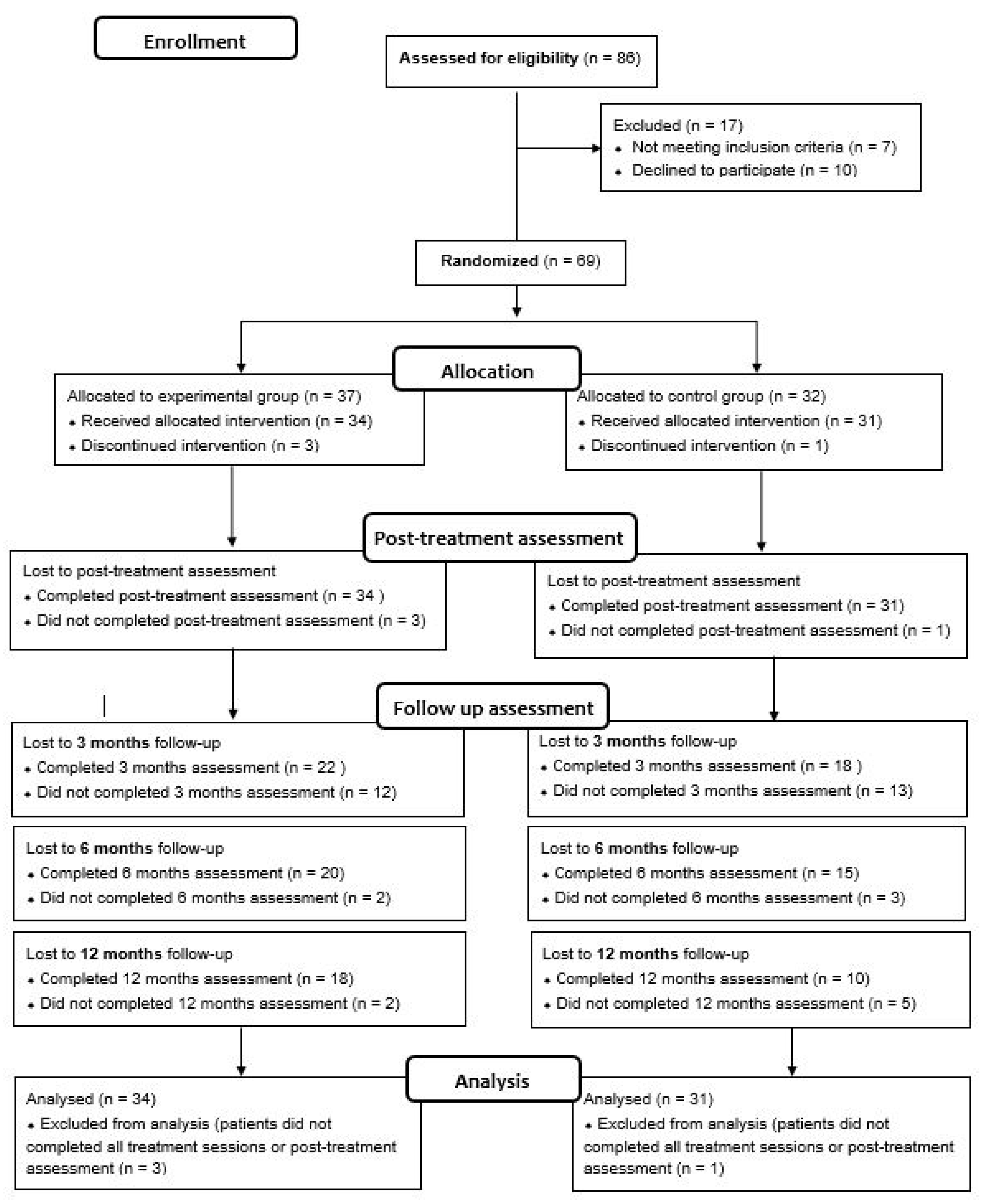
This is a single-blind RCT registered in 2014 (ClinicalTrials.gov, Identifier NCT02319746). The study protocol is described in a previous article conducted by González-Ortega et al. [34]. This RCT fulfills the CONSORT (CONsolidated Standards of Reporting Trials) guidelines, checklist, and flow diagram (Figure 1).
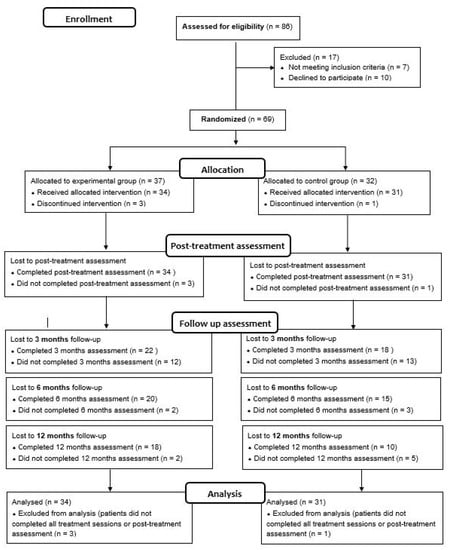
Figure 1.
The CONSORT flow diagram.
The study was carried out in accordance with the Helsinki Declaration of 1975 and was approved by the Clinical Research Ethics Committees of Araba University Hospital (HS/EC/2012-003) and the Clinic Hospital of Barcelona (HCB/2016/0639).
2.2. Participants
The study was conducted on FEP patients who were cannabis users recruited between 2013 and 2019 from Araba University Hospital and Clinic Hospital of Barcelona.
The sample size calculation was performed using Ene 2.0 software and based on previous studies related to this population [27,28,29,30,31]. To achieve an 80% power to detect mean differences from the null hypothesis, H0: μ1 = μ2, using a bilateral Student’s t-test for two independent samples, with a significance level of 5%, an enrollment of 30 patients for each group was estimated, meaning a total of 60 patients for the study.
2.2.1. Inclusion Criteria
The study inclusion criteria for patients were:
- Being diagnosed as FEP according to the revised fourth edition of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [35] (i.e., schizophreniform disorder, schizoaffective disorder, delusional disorder, bipolar disorder with psychotic symptoms, atypical psychosis, brief psychotic disorder, non-specified psychotic disorder, or major depressive disorder with psychotic symptoms).
- Meeting dependence or abuse of cannabis criteria according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IVTR) [35] and the scores of the European Addiction (Europ-ASI) [36,37] (scores of 4 to 7: abuse; scores of 8 to 9: dependence) (Table 1).
 Table 1. Classification of cannabis use for selection of participants.
Table 1. Classification of cannabis use for selection of participants. - Aged between 15 and 40 years. In the case of minors (under 18 years of age), written informed consent was requested from their parents or guardians.
2.2.2. Exclusion Criteria
The study exclusion criteria for patients included organic brain pathology and/or mental disability according to DSM-IVTR criteria.
2.3. Procedure
Patients, who met inclusion criteria and signed the informed consent to participate in the study, were assessed and randomly assigned to one of the treatment groups by permuted block randomization with a block size of 4 and a 1:1 allocation using a computer-generated random sequence. The allocation sequence was prepared by an independent person not otherwise involved in the clinical trial.
All patients were assessed at baseline, post-treatment, and in the follow-up period (at 3 and 6 months and at 1 year of follow-up from the end of the treatment program). The assessment was carried out by a researcher who was blind to the patient allocation process. The evaluators of two participating centers were trained to use scales for inter-rater reliability by rating each of the scales with practical cases. The therapists from both centers received a face-to-face training session and were provided with the same materials that would later be offered to the patients in the intervention program.
2.4. Measures
The assessment protocol is widely described in a previous article by González-Ortega et al. [34]. Sociodemographic variables (age, gender, educational level, socioeconomic level, employment status, family history of psychiatric disorders) were collected using a clinical interview at baseline. Clinical and cannabis/other substance use-related variables were assessed at baseline, post-treatment, and in the follow-up (3, 6, and 12 months of follow-up from the end of the intervention program).
2.4.1. Primary Outcome
The primary outcome was the reduction in cannabis use severity measured by Europ-ASI [36,37], (which assesses the severity of the substance use problem) and DSM-IVTR criteria.
2.4.2. Secondary Outcomes
Cannabis Use
Cannabis and other substance use, including the frequency of use (daily, weekend, weekly, monthly), the amount of cannabis use, the age of onset of use, the history of use (years), and urine samples were collected.
Cannabis use was monitored by patient reports and with repeated urine toxicology tests. Toxins in urine analysis were performed with an immunochromatographic test to detect qualitatively drug metabolites at baseline, at sessions 4 and 8 of the psychological treatment, at post-treatment, and at 3 and 6 months and 1 year of follow-up.
Clinical Variables and Functioning
Patients were diagnosed according to the DSM-IV-TR criteria using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Axis I Disorders (SCID-I) [38].
The Clinical Global Impression Scale (CGI) [39] was used to assess symptom severity (CGI-Severity) and global improvement (CGI-Improvement).
The illness awareness of patients was measured using the scale to assess Unawareness in Mental Disorders (SUMD) [40,41] and medication adherence was assessed with the 4-item Morisky Medication Adherence Scale (MMAS) [42,43].
Positive, negative, and general psychotic symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) [44,45].
Depressive symptoms were assessed with the Hamilton Depression Rating Scale (HDRS) [46,47] and anxiety symptoms with the Hamilton Anxiety Scale (HAM-A) [48,49].
The Young Mania Rating Scale (YMRS) [50,51] was used to measure the manic symptoms.
The functioning of patients was measured using the Functioning Assessment Short Test (FAST) [52], which assesses six specific areas of functioning: autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships, and leisure time.
Determination of Treatment Response
The efficacy of the therapy was assessed analyzing the treatment response of patients. The treatment response in relation to the cannabis cessation was determined by comparing the time it took for patients in both groups to decrease the severity of consumption, evaluated through the decrease in the Europ-ASI scale score (one category of severity) from baseline to one year of follow-up.
The clinical response was defined as a significant improvement in the psychopathology, assessed by a decrease in symptoms from baseline to endpoint of follow-up. The primary efficacy measure was the treatment response, defined according to other studies, as at least a 20% reduction in PANSS total score from baseline to the endpoint [53]. Secondary efficacy variables included the response according to a decrease in the total score ≥ 50% in HDRS [54], HAM-A [55], and YMRS [56] scales.
The functional response was defined as the reduction of one category of severity of functional impairment, according to the cut-off values of the FAST scale [57], which has been validated in this population [58,59].
2.5. Intervention Programs
Patients were randomized into two treatment groups (TAU or CBT-CC).
2.5.1. Treatment as Usual (TAU)
TAU refers to the combined clinical treatment provided to FEP patients that included the pharmacological treatment prescribed by the psychiatrist and an individual psychological therapy involving psychoeducation and CBT, following the same format as the CBT-CC group, that is, 1-h sessions once a week for 16 weeks.
Therapy sessions include career counseling and information to enable patients to understand and be able to manage their disease, providing them cognitive–behavioral tools for symptom management and relapses and to contribute to their well-being.
The first part of the psychological program (sessions 1–9) was composed of psychoeducational sessions aimed at improving patients’ insight into their illness, treatment adherence, prodromal identification, early intervention to prevent relapses, and a healthy lifestyle. The second part of the intervention (sessions 10–16) included cognitive–behavioral techniques for symptom and thought management (anxiety management techniques) and social and problem-solving skills.
2.5.2. Specific CBT for Cannabis Cessation (CBT-CC)
The CBT-CC group received a specific individual CBT for cannabis cessation composed of 1-h sessions once a week for 16 weeks, in addition to the pharmacological treatment prescribed by the psychiatrist.
The treatment included a cognitive–behavioral approach that integrated three fundamental aspects: (1) motivational strategies to develop a good therapeutic alliance and motivation for change, (2) cognitive–behavioral techniques for cannabis abstinence, symptom management, and improvement in psychosocial functioning, and (3) a specific intervention for change maintenance and relapse prevention. The content of the sessions is as follows:
Sessions 1–4: The first four sessions involved motivational interviewing [60], followed by brief psychoeducation focused on general information about cannabis and psychosis: (a) psychosis and substance use, (b) medication and treatment adherence, (c) awareness of the vulnerability, (d) recognition of symptoms, (e) healthy lifestyle, and (f) risk and protective factors.
Sessions 5–14: The second part of the program was focused on commitment to change [61] and included the following aspects:
Behavioral therapy:
- -
- Anxiety management techniques;
- -
- Stimulus control;
- -
- In vivo exposure therapy with response prevention, identifying triggers and beliefs that could lead to substance use and exacerbation of psychotic symptoms and exposure to such triggers.
Cognitive therapy:
- -
- Specific techniques for managing thoughts about the consumption and use of cannabis and other substances (craving/abstinence) and symptom management;
- -
- Cognitive restructuring; identifying and refuting cognitive distortions;
- -
- Training in problem-solving;
- -
- Training in social skills; assertiveness; skills to refuse drugs and changes in lifestyle.
Sessions 15–16: The third part of the program included a specific intervention for relapse prevention, focused on the identification of high-risk situations that could lead to maintenance of substance use and increased severity and chronicity of psychotic symptoms, as well as the teaching of coping skills for such situations.
2.6. Statistical Analysis
SPSS Statistics for Windows (version 23.0, IBM, Armonk, NY, USA) was used for the statistical analysis, with the significance level set at p ≤ 0.05.
Differences in baseline sociodemographic and clinical characteristics between intervention groups were analyzed using the χ2 test for categorical variables and Student’s t-test for continuous variables.
With respect to cannabis consumption, the concordance between results identified in urine toxicology tests and clinician rating scale determined by participant-reported cannabis use and DSM-IVTR [35]/Europ-ASI [36,37] criteria was evaluated using the Kappa Coefficient.
Ordinal mixed-effects models were used to analyze the differences in cannabis use reduction over time (from baseline to the 12 months of follow-up) between the two intervention groups. The efficacy of the therapy for cannabis cessation and the effect of cannabis abstinence/reduction in terms of clinical and functional outcomes for patients were analyzed with lineal mixed-effects models for the analyses of repeated measures. First, we assessed the individual effect of gender, age, civil status, socioeconomic level, educational level, family history, adherence to treatment, age at onset of cannabis use, and other substance use. Those variables for which the ANOVA test had a p-value < 0.1 were included in the final model. Finally, multivariate mixed-effects models were fitted, including the intervention group (CBT-CC vs. TAU) or the cannabis abstinence/reduction vs. continued use group, those confounding variables previously selected, and the time variable. The analysis of cannabis abstinence/reduction vs. continued use was performed with all samples and the effect of the intervention group was controlled. The reference category for the grouping variables was the TAU group and the continued cannabis use, respectively. A random effect was included in these models to account for the repeated measure structure of the data and was performed with maximum likelihood methods.
Survival analysis (Kaplan–Meier and adjusted Cox regression) was used to determine differences between intervention groups in terms of time to treatment response.
3. Results
3.1. Baseline Characteristics of the Sample
A total of 69 FEP patients (35 for the CBT-CC group and 34 for the TAU group) met inclusion criteria, consented to participate, and were initially included in the study. Of these, four (three in the CBT-CC group and one in the TAU group) dropped out or did not complete all intervention sessions. The analyses were performed with patients who completed all treatment sessions, and performed at least the post-treatment assessment, so the final study sample consisted of 65 patients (34 were assigned in the CBT-CC group and 31 in the TAU group) (Figure 1).
The majority of the sample (72.3%) were men and (27.7%) were women. The mean age of the sample was 25.78 years (7.08), and the age of cannabis onset was 15.55 (2.55). Thirty-nine (60%) of patients met the criteria for cannabis abuse and 26 (40%) for cannabis dependence.
There were no statistically significant differences between the two treatment groups on any baseline measures. The sociodemographic, clinical, and cannabis use baseline characteristics of the participants of the two groups are presented in Table 2.

Table 2.
Baseline sociodemographic and clinical characteristics of the sample.
3.2. Outcomes
3.2.1. Primary Outcome: Evolution of Cannabis Use Severity over 12 Months
When cannabis use of the CBT-CC and TAU groups was compared over time (from baseline to the 12 months of follow-up), the ordinal mixed models’ results revealed significant differences between the two groups regarding the change in cannabis use, assessed by Europ-ASI scores. The CBT-CC group had a greater decrease in the severity of consumption compared to the TAU group (β = 0.190; p < 0.001). Patients who received specific therapy for cannabis use cessation had a greater reduction in the cannabis use severity than TAU at post-treatment (β = −1.418, p < 0.001) and in all follow-up visits (3 months: β = −0.990, p = 0.002; 6 months: β = −1.167, p ≤ 0.001; 12 months: β = −1.091, p = 0.004).
Specifically, 50% of the CBT-CC group sample achieved abstinence at post-treatment versus 12.9% of TAU, and the severity of their cannabis use was also lower in the follow-up compared to TAU (χ2 = 29.055; p < 0.001). Namely, 29.4% of patients used, 17.6 abused, and only 2.9% met the criteria for cannabis dependence, whereas the majority of TAU group patients were dependent (48.4%) on or abused (35.5%) cannabis. These results were maintained at follow-up, with a higher percentage of abstinence or reduction in the severity of consumption in patients of the CBT-CC group compared to TAU (Table 3).

Table 3.
Cannabis use severity at follow-up.
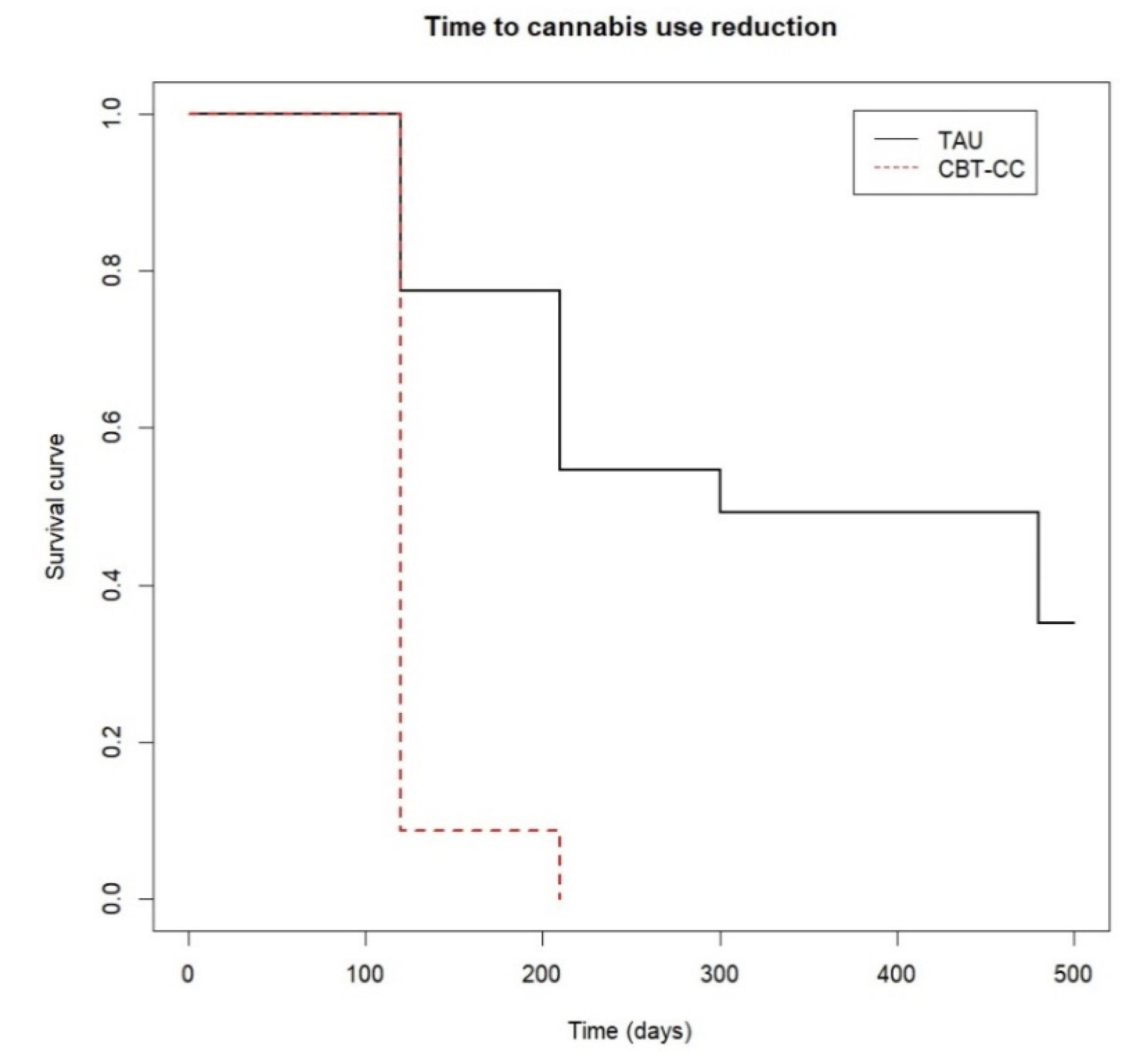
The survival analysis also showed that the CBT-CC group achieved a decrease in cannabis use much earlier than the TAU group (log-rank test: χ2 = 34.34, p ≤ 0.001). In fact, the CBT-CC group had seven times more likely to decrease a category of the severity of consumption than the TAU group (HR = 7.681, 95% CI: 3.58, 16.48). Specifically, the median time indicated that 50% of the CBT-CC group patients reduced cannabis use in less than 120 days, (which coincided with the end of therapy), while in the TAU group the median time was 300 days (Figure 2).
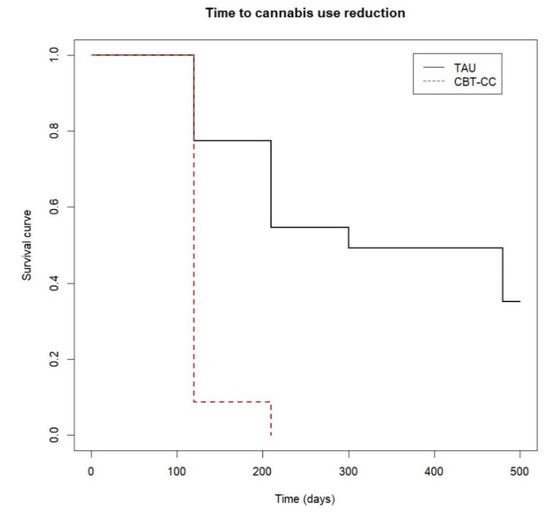
Figure 2.
Time to cannabis use reduction.
There was a high concordance (Kappa index) between cannabis use identified in urine toxicology tests and clinician rating scale determined by participant-reported cannabis use and Europ-ASI/DSM-IVTR criteria, at post-treatment (k = 0.893) and in each follow-up visit (3 months: k = 1; 6 months: k = 0.941; 12 months: k = 0.924, p < 0.001).
3.2.2. Secondary Outcomes
Frequency and Amount of Cannabis Use Thought the Follow-Up
At baseline, patients in both groups consumed cannabis daily and there were no differences in the amount of cannabis consumed, with an average of 2.204 (0.956) grams per day in the TAU group and a mean of 1.883 (0.956) grams in the CBT-CC group. However, there were statistically significant differences between two groups at post-treatment and in the follow-up, with a greater reduction in the amount and frequency of cannabis used in the CBT-CC group compared to TAU. Forty-seven percent of the CBT-CC group patients were abstinent post-treatment and more than half remained abstinent at 3, 6, and 12 months of follow-up. However, only 12.9% of TAU group patients discontinued cannabis post-treatment and the majority of them continued to use cannabis daily at follow-up (Table 4).

Table 4.
Frequency and amount of cannabis use throughout the follow-up.
Clinical and Functional Outcomes
Regarding clinical and functional outcomes of patients, although both groups improved their outcomes throughout the follow-up, the experimental group obtained a greater reduction in psychotic symptoms over time (except at the 12-month follow-up visit) than the control group (post-treatment: β = −5.225, p < 0.001; 3 months: β = −4.143, p = 0.007; 6 months: β = −5.045, p = 0.003). The CBT-CC group also showed a greater improvement in functional outcome (β =−8.111; p = 0.036) compared to the TAU group at post-treatment, with no significant differences between groups in the follow-up. There were no differences between groups in other clinical outcomes evolution (negative and general psychotic symptoms, depressive, manic, anxiety symptoms, or awareness of disease) over the 12 months of follow-up.
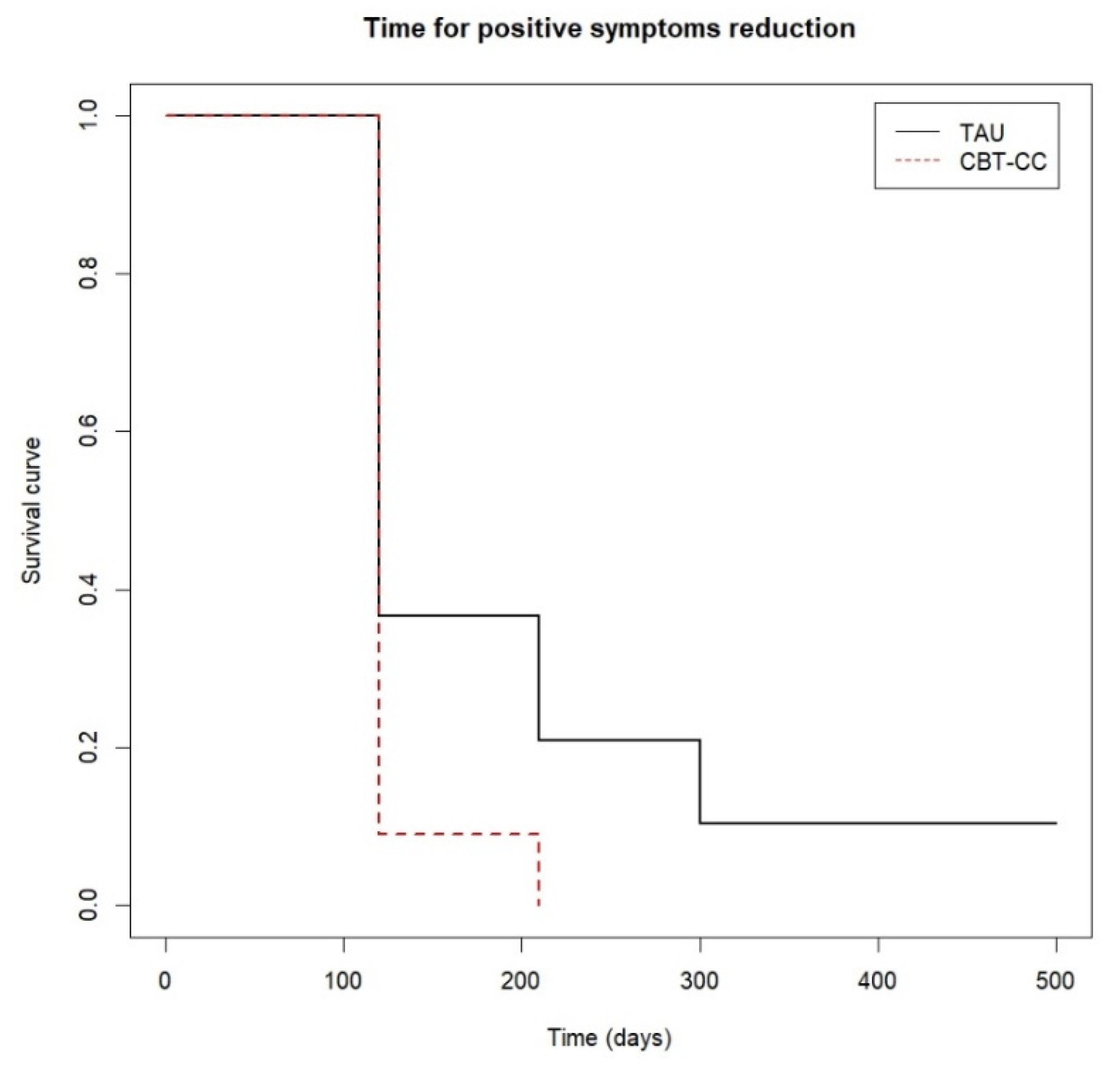
When we analyzed the survival analysis of the time it took each group to reach the clinical improvement criterion, the CBT-CC group showed a greater acceleration in the achievement of the improvement criterion, according to the decrease in the score of the PANSS P and G, HAM-A and FAST scales, compared to the TAU group (Figure 3, Figure 4, Figure 5 and Figure 6). Regarding positive symptoms, Figure 3 shows that the CBT-CC group achieved the reduction earlier than the TAU group (log-rank test: χ2 = 15.78, p = 0.003). In fact, 90% of CBT-CC group patients achieved a response at post-treatment (120 days), while the TAU group needed 6 months (300 days). The CBT-CC experimental group was twice as likely to reduce positive symptoms by at least 20%, compared to the TAU group (HR = 2.148, 95%CI: 1.209, 3.817).
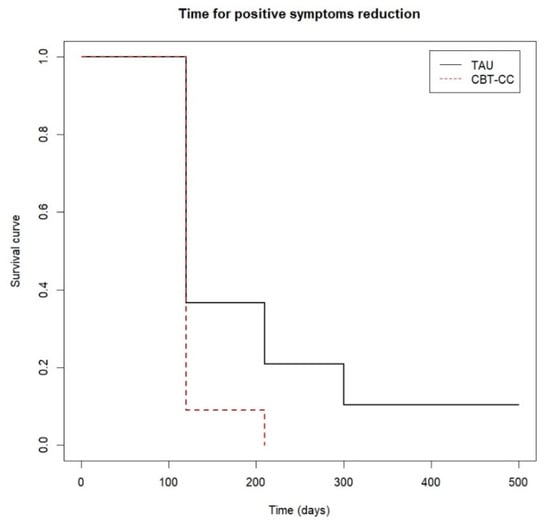
Figure 3.
Time to positive psychotic symptoms reduction.
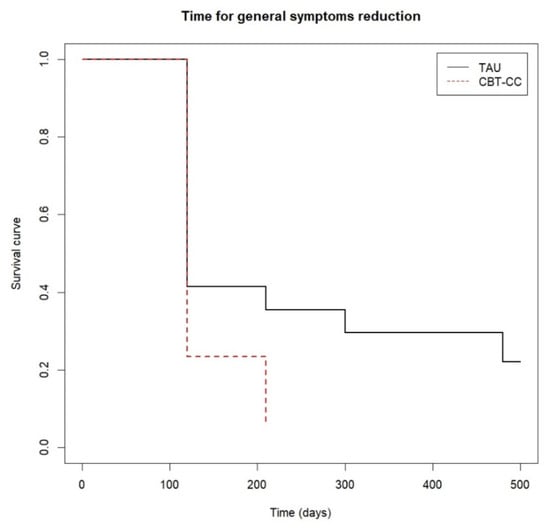
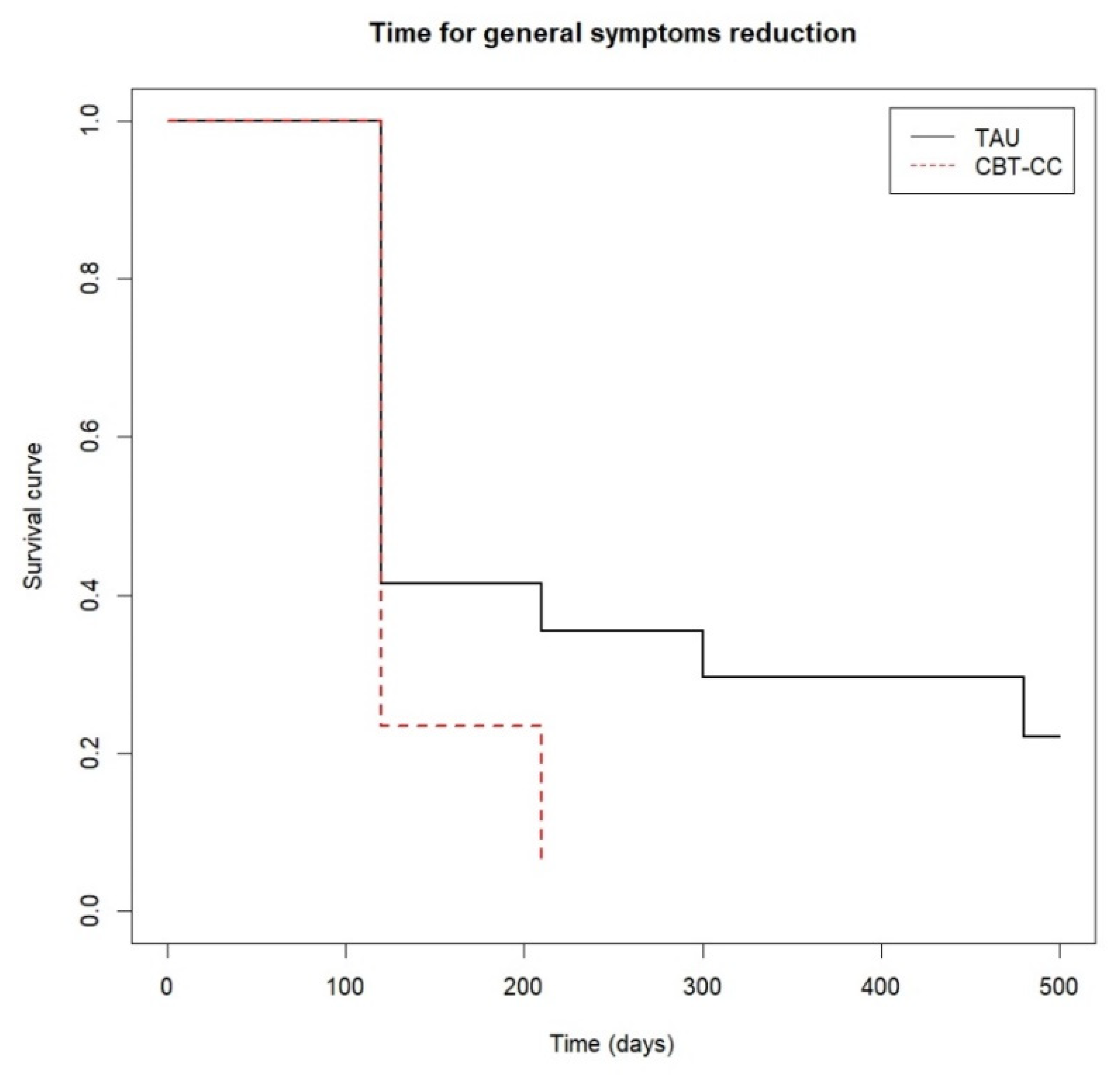
Figure 4.
Time to general psychotic symptoms reduction.
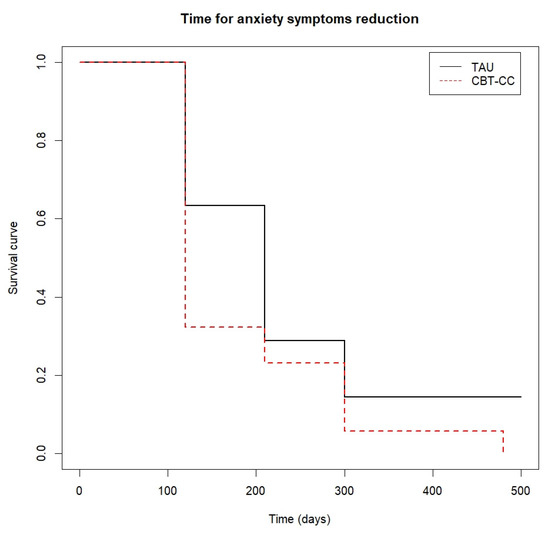
Figure 5.
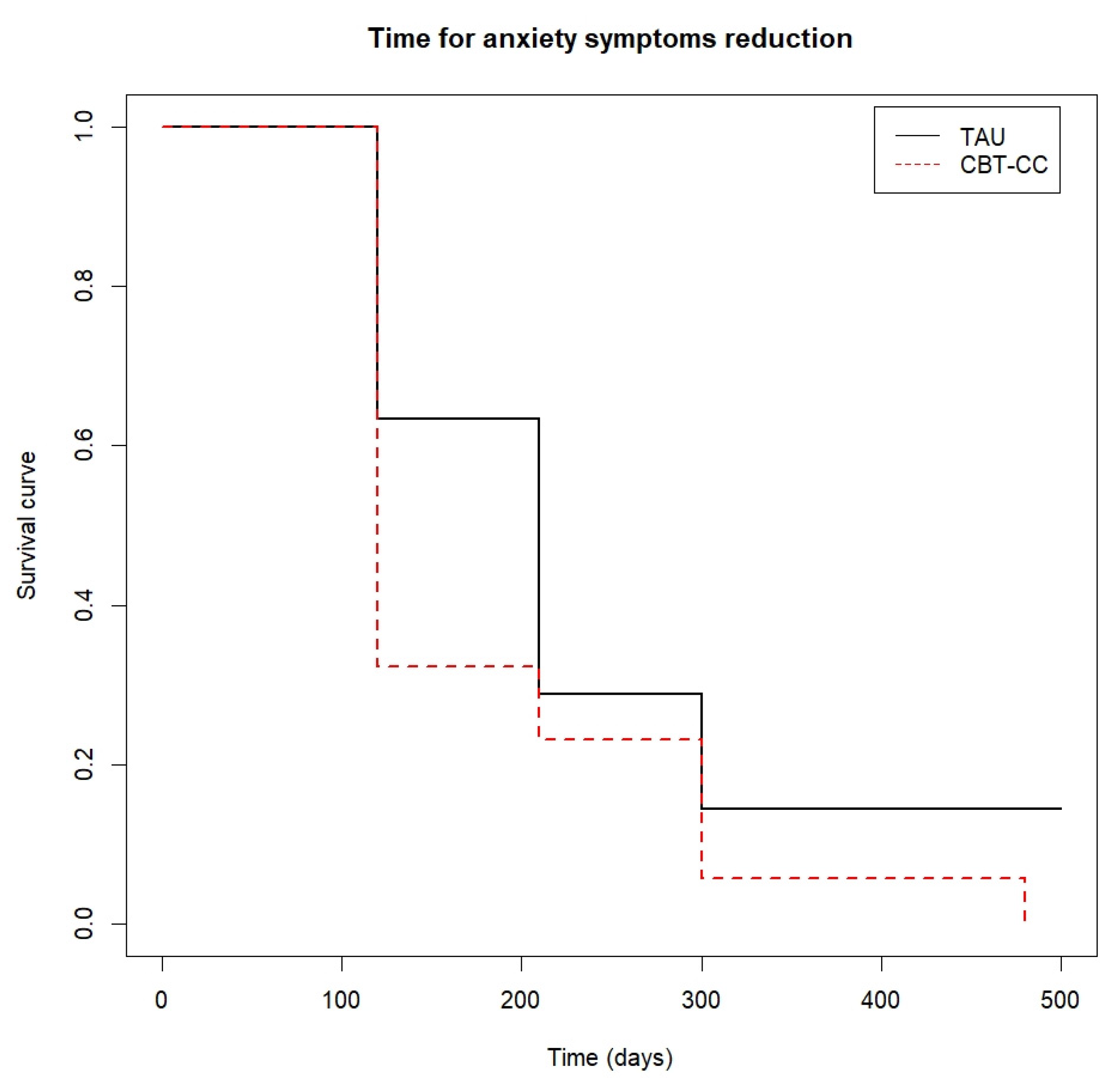
Time to anxiety symptoms reduction.
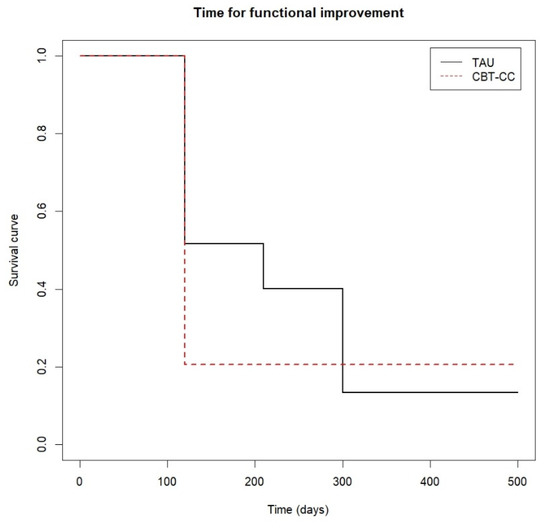
Figure 6.
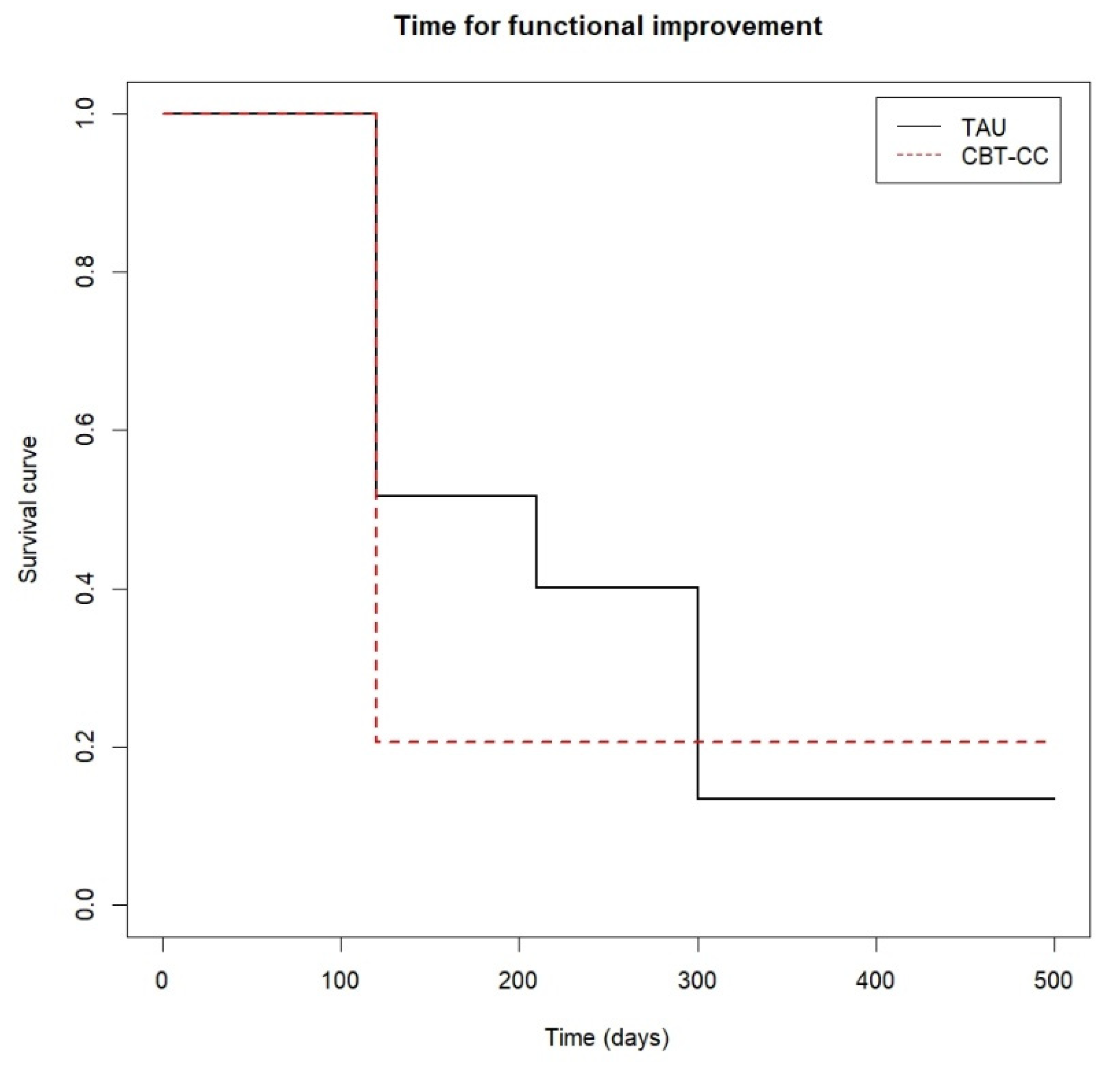
Time to functional improvement.
There were significant differences between the curves of both groups at follow-up (log-rank test: χ2 = 8.87, p = 0.010). Approximately 80% of the CBT-CC group achieved the response criterion post-treatment, while the 80% of the TAU group decreased the general psychotic symptoms at 12 months of follow-up, with a greater probability of reducing symptoms in the CBT-CC group in the follow-up (HR = 1.74, 95%CI: 0.963, 3.162) (Figure 4).
The CBT-CC group was also more likely to achieve a 50% reduction in anxiety score (log-rank test: χ2 = 3.85, p = 0.05), being the median time in this group of 120 days versus 210 days in the TAU group (HR = 1.775, 95%CI: 0.993, 3.172) (Figure 5).
Finally, Figure 6 shows that approximately 80% of the CBT-CC group patients managed to reduce one category in the FAST scale at 120 days (post-treatment) from baseline, while in the TAU group at 210 days (3 months) only 60% of patients achieved improvement criteria. That is, the CBT-CC group managed to improve functionality in less time than the TAU group (log-rank test: χ2 = 7.68, p = 0.05, HR = 1.686, 95%CI: 0.922, 3.081).
Relationship between Cannabis Cessation/Reduction and Clinical and Functional Outcomes
The relation between cannabis cessation and/or reduction and clinical and functional outcomes of patients was analyzed, and the results showed that patients who stopped or reduced cannabis use during the follow-up, decreased general psychotic (β = −0.754; p = 0.022) compared to those who continued to cannabis use. In addition, patients who stopped and/or reduced their consumption had a better awareness of the disease than those who continued to consume during follow-up (β = 0.197; p = 0.050).
4. Discussion
This randomized controlled trial aimed to compare the efficacy of a specific CBT program for cannabis use cessation with standard treatment in patients with FEP cannabis users.
The primary outcome of the study was that CBT-CC patients had a greater decrease in the severity of cannabis use over time, compared to the TAU group. Moreover, the treatment response was faster in the CBT-CC group, reducing their cannabis use in around 120 days, (which coincided with post-treatment), while in the control group the time to reduce the consumption was 300 days (around 6 months of follow-up). Very few RCTs have evaluated psychosocial interventions for cannabis use cessation in FEP, and these have not found benefits in terms of reduction in cannabis use in the follow-up, compared with TAU [27,28,29,30,31,32]. Therefore, our results are promising and suggest that our intervention could be useful for cannabis cessation in FEP patients.
The second main finding of the study is that our psychological therapy also proved to be effective in improving clinical and functional outcomes of FEP. The CBT-CC group had a greater reduction in positive psychotic symptoms and a greater improvement in functional outcomes post-treatment compared to the TAU group. Moreover, the CBT-CC group reached the treatment response, that is, the clinical and functional improvement criterion, faster in the follow-up than the TAU group. Specifically, the majority of patients (80–90%) who received CBT-CC reduced the positive and general psychotic symptoms earlier (at post-treatment), while the TAU group needed between 6 and 12 months to reach the same percentages of response. In addition, the CBT-CC group also decreased anxiety symptoms and achieved functional response in less time (at post-treatment versus 3 and 6 months, respectively). These results have not been tested in other RCTs that have assessed the efficacy of an integrated psychological intervention to reduce cannabis use and improve the outcome of FEP [27,29,31,32]. Only in the study conducted by Madigan et al. [30], the intervention improved the quality of life of patients at 3 months and one-year follow-up; however, this was not associated with a reduction in cannabis use or improvement in clinical outcomes. Our findings revealed that the success of therapy in symptom improvement may be related to the dual therapeutic approach, aimed at both cannabis cessation and symptom improvement so that the cannabis reduction/cessation enhanced the efficacy of strategies aimed at managing symptoms.
The third finding of the study was that cannabis cessation influenced the clinical and functional outcomes of FEP. Patients who stopped and/or reduced cannabis use during the follow-up decreased psychotic symptoms and had a better awareness of the disease compared to those who continued cannabis use during follow-up. Other observational longitudinal studies confirm that cannabis use cessation has been related to an improvement in clinical and functional outcomes of FEP in the follow-up [18,20]. In a recent study, Setién-Suero et al. [26] also found that persistent cannabis FEP users had more severe symptoms and poorer functionality compared to ex-users and never-users at a 10-year follow-up. Moreover, patients who stopped cannabis do not differ from those who had never consumed.
Our findings have several strengths and relevant implications for clinical practice and underscore the importance of treating early FEP cannabis users in order to improve the prognosis of their disease. Since cannabis has a significant role in the prognosis of FEP patients and given that the deleterious effect of cannabis could be reversed with the decrease or cessation of consumption, there is a need for early intervention for cannabis cessation after the onset of FEP. Although CBT has previously proven to be a useful therapeutic approach to treat and improve clinical and functional outcomes in FEP [62], to date no specific therapies had been found that have been shown to be effective for FEP cannabis users. This integrated psychological CBT intervention has been shown to be effective both in cannabis cessation use and in improving clinical and functional outcomes of FEP cannabis users, following the paradigm of precision psychotherapy [63].
The key aspects of the therapy are developing a good therapeutic alliance and motivation for change, training in coping skills and self-management strategies, and relapse prevention. Our results support the importance of developing a good therapeutic alliance in the first therapy sessions, to facilitate motivation for change in this type of patient, who often have little illness awareness, and minimize the consequences of cannabis use [64]. A crucial psychological treatment target is to increase awareness about the problematic cannabis use behavior and the impact of cannabis use on the prognosis of the psychosis. The objective is to know the cannabis use behavior, the perceptions, and knowledge about cannabis use as well as provide them with a brief psychoeducation focused on general information about cannabis use, psychosis, and the relationship between cannabis use and psychosis. It is also essential to establish a change plan with the patient [60,61], considering not only abstinence but also the reduction in cannabis use severity for those patients who are not ready to reduce or quit cannabis, as a change goal and therapeutic objective [65]. The strategies aimed at improving the awareness of the adverse impact of cannabis use are critical to cannabis cessation and maintenance of change [66] (Hides et al., 2016). In our FEP cannabis users’ sample, patients who stopped and/or reduced their consumption, had a better awareness of the disease compared to those who continued to consume during follow-up, so increased awareness of cannabis use may be critical to increasing motivation for cannabis cessation and strengthen the commitment to change and ultimately, improve clinical and functional outcomes.
In the second phase of treatment, the aim was to help the patient make the proposed changes through training in coping skills and self-control strategies, applying specific cognitive–behavioral techniques to treat both the psychotic disorder and cannabis abstinence. Our findings showed that psychological therapy, with a dual approach aimed at both cannabis cessation and psychosis, proved to be effective in both reducing cannabis and improving psychotic symptoms and functional outcomes. Since cannabis use has been related to higher severity of positive psychotic symptoms (11, 18, 19) and a worse prognosis in patients with FEP (20–22), it is essential to teach the patient coping strategies such as cognitive distraction or cognitive restructuring techniques to learn to manage positive symptoms and reduce the likelihood of relapse. Likewise, providing the patient with strategies to deal with both situations of risk of consumption (training in skills to reject cannabis use) and situations of daily life (communication skills, assertiveness training, skills to improve social cognition), will improve patient functioning and the course of the disease.
Finally, it is common that during the recovery process relapses occur, so a key aspect of our therapy is to develop strategies for change maintenance and relapse prevention [67]. In our FEP sample, the CBT-CC group achieved a significant decrease in the severity of cannabis use post-treatment and it was maintained at follow-up. The treatment response was also reached post-treatment and was maintained over time, reinforcing the importance of developing a relapse plan.
This study has some limitations. One of the limitations of the study is related to the usual loss of patients in the follow-up in this type of clinical trial with a longitudinal design, which should be taken into consideration in interpreting the efficacy of the therapy. In future studies, it is recommended to develop motivational strategies for patient retention in follow-up evaluations. Our study had a follow-up of 1 year; a longer follow-up period should be considered in future research. Another limitation is that the psychological treatment programs had an individual format, while others employed a group or family approach, given the relevance of the family environment [68]. Finally, the influence of the type of cannabis used (marijuana, hashish) and/or the form of consumption (smoked or inhaled) on the prognosis of FEP could be considered in future studies.
5. Conclusions
The results of this study suggest that early intervention based on a specific CBT program for cannabis cessation, that combines therapeutic strategies aimed at addressing both mental and addictive disorders for patients with FEP and cannabis use comorbidity, may be effective in reducing the cannabis use severity, in addition to improving clinical and functional outcomes of FEP patients.
Author Contributions
Conceptualization, I.G.-O., E.E. and A.G.-P.; methodology, I.G.-O., E.E. and A.G.-P.; software, formal analysis, data curation, and visualization, S.A.; investigation, I.G.-O., E.E., M.B. and A.G.-P.; writing—original draft preparation, I.G.-O.; writing—review and editing, I.G.-O., E.E., E.V., G.S.d.P. and A.G.-P.; validation, supervision, project administration, and funding acquisition, I.G.-O. and A.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Spanish Ministry of Economy and Competitiveness through the Carlos III Health Institute (ISCIII) and the European Regional Development Fund (ERDF) (PI13/02252, PI18/01055, PI21/00713, PI19/00569).
Institutional Review Board Statement
The study was approved by the Clinical Research Ethics Committees of all the participating centers: Araba University Hospital (HS/EC/2012-003), Clinic Hospital of Barcelona (HCB/2016/0639).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are contained within the article.
Acknowledgments
We would like to thank the following institutions: the Basque Foundation for Health Innovation and Research (BIOEF), the Centre for Biomedical Research in the Mental Health Network (CIBERSAM), the University of the Basque Country, and Bioaraba Research Institute. The authors also want to thank the student Ivan Ballester for his collaboration in the study.
Conflicts of Interest
Ana González-Pinto has received grants from or served as a consultant, advisor, or CME speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Ferrer, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministries of Science and Innovation (through CIBERSAM) and of Science (through the Carlos III Institute of Health), the Basque Government, the Stanley Medical Research Institute, and Wyeth. Miguel Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Rovi, and Takeda. Eduard Vieta has received grants and served as consultant, advisor, or CME speaker for the following entities: AB-Biotics, AbbVie, Angelini, Biogen, Boehringer-Ingelheim, Celon Pharma, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, Janssen, Lundbeck, Novartis, Orion Corporation, Organon, Otsuka, Sage, Sanofi-Aventis, Sunovion, and Takeda, outside the submitted work. The other authors have no other conflict of interest to declare.
References
- Oluwoye, O.; Monroe-DeVita, M.; Burduli, E.; Chwastiak, L.; McPherson, S.; McClellan, J.M.; McDonell, M.G. The impact of tobacco, alcohol, and cannabis use in patients with first-episode psychosis: Data from the national RAISE-ETP study. Early Interv. Psychiatry 2019, 13, 142–146. [Google Scholar] [CrossRef]
- Plan Nacional Sobre Drogas. Alcohol, Tabaco y Drogas Ilegales en España. Informe 2020; Delegación del Gobierno para el Plan Nacional sobre Drogas, Ministerio de Sanidad: Madrid, Spain, 2020. [Google Scholar]
- Batalla, A.; Maat, A. Cannabis use and psychosis susceptibility: A call to action. Eur. Neuropsychopharmacol. 2022, 54, 70–71. [Google Scholar] [CrossRef]
- González-Pinto, A.; Vega, P.; Ibañez, B.; Mosquera, F.; Barbeito, S.; Gutierrez, M.; Ruiz de Azúa, S.; Ruiz, I.; Vieta, E. Impact of cannabis and other drugs on age at onset psychosis. J. Clin. Psychiatry 2008, 69, 1210–1216. [Google Scholar] [CrossRef]
- Myles, H.; Myles, N.; Large, M. Cannabis use in first episode psychosis: Meta-analysis of prevalence, and the time course of initiation and continued use. Aust. N. Z. J. Psychiatry 2016, 50, 208–219. [Google Scholar] [CrossRef]
- Stone, J.M.; Fisher, H.L.; Major, B.; Chisholm, B.; Woolley, J.; Lawrence, J.; Rahaman, N.; Joyce, J.M.; Hinton, M.; Johnson, S.; et al. Cannabis use and first-episode psychosis: Relationship with manic and psychotic symptoms, and with age at presentation. Psychol. Med. 2014, 44, 499–506. [Google Scholar] [CrossRef]
- van der Steur, S.J.; Batalla, A.; Bossong, M.G. Factors Moderating the Association Between Cannabis Use and Psychosis Risk: A Systematic Review. Brain Sci. 2020, 10, 97. [Google Scholar] [CrossRef]
- Di Forti, M.; Quattrone, D.; Freeman, T.P.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.E.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [Google Scholar] [CrossRef]
- Marconi, A.; di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef]
- Murray, R.M.; Quigley, H.; Quattrone, D.; Englund, A.; Di Forti, M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: Increasing risk for psychosis. World J. Psychiatry 2016, 15, 195–204. [Google Scholar] [CrossRef]
- Hasan, A.; von Keller, R.; Friemel, C.M.; Hall, W.; Schneider, M.; Koethe, D.; Leweke, F.M.; Strube, W.; Hoch, E. Cannabis use and psychosis: A review of reviews. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 403–412. [Google Scholar] [CrossRef]
- Arranz, S.; Mané, A.; Bergé, D.; Monserrat, C.; Cabezas, A.; Vilella, E.; Sanchez-Gistau, V. The impact of sex and cannabis on clinical features in first-admitted patients with psychosis. Eur. Neuropsychopharmacol. 2020, 36, 235–243. [Google Scholar] [CrossRef]
- Bioque, M.; Mas, S.; Costanzo, M.C.; Cabrera, B.; Lobo, A.; González-Pinto, A.; Rodriguez-Toscano, E.; Corripio, I.; Vieta, E.; Baeza, I.; et al. Gene-environment interaction between an endocannabinoid system genetic polymorphism and cannabis use in first episode of psychosis. Eur. Neuropsychopharmacol. 2019, 29, 786–794. [Google Scholar] [CrossRef]
- Sami, M.B.; Bhattacharyya, S. Are cannabis-using and non-using patients different groups? Towards understanding the neurobiology of cannabis use in psychotic disorders. J. Psychopharmacol. 2018, 32, 825–849. [Google Scholar] [CrossRef]
- García, S.; Martínez-Cengotitabengoa, M.; López-Zurbano, S.; Zorrilla, I.; López, P.; Vieta, E.; González-Pinto, A. Adherence to Antipsychotic Medication in Bipolar Disorder and Schizophrenic Patients: A Systematic Review. J. Clin. Psychopharmacol. 2016, 36, 355–371. [Google Scholar] [CrossRef]
- Foglia, E.; Schoeler, T.; Klamerus, E.; Morgan, K.; Bhattacharyya, S. Cannabis use and adherence to antipsychotic medication: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1691–1705. [Google Scholar] [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Klamerus, E.; Foglia, E.; Murray, R.; Bhattacharyya, S. Effect of continued cannabis use on medication adherence in the first two years following onset of psychosis. Psychiatry Res. 2017, 255, 36–41. [Google Scholar] [CrossRef]
- Clausen, L.; Hjorthøj, C.R.; Thorup, A.; Jeppesen, P.; Petersen, L.; Bertelsen, M.; Nordentoft, M. Change in cannabis use, clinical symptoms and social functioning among patients with first-episode psychosis: A 5-year follow-up study of patients in the OPUS trial. Psychol. Med. 2014, 44, 117–126. [Google Scholar] [CrossRef]
- Seddon, J.L.; Birchwood, M.; Copello, A.; Everard, L.; Jones, P.B.; Fowler, D.; Amos, T.; Freemantle, N.; Sharma, V.; Marshall, M.; et al. Cannabis Use Is Associated with Increased Psychotic Symptoms and Poorer Psychosocial Functioning in First-Episode Psychosis: A Report from the UK National EDEN Study. Schizophr. Bull. 2016, 42, 619–625. [Google Scholar] [CrossRef]
- González-Pinto, A.; Alberich, S.; Barbeito, S.; Gutierrez, M.; Vega, P.; Ibáñez, B.; Haidar, M.K.; Vieta, E.; Arango, C. Cannabis and first-episode psychosis: Different long-term outcomes depending on continued or discontinued use. Schizophr. Bull. 2011, 37, 631–639. [Google Scholar] [CrossRef]
- Patel, R.; Wilson, R.; Jackson, R.; Ball, M.; Shetty, H.; Broadbent, M.; Stewart, R.; McGuire, P.; Bhattacharyya, S. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: An observational study. BMJ Open 2016, 6, e009888. [Google Scholar] [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Klamerus, E.; Foglia, E.; Ajnakina, O.; Gayer-Anderson, C.; Colizzi, M.; Quattrone, D.; Behlke, I.; et al. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: An observational study. Lancet Psychiatry 2016, 3, 947–953. [Google Scholar] [CrossRef]
- Bahorik, A.L.; Newhill, C.E.; Eack, S.M. Characterizing the longitudinal patterns of substance use among individuals diagnosed with serious mental illness after psychiatric hospitalization. Addiction 2013, 108, 1259–1269. [Google Scholar] [CrossRef]
- Faber, G.; Smid, H.G.; Van Gool, A.R.; Wunderink, L.; van den Bosch, R.J.; Wiersma, D. Continued cannabis use and outcome in first-episode psychosis: Data from a randomized, open-label, controlled trial. J. Clin. Psychiatry 2012, 73, 632–638. [Google Scholar] [CrossRef]
- González-Blanch, C.; Gleeson, J.F.; Koval, P.; Cotton, S.M.; McGorry, P.D.; Alvarez- Jimenez, M. Social functioning trajectories of young first-episode psychosis patients with and without cannabis misuse: A 30-month follow-up study. PLoS ONE 2015, 10, e0122404. [Google Scholar] [CrossRef]
- Setién-Suero, E.; Neergaard, K.; Ortiz-García de la Foz, V.; Suárez-Pinilla, P.; Martínez-García, O.; Crespo-Facorro, B.; Ayesa-Arriola, R. Stopping cannabis use benefits outcome in psychosis: Findings from 10-year follow-up study in the PAFIP-cohort. Acta Psychiatr. Scand. 2019, 140, 349–359. [Google Scholar] [CrossRef]
- Bonsack, C.; Gibellini, S.; Favrod, J.; Montagrin, Y.; Besson, J.; Bovet, P.; Conus, P. Motivational intervention to reduce cannabis use in young people with psychosis: A randomized controlled trial. Psychother. Psychosom. 2011, 80, 287–297. [Google Scholar] [CrossRef]
- Edwards, J.; Elkins, K.; Hinton, M.; Harrigan, S.M.; Donovan, K.; Athanasopoulos, O.; McGorry, P.D. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr. Scand. 2006, 114, 109–117. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Fohlmann, A.; Larsen, A.; Gluud, C.; Arendt, M.; Nordentoft, M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: The CapOpus randomized trial. Psychol. Med. 2013, 43, 1499–1510. [Google Scholar] [CrossRef]
- Madigan, K.; Brennan, D.; Lawlor, E.; Turner, N.; Kinsella, A.; O’Connor, J.J.; Russell, V.; Waddington, J.L.; O’Callaghan, E. A multi-center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophr Res. 2013, 143, 138–142. [Google Scholar] [CrossRef]
- Barrowclough, C.; Marshall, M.; Gregg, L.; Fitzsimmons, M.; Tomenson, B.; Warburton, J.; Lobban, F. A phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: A randomized controlled trial. Psychol. Med. 2014, 44, 2749–2761. [Google Scholar] [CrossRef]
- Cather, C.; Brunette, M.F.; Mueser, K.T.; Babbin, S.F.; Rosenheck, R.; Correll, C.U.; Kalos-Meyer, P. Impact of comprehensive treatment for first episode psychosis on substance use outcomes: A randomized controlled trial. Psychiatry Res. 2018, 268, 303–311. [Google Scholar] [CrossRef]
- Vieta, E.; Berk, M. Early intervention comes late. Eur. Neuropsychopharmacol. 2022, 59, 1–3. [Google Scholar] [CrossRef]
- González-Ortega, I.; Echeburúa, E.; García-Alocén, A.; Vega, P.; González-Pinto, A. Cognitive behavioral therapy program for cannabis use cessation in first-episode psychosis patients: Study protocol for a randomized controlled trial. Trials 2016, 17, 372. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders (DSM IV-TR), 4th ed.; text rev.; American Psychiatric Press: Washington, DC, USA, 2000. [Google Scholar]
- Kokkevi, A.; Hartgers, C. EuropASI: European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. Eur. Addict. Res. 1995, 1, 208–210. [Google Scholar] [CrossRef]
- Bobes, J.; González, M.P.; Sáiz, P.A.; Bousoño, M. (Eds.) Índice europeo de la severidad de la adicción: EuropASI, Versión española. In Actas IV Reunión Interregional de Psiquiatría; 1996; pp. 201–218. Available online: https://15f8034cdff6595cbfa1-1dd67c28d3aade9d3442ee99310d18bd.ssl.cf3.rackcdn.com/8858b1de688c412047067d05fbfcba70/EUROPASI.pdf (accessed on 1 June 2022).
- First, M.B.; Spitzer, R.; Gibbon, M. Structured Clinical Interview for DSM-IV. Axis I Disorders; American Psychiatric Press Inc.: Washington, DC, USA, 1997. [Google Scholar]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology-Revised; Department of Health, Education and Welfare: Rockville, MD, USA, 1976. [Google Scholar]
- Amador, X.F.; Strauss, D.H.; Yale, S.A.; Flaum, M.M.; Endicott, J.; Gorman, J.M. Assessment of insight in psychosis. Am. J. Psychiatry 1993, 150, 873–879. [Google Scholar]
- Ruiz, A.; Pousa, E.; Duñó, R.; Crosas, J.; Cuppa, S.; García, C. Spanish adaptation of the Scale to Assess Unawareness of Mental Disorder (SUMD). Actas Esp. Psiquiatr. 2008, 36, 1191–1198. [Google Scholar]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef]
- Val, A.; Amorós, G.; Martínez, P.; Fernández, M.L.; León, M. Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test. Aten. Primaria 1992, 10, 767–770. (In Spanish) [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizoph. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Peralta-Martin, V.; Cuesta-Zorita, M.J. Validation of Positive and Negative Symptom Scale (PANSS) in a sample of Spanish schizophrenic patients. Actas Luso-Esp. Neurol. Psiquiatr. Cienc. Afines 1994, 22, 171–177. [Google Scholar]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Ramos-Brieva, J.C.A. Validación de la versión castellana de la escala de Hamilton para la depresión. Actas Luso-Esp. Neurol. Psiquiatr. Cienc. Afines 1986, 14, 324–334. [Google Scholar]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Lobo, A.; Chamorro, L. Validación de las versiones en español de la Montgomery-Asberg Depression Rating Scale y la Hamilton Anxiety Rating Scale para la evaluación de la depresión y de la ansiedad. Med. Clin. 2002, 118, 493–499. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatr. 1978, 1, 429–435. [Google Scholar] [CrossRef]
- Colom, F.; Vieta, E.; Martínez-Arán, A.; García-García, M.; Reinares, M.; Torrent, C.; Goikolea, J.M.; Banús, S.; Salamero, S. Versión española de una escala de evaluación de la manía: Validez y fiabilidad de la Escala de Young. Med. Clin. 2002, 119, 366–371. [Google Scholar] [CrossRef]
- Rosa, A.R.; Sánchez-Moreno, J.; Martıínez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.L.; et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin. Pract. Epidemiol. Ment. Health 2007, 3, 5. [Google Scholar] [CrossRef]
- Leucht, S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J. Clin. Psychiatry 2014, 75, 8–14. [Google Scholar] [CrossRef]
- Shelton, R. Management of major depressive disorders following failure of antidepressant treatment. Prim. Psichiatry 2006, 12, 73–82. [Google Scholar]
- Stein, D.J.; Khoo, J.P.; Picarel-Blanchot, F.; Olivier, V.; Van Ameringen, M. Efficacy of Agomelatine 25–50 mg for the Treatment of Anxious Symptoms and Functional Impairment in Generalized Anxiety Disorder: A Meta-Analysis of Three Placebo-Controlled Studies. Adv. Ther. 2021, 38, 1567–1583. [Google Scholar] [CrossRef]
- Bourin, M.; Thibaut, F. How assess drugs in the treatment of acute bipolar mania? Front Pharmacol. 2013, 29, 4. [Google Scholar] [CrossRef]
- González-Ortega, I.; Rosa, A.; Alberich, S.; Barbeito, S.; Vega, P.; Echeburúa, E.; Vieta, E.; González-Pinto, A. Validation and use of the functioning assessment short test in first psychotic episodes. J. Nerv. Ment. Dis. 2010, 198, 836–840. [Google Scholar] [CrossRef]
- Amoretti, S.; Mezquida, G.; Rosa, A.R.; Bioque, M.; Cuesta, M.J.; Pina-Camacho, L.; Garcia-Rizo, C.; Barcones, F.; González-Pinto, A.; Merchán-Naranjo, J.; et al. The functioning assessment short test (FAST) applied to first-episode psychosis: Psychometric properties and severity thresholds. Eur. Neuropsychopharmacol. 2021, 47, 98–111. [Google Scholar] [CrossRef]
- Bonnín, C.M.; Martínez-Arán, A.; Reinares, M.; Valentí, M.; Solé, B.; Jiménez, E.; Montejo, L.; Vieta, E.; Rosa, A.R. Thresholds for severity, remission and recovery using the functioning assessment short test (FAST) in bipolar disorder. J. Affect. Disord. 2018, 240, 57–62. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Preparing People to Change Addictive Behavior, 3rd ed.; Guildford Press: New York, NY, USA, 2013. [Google Scholar]
- Prochaska, J.O.; DiClemente, C.C. Toward a comprehensive model of change. In Addictive Behaviors: Processes of Change; Miller, W.R., Heather, N., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 3–28. [Google Scholar]
- González-Ortega, I.; Vega, P.; Echeburúa, E.; Alberich, S.; Fernández-Sevillano, J.; Barbeito, S.; Balanzá-Martínez, V.; Vieta, E.; Lorente-Rovira, E.; Luengo, A.; et al. A Multicentre, Randomised, Controlled Trial of a Combined Clinical Treatment for First-Episode Psychosis. Int. J. Env. Res. Pub. Health 2021, 18, 7239. [Google Scholar] [CrossRef]
- Martinez-Aran, A.; Vieta, E. Precision psychotherapy. Eur. Neuropsychopharmacol. 2022, 55, 20–21. [Google Scholar] [CrossRef]
- Berry, K.; Gregg, L.; Lobban, F.; Barrowclough, C. Therapeutic alliance in psychological therapy for people with recent onset psychosis who use cannabis. Compr. Psychiatry 2016, 67, 73–80. [Google Scholar] [CrossRef]
- Lozano, B.E.; Stephens, R.S.; Roffman, R.A. Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction 2006, 101, 1589–1597. [Google Scholar] [CrossRef]
- Rebgetz, S.; Hides, L.; Kavanagh, D.J.; Choudhary, A. Prospective recovery of cannabis use in a psychotic population: A qualitative analysis. Addict. Behav. Rep. 2016, 17, 31–36. [Google Scholar] [CrossRef][Green Version]
- Marlatt, G.A.; Gordon, J.R. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors; Guilford Press: New York, NY, USA, 1985. [Google Scholar]
- Verdolini, N.; Amoretti, S.; Mezquida, G.; Cuesta, M.J.; Pina-Camacho, L.; García-Rizo, C.; Lobo, A.; González-Pinto, A.; Merchán-Naranjo, J.; Corripio, I.; et al. The effect of family environment and psychiatric family history on psychosocial functioning in first-episode psychosis at baseline and after 2 years. Eur. Neuropsychopharmacol. 2021, 49, 54–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).