The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Materials
2.2. Methods
2.2.1. Composite Visualization
2.2.2. Assessment of Microbiological Contamination of SAH
2.2.3. Polishing of the Model Composites
2.2.4. Laser Irradiation
Irradiation of Polished Model Composites for Roughness Testing
2.2.5. Assessment of Decontamination Effectiveness on the Polished Composites
2.2.6. Statistical Analysis
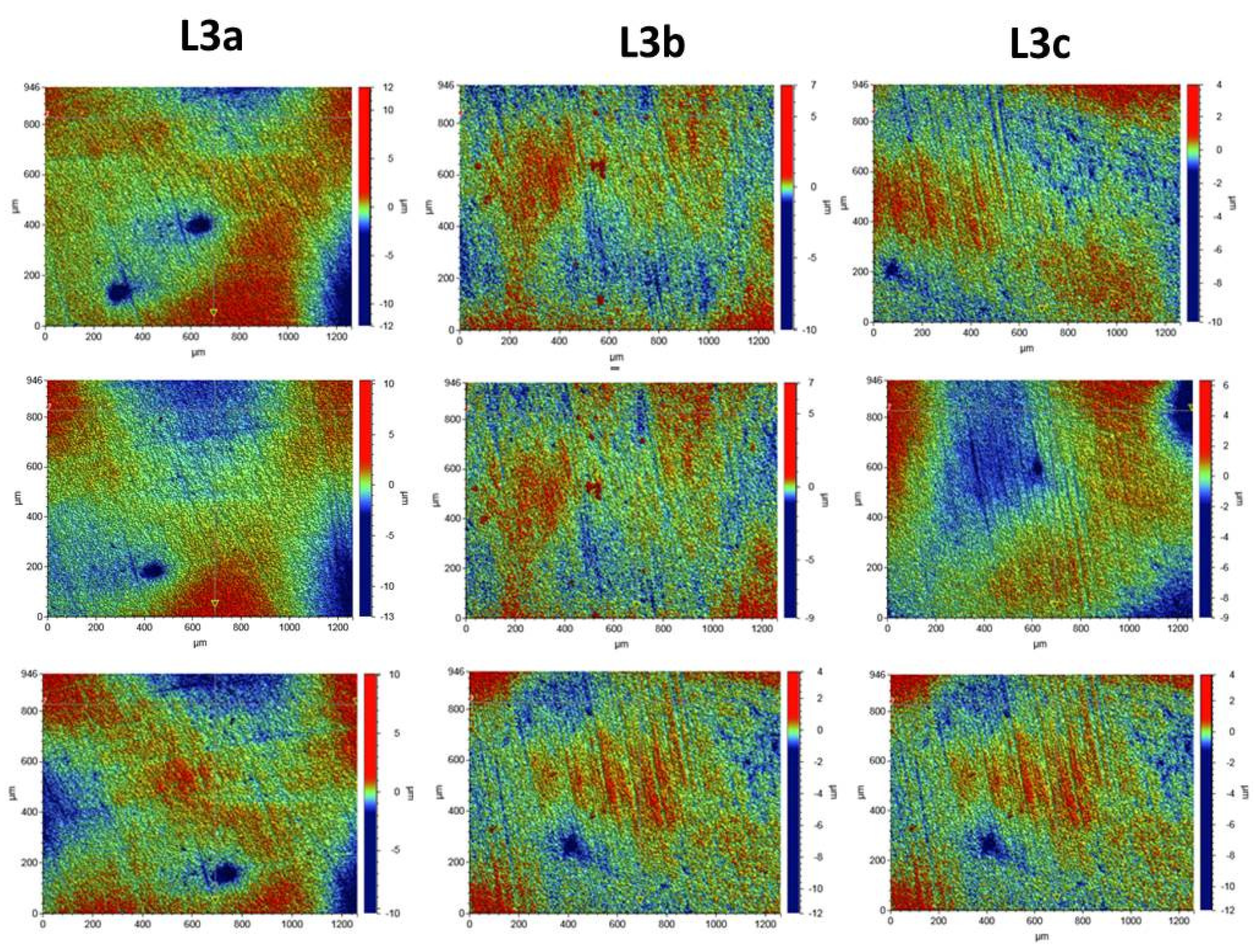
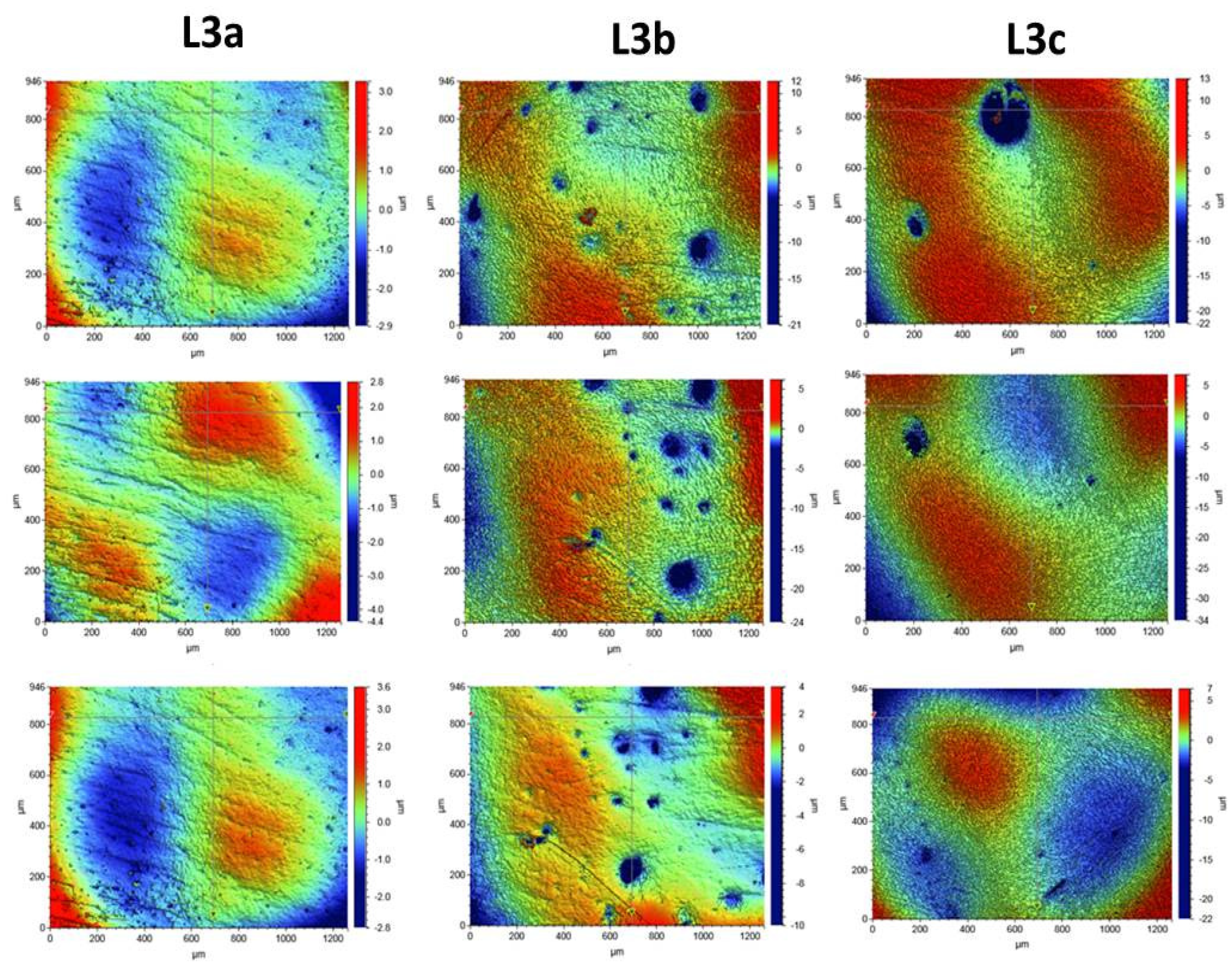
2.2.7. Surface Morphology Analysis
Optical Profilometry
3. Results
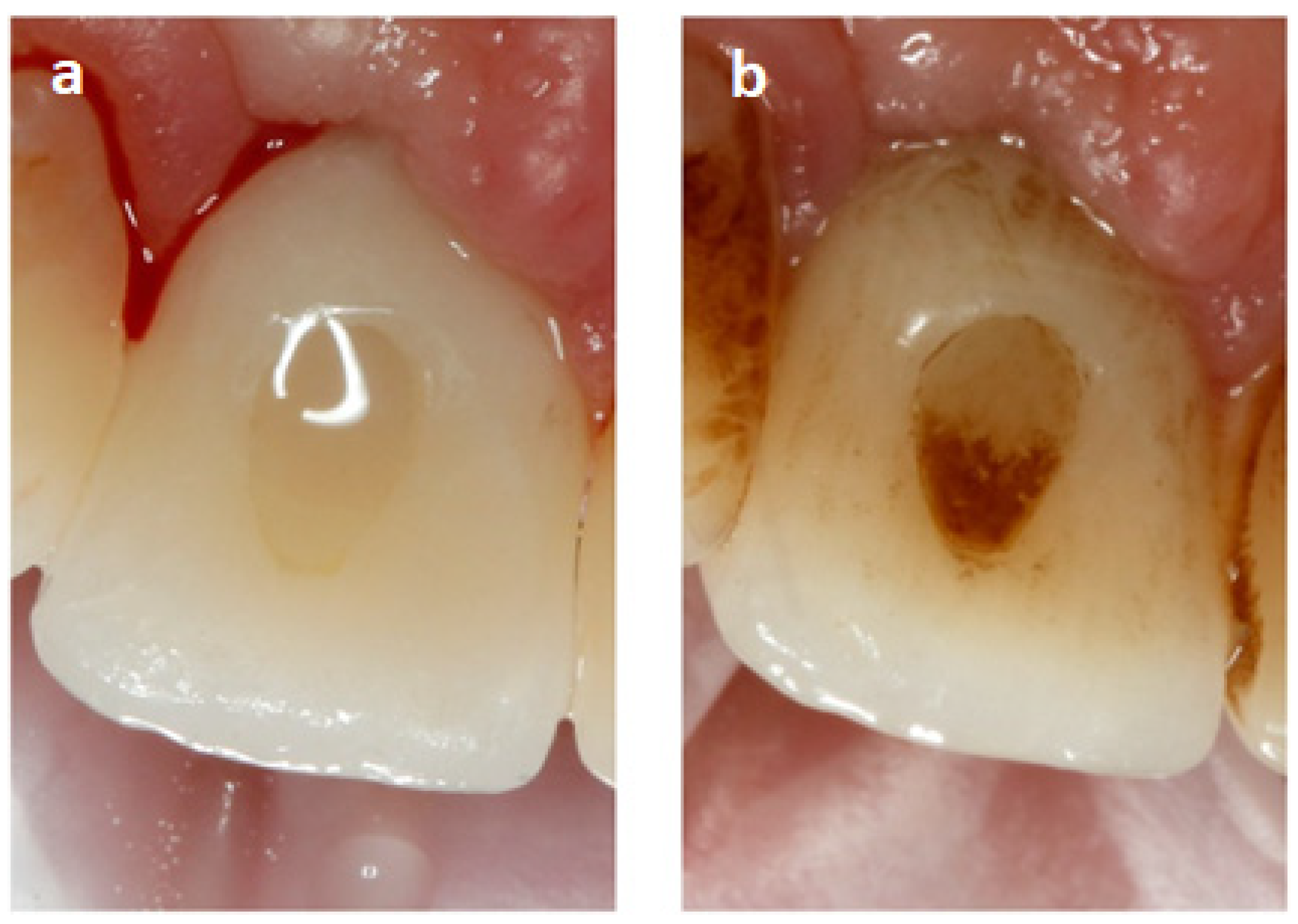
3.1. Visualization of the Place Where the Composite Was Used to Fill SAH before and after Sandblasting
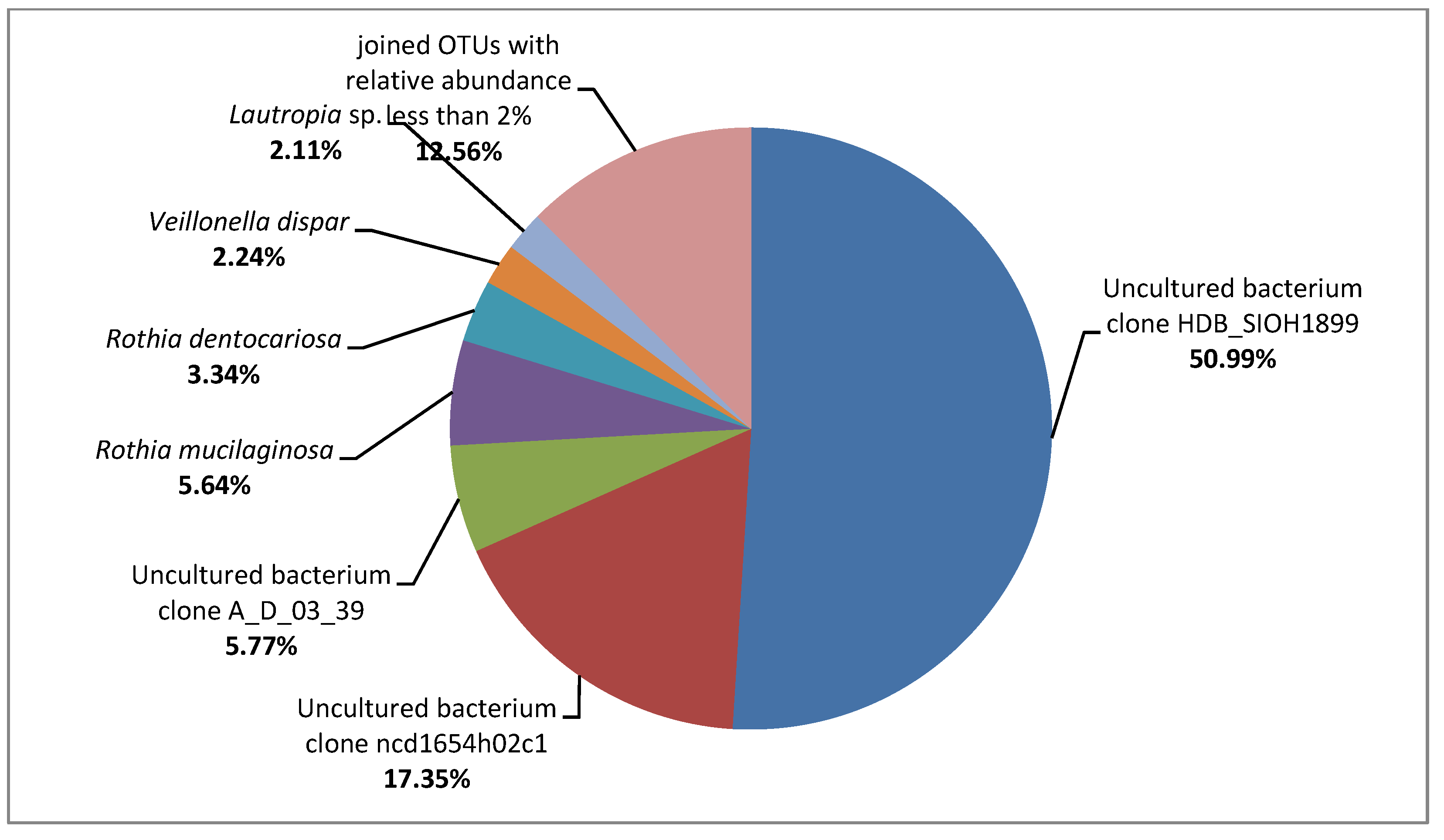
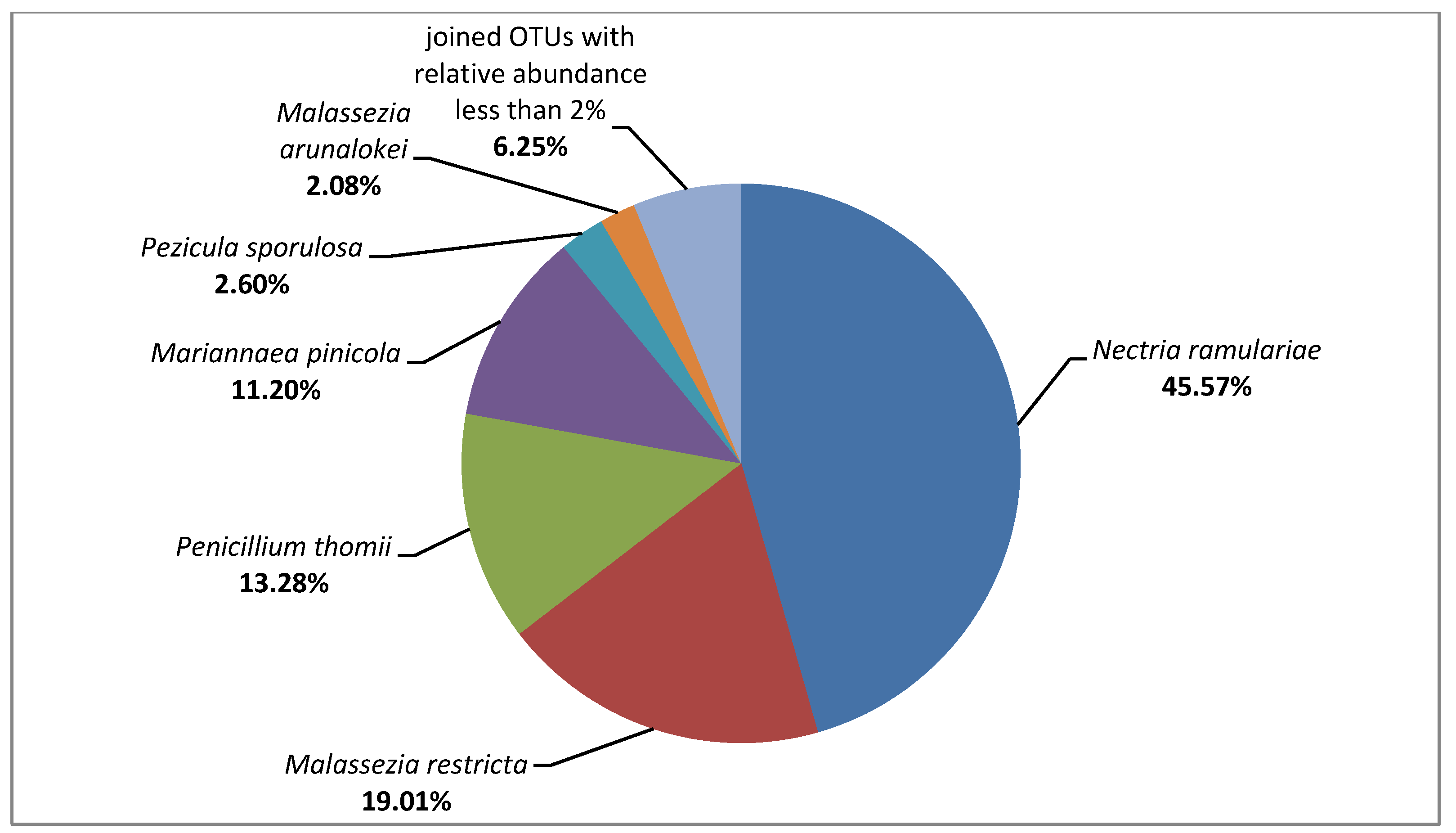
3.2. Metagenome on the Composites Surface
3.3. Quantitative and Qualitative Analysis of Microbial Contamination of Composites, Porcelain, Zirconium and Teeth Surfaces
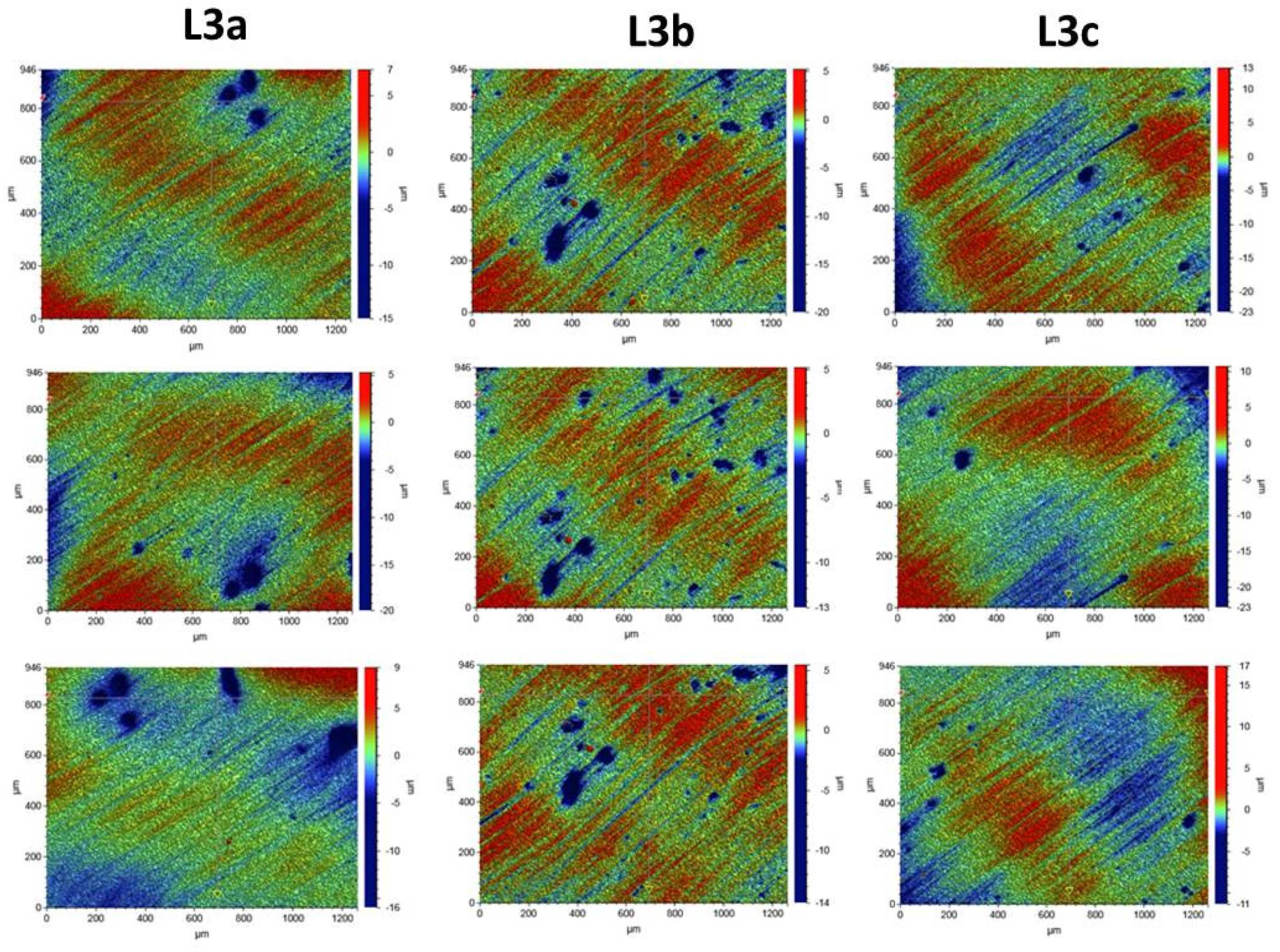
3.4. Surface Morphology Analysis
Composite Roughness Tests
3.5. Evaluation of the Biocidal Effect of Laser Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Scarano, A.; Murmura, G.; Sinjiari, B.; Sollazzo, V.; Spinelli, G.; Carinci, F. Analysis and structural examination of screw loosening in oral implants. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. S2), 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; Traini, T.; Varvara, G.; Scarano, A.; Murmura, G.; Caputi, S. A 10-year retrospective comparative human study on screw-retained versus cemented dental implant abutments. J. Biol. Regul. Homeost. Agents 2019, 33, 787–797. [Google Scholar] [PubMed]
- Lee, A.; Wang, H. Biofilm related to dental implants. Implant. Dent. 2010, 19, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Paquette, D.W.; Brodala, N.; Williams, R.C. Risk factors for endosseous dental implant failure. Dent. Clin. N. Am. 2006, 50, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Van Aelst, L.; Collaert, B.; Persson, G.; De Bruyn, H. The peri-implant sulcus compared with internal implant and suprastructure components: A microbiological analysis. Clin. Implant. Dent. Relat. Res. 2011, 13, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Turkyilmaz, I. Evaluation of the sealing capability of implants to titanium and zirconia abutments against Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum under different screw torque values. J. Prosthet. Dent. 2014, 112, 561–567. [Google Scholar] [CrossRef]
- Mahony, A.; MacNeill, S.; Cobb, C. Design features that may influence bacterial plaque retention: A retrospective analysis of failed implants. Quintessence Int. 2000, 31, 249–256. [Google Scholar]
- Subramani, K.; Jung, R.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef]
- Nedeljkovic, J.; DeMunck, A.; Ungureanu, V.; Slomka, C.; Bartic, A.; Vananroye, C.; Clasen, W.; Teughels, B.; Van Meerbeek, K.; Van Landuyt, K.L. Biofilm-induced changes to the composite surface. J. Dent. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Fugolin, A.; Pfeifer, C. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.; Laurence, J. Proteins, pathogens, and failure at the composite-tooth interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qing Yu, R. Dental Composite—An overview Science Direct Topics. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Endo, T.; Finger, W.J.; Kanehira, M.; Utterodt, A.; Komatsu, M. Surface texture and roughness of polished nanofill and nanohybrid resin composites. Dent. Mater. J. 2010, 29, 213–223. [Google Scholar] [CrossRef][Green Version]
- Ergücü, Z.; Türkün, L.S. Surface roughness of novel resin composites polished with one-step systems. Oper. Dent. 2007, 32, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Sancakli, H.; Yildiz, E. The effect of one-step and multi-step polishing systems on the surface roughness and microhardness of novel resin composites. Eur. J. Dent. 2012, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Cavalcanti, A.; Fonseca, F.; Zago, C.; Junior, R.; França, F. Efficacy of Gutta-Percha and Polytetrafluoroethylene Tape to Microbiologically Seal the Screw Access Channel of Different Prosthetic Implant Abutments. Clin. Implant. Dent. Relat. Res. 2016, 18, 778–787. [Google Scholar] [CrossRef]
- Baixe, S.; Tenenbaum, H.; Etienne, O. Microbial contamination of the implant-abutment connections: Review of the literature. Rev. Stomatol. Chir. Maxillo-Faciale Chir. Orale 2016, 117, 20–25. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Rybitwa, D.; Wilczyński, S.; Machoy, M.; Łobacz, M. Effective microbiological decontamination of dental healing abutments colonised with Rothia aeria by a diode laser as a helpful step towards successful implantoprosthetic therapy. Lasers Med. Sci. Res. 2020, 36, 875–887. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The efficacy of a diode laser on titanium implants for the reduction of microorganisms that cause periimplantitis. Materials 2021, 14, 7215. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The Use of a Diode Laser for Removal of Microorganisms from the Surfaces of Zirconia and Porcelain Applied to Superstructure Dental Implants. Microorganisms 2021, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Kocaagaoglu, H.; Aslan, T.; Gürbulak, A.; Albayrak, H.; Taşdemir, Z.; Gumus, H. Efficacy of polishing kits on the surface roughness and color stability of different composite resins Niger. J. Clin. Pract. 2017, 20, 557–565. [Google Scholar] [CrossRef]
- Sarac, D.; Sarac, Y.S.; Kulunk, S.; Ural, C.; Kulunk, T. The effect of polishing techniques on the surface roughness and color change of composite resins. J. Prosthet. Dent. 2006, 96, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yasser, A. Impact of Polishing Systems on the Surface Roughness and Microhardness of Nanocomposites. J. Contemp. Dent. Pract. 2017, 18, 647–651. [Google Scholar] [CrossRef]
- Human Oral Microbiome Database. Available online: http://www.homd.org./ (accessed on 7 April 2020).
- Nascimento, C.; Pita, M.; Calef, P.; Silva, T.; Santos, J.; Pedrazzi, V. Different sealing materials preventing the microbial leakage into the screw-retained implant restorations: An in vitro analysis by DNA checkerboard hybridization. Clin. Oral Implant. Res. 2017, 28, 242–250. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Rahnama, M. Corrigendum: Application of a Medical Diode Laser (810 nm) for Disinfecting Small Microbiologically Contaminated Spots on Degraded Collagenous Materials for Improved Biosafety in Objects of Exceptional Historical Value from the Auschwitz-Birkenau State Museum and Protection of Human Health. Front. Microbiol. 2021, 12, 673867, Erratum in Front. Microbiol. 2020, 11, 596852. [Google Scholar] [CrossRef]
- Szulc, J.; Okrasa, M.; Nowak, A.; Nizioł, J.; Ruman, T.; Kuberski, S. Assessment of Physicochemical, Microbiological and Toxicological Hazards at an Illegal Landfill in Central Poland. Int. J. Environ. Res. Public Health 2022, 19, 4826. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.K.; Shi, W.; McLean, J.S.; He, X. Saccharibacteria (TM7) in the Human Oral Microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef]
- Ereifej, N.S.; Oweis, Y.G.; Eliades, G. The Effect of Polishing Technique on 3-D Surface Roughness and Gloss of Dental Restorative Resin Composites. Oper. Dent. 2013, 38, E9–E20. [Google Scholar] [CrossRef]
- Scheibe, K.; Almeida, K.; Medeiros, I.S.; Costa, J.F.; Alves, C. Effect of different polishing systems on the surface roughness of microhybrid composites. J. Appl. Oral Sci. 2009, 17, 21–26. [Google Scholar] [CrossRef]
- Janus, J.; Fauxpointa, G.; Arntzc, Y.; Pelletier, H.; Etienne, O. Surface roughness and morphology of three nanocomposites after two different polishing treatments by a multitechnique approach. Dent. Mater. 2010, 26, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, M.; Oh, S.; Kim, K. Changes in the physical properties and color stability of aesthetic restorative materials caused by various bevarages. Dent. Mater. J. 2019, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Forjanic, M.; Ohmiti, K.; Rousson, V. Surface deterioration of dental materials after simulated toothbrushing in relation to brushing time and load. Dent Mater. 2010, 26, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Kakaboura, A.; Fragouli, M.; Rahiotis, C.; Silikas, N. Evaluation of surface characteristics of dental composites using profilometry, scanning electron, atomic force microscopy and gloss-meter. J. Mater. Sci. Mater. Med. 2007, 18, 155–163. [Google Scholar] [CrossRef]
- Willems, G.; Lambrechts, P.; Braem, M.; Vanherle, G. Composite resins in the 21st century. Quintessence Int. 1993, 24, 641–658. [Google Scholar]
- Beyth, N.; Bahir, R.; Matalon, S.; Abraham, J.; Domb, E.; Weiss, I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef]
- Rinastiti, M.; Özcan, M.; Siswomihardjo, W. Effect of Biofilm on the Repair Bond Strengths of Composites October 12. J. Dent. Res. 2010, 89, 1476–1481. [Google Scholar] [CrossRef]
- Gutknecht, N.; Van Gogswaardt, D.; Conrads, G.; Apel, C.; Schubert, C.; Lampert, F. Diode laser radiation and its bactericidal effect in root canal wall dentin. J. Clin. Laser Med. Surg. 2000, 18, 57–60. [Google Scholar] [CrossRef]
- Dai, S.; Xiao, G.; Dong, N.; Liu, F.; He, S.; Guo, Q. Bactericidal effect of a diode laser on Enterococcus faecalis in human primary teeth—An in vitro study. BMC Oral Health 2018, 18, 154. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Wilczyński, S.; Łobacz, M. Irradiation with medical diode laser as a new method of spot-elimination of microorganisms to preserve historical cellulosic objects and human health. Int. Biodeterior. Biodegrad. 2020, 154, 105055. [Google Scholar] [CrossRef]
- Noort, R.; Davis, L.G. The surface finish of composite resin restorative materials. Br. Dent. J. 1984, 157, 360–364. [Google Scholar] [PubMed]
- Dijken, J.W.; Ruyter, I.E. Surface characteristics of posterior composites after polishing and toothbrushing. Acta Odontol. Scand. 1987, 45, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Carlén, A.; Nikdel, K.; Wennerberg, A.; Holmberg, K.; Olsson, J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials 2001, 22, 481–487. [Google Scholar] [CrossRef]

| Patient | Teeth (CFU/0.4 cm2) | Porcelain (CFU/0.4 cm2) | Zirconium (CFU/0.4 cm2) | Composite (CFU/0.4 cm2) |
|---|---|---|---|---|
| 1 | 4.50 × 106 ± 0.36 × 106 | 3.98 × 107 ± 0.33 × 107 | 1.10 × 108 ± 0.30 × 108 | 1.65 × 108 ± 0.09 × 108 |
| 2 | 1.44 × 104 ± 0.61 × 104 | 5.16 × 104 ± 0.55 × 104 | 2.47 × 105 ± 0.60 × 105 | 1.21 × 106 ± 0.60 × 105 |
| 3 | 3.58 × 103 ± 0.45 × 103 | 3.20 × 104 ± 0.46 × 104 | 1.16 × 106 ± 0.54 × 106 | 2.92 × 106 ± 0.78 × 106 |
| 4 | 1.60 × 106 ± 2.77 × 106 | 1.20 × 107 ± 2.1 × 107 | 4.50 × 107 ± 7.6 × 107 | 5.30 × 107 ± 8.80 × 107 |
| 5 | 1.30 × 106 ± 2.30 × 106 | 1.40 × 107 ± 2.4 × 107 | 4.10 × 107 ± 6.9 × 107 | 5.70 × 107 ± 9.70 × 107 |
| 6 | 1.50 × 106 ± 2.6 × 106 | 1.30 × 107 ± 2.3 × 107 | 2.50 × 107 ± 4.3 × 107 | 5.80 × 107 ± 6.90 × 107 |
| Microorganism | Patient | Teeth (CFU/0.4 cm2) | Porcelain (CFU/0.4 cm2) | Zirconium (CFU/0.4 cm2) | Composite (CFU/0.4 cm2) |
|---|---|---|---|---|---|
| Candida guillermondi | 1 | 1.40 × 103 ± 1.56 × 103 | 1.50 × 105 ± 0.96 × 105 | 1.18 × 106 ± 0.95 × 106 | 6.00 × 107 ± 0.79 × 107 |
| 2 | 1.00 × 103 ± 0.70 × 103 | 1.26 × 103 ± 1.23 × 103 | 1.38 × 103 ± 1.07 × 103 | 1.89 × 103 ± 0.96 × 103 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 1.67 × 102 ± 1.42 × 102 | 1.40 × 103 ± 1.09 × 103 | 3.20 × 104 ± 0.73 × 104 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 4.80 × 106 ± 3.95 × 106 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 1.40 × 106 ± 0.95 × 106 | 3.08 × 106 ± 1.85 × 106 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 3.20 × 106 ± 1.95 × 106 | 3.65 × 106 ± 0.95 × 106 | |
| Klebsiella oxytoca | 1 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 3 | 1.20 × 103 ± 1.40 × 102 | 3.20 × 104 ± 2.07 × 104 | 6.13 × 105 ± 3.24 × 105 | 3.00 × 106 ± 2.09 × 105 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 2.20 × 101 ± 0,30 × 101 | 1.20 × 102 ± 2,30 × 101 | 4.30 × 102 ± 2,30 × 101 | 6.00 × 102 ± 0.75 × 102 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Neisseria flavescens | 1 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 1.00 × 102 ± 1.54 × 102 | 7.67 × 102 ± 1.62 × 102 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Neisseria perflava | 1 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 6.67 × 102 ± 2.32 × 102 | 4.33 × 102 ± 2.71 × 102 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Neisseria subflava | 1 | 0.00 × 100 ± 0.00 × 100 | 3.00 × 106 ± 2.91 × 106 | 5.60 × 106 ± 2.43 × 106 | 1.40 × 106 ± 1.35 × 106 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 4 | 3.84 × 103 ± 2.00 × 103 | 3.94 × 103 ± 1.02 × 102 | 2.04 × 104 ± 3.02 × 102 | 2.08 × 105 ± 1.33 × 103 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Rothia aeria | 1 | 2.00 × 105 ± 1.64 × 105 | 8.00 × 105 ± 1.37 × 105 | 2.00 × 106 ± 1.23 × 106 | 3.40 × 106 ± 1.57 × 106 |
| 2 | 1.09 × 104 ± 1.29 × 104 | 1.83 × 104 ± 1.01 × 104 | 8.83 × 104 ± 3.28 × 104 | 5.45 × 105 ± 3.30 × 105 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 4 | 5.40 × 103 ± 1.40 × 101 | 1.30 × 104 ± 2.70 × 104 | 1.20 × 105 ± 1.10 × 104 | 6.20 × 105 ± 1.30 × 105 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 6.30 × 103 ± 1.70 × 104 | 5.30 × 105 ± 1.40 × 101 | |
| 6 | 1.30 × 103 ± 1.70 × 101 | 3.60 × 103 ± 2.40 × 102 | 3.90 × 103 ± 1.90 × 102 | 0.00 × 100 ± 0.00 × 100 | |
| Rothia dentocariosa | 1 | 1.20 × 106 ± 0.91 × 106 | 3.40 × 106 ± 0.8 × 106 | 9.00 × 106 ± 5.92 × 106 | 6.00 × 107 ± 3.82 × 107 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 3 | 1.43 × 103 ± 1.11 × 103 | 1.60 × 104 ± 0.85 × 104 | 6.13 × 105 ± 4.67 × 105 | 1.65 × 106 ± 0.80 × 106 | |
| 4 | 5.40 × 103 ± 1.14 × 101 | 1.20 × 104 ± 1.05 × 104 | 1.20 × 105 ± 2.10 × 104 | 1.60 × 106 ± 3.50 × 104 | |
| 5 | 2.20 × 102 ± 1.30 × 101 | 4.20 × 103 ± 1.30 × 105 | 6.20 × 105 ± 1.30 × 105 | 5.80 × 108 ± 8.20 × 107 | |
| 6 | 4.6 × 102 ± 2.52 × 102 | 5.6 × 103 ± 2.52 × 102 | 8.00 × 105 ± 1.54 × 104 | 2.10 × 106 ± 5.09×104 | |
| Staphylococcus epidermidis | 1 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 2 | 1.22 × 103 ± 0.87 × 103 | 2.67 × 102 ± 1.29 × 102 | 6.67 × 103 ± 2.35 × 103 | 1.00 × 104 ± 0.72 × 104 | |
| 3 | 1.67 × 102 ± 1.94 × 102 | 1.00 × 103 ± 0.82 × 103 | 1.33 × 104 ± 0.68 × 104 | 2.00 × 103 ± 1.45 × 103 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Streptococcus parasanguinis | 1 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 2 | 1.30 × 103 ± 1.03 × 103 | 3.20 × 104 ± 1.50 × 104 | 1.52 × 105 ± 0.92 × 105 | 5.53 × 105 ± 1.30 × 105 | |
| 3 | 1.88 × 103 ± 0.81 × 103 | 1.40 × 104 ± 0.58 × 104 | 4.33 × 105 ± 1.24 × 105 | 1.17 × 106 ± 2.05 × 105 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| Streptococcus pneumoniae | 1 | 3.00 × 106 ± 2.02 × 106 | 3.16 × 107 ± 1.65 × 107 | 8.36 × 107 ± 1.10 × 107 | 9.00 × 107 ± 1.57 × 107 |
| 2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 3 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 4 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | |
| 5 | 1.30 × 103 ± 2.07 × 103 | 5.40 × 103 ± 1.40 × 101 | 9.10 × 104 ± 6.60 × 103 | 6.30 × 105 ± 1.70 × 104 | |
| 6 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| Roughness Parameter Ra (µm) | ||||
|---|---|---|---|---|
| Measurement | K1 | L3a | L3b | L3c |
| 1 | 0.760 ± 0.07 | 1.041 ± 0.10 | 0.735 ± 0.07 | 0.840 ± 0.08 |
| 2 | 0.714 ± 0.07 | 1.052 ± 0.10 | 0.678 ± 0.07 | 1.044 ± 0.10 |
| 3 | 0.665 ± 0.06 | 1.354 ± 0.13 | 0.646 ± 0.06 | 0.903 ± 0.09 |
| 4 | 0.757 ± 0.07 | - | - | - |
| 5 | 0.584 ± 0.06 | - | - | - |
| Average value | 0.696 ± 0.07 | 1.149 ± 0.11 | 0.686 ± 0.07 | 0.930 ± 0.09 |
| Measurement | K2 | L3a | L3b | L3c |
| 1 | 0.486 ± 0.05 | 0.664 ± 0.06 | 0.325 ± 0.03 | 0.359 ± 0.03 |
| 2 | 0.724 ± 0.07 | 0.850 ± 0.08 | 0.306 ± 0.03 | 0.646 ± 0.06 |
| 3 | 0.516 ± 0.05 | 0.544 ± 0.05 | 0.330 ± 0.03 | 0.330 ± 0.03 |
| 4 | 0.532 ± 0.05 | - | - | - |
| 5 | 0.632 ± 0.06 | - | - | - |
| Average value | 0.578 ±0.06 | 0.686 ± 0.07 | 0.320 ± 0.03 | 0.445 ± 0.04 |
| Measurement | K3 | L3a | L3b | L3c |
| 1 | 1.080 ± 0.10 | 0.452 ± 0.04 | 0.982 ± 0.09 | 1.879 ± 0.18 |
| 2 | 0.576 ± 0.06 | 0.563 ± 0.05 | 0.658 ± 0.06 | 1.860 ± 0.18 |
| 3 | 0.591 ± 0.06 | 0.563 ± 0.05 | 0.662 ± 0.06 | 1.246 ± 0.12 |
| 4 | 0.481 ± 0.05 | - | - | - |
| 5 | 0.322 ± 0.03 | - | - | - |
| Average value | 0.610 ± 0.06 | 0.526 ± 0.05 | 0.767 ± 0.07 | 1.661 ± 0.16 |
| Microorganism | Type of Sample | |||||
|---|---|---|---|---|---|---|
| Composite | Time of Exposure | |||||
| Unirradiated | L1 (1 min) | L2 (1 min) | L3 (1 min) | L3 (2 × 30 s) | ||
| Average Number of Microorganisms (CFU/0.4 cm2) | Reduction (%) | |||||
| Candida guillermondi | K1 | 5.05 × 107 ± 2.25 × 107 | 41.09 * | 57.38 * | 75.91 * | 83.06 * |
| K2 | 69.77 * | 75.45 * | 93.61 * | 94.92 * | ||
| K3 | 53.66 * | 73.27 * | 86.74 * | 94.16 * | ||
| Klebsiella oxytoca | K1 | 8.91 × 105 ± 3.17 × 105 | 38.38 * | 62.66 * | 70.75 * | 74.35 * |
| K2 | 55.28 * | 78.66 * | 87.83 * | 87.89 * | ||
| K3 | 47.22 * | 78.68 * | 75.48 * | 75.94 * | ||
| Rothia dentocariosa | K1 | 2.33 × 105 ± 6.54 × 105 | 40.75 * | 58.04 * | 98.36 * | 99.28 * |
| K2 | 44.41 * | 74.35 * | 87.89 * | 99.97 * | ||
| K3 | 41.55 * | 64.66 * | 99.28 * | 99.41 * | ||
| Streptococcus pneumoniae | K1 | 5.15 × 107 ± 2.15 × 107 | 42.96 * | 80.45 * | 96.87 * | 99.00 * |
| K2 | 67.44 * | 85.26 * | 100.00 * | 99.90 * | ||
| K3 | 58.34 * | 82.77 * | 100.00 * | 99.22 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Łobacz, M. The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies. Int. J. Environ. Res. Public Health 2022, 19, 7494. https://doi.org/10.3390/ijerph19127494
Wawrzyk A, Rahnama M, Sofińska-Chmiel W, Wilczyński S, Łobacz M. The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies. International Journal of Environmental Research and Public Health. 2022; 19(12):7494. https://doi.org/10.3390/ijerph19127494
Chicago/Turabian StyleWawrzyk, Anna, Mansur Rahnama, Weronika Sofińska-Chmiel, Sławomir Wilczyński, and Michał Łobacz. 2022. "The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies" International Journal of Environmental Research and Public Health 19, no. 12: 7494. https://doi.org/10.3390/ijerph19127494
APA StyleWawrzyk, A., Rahnama, M., Sofińska-Chmiel, W., Wilczyński, S., & Łobacz, M. (2022). The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies. International Journal of Environmental Research and Public Health, 19(12), 7494. https://doi.org/10.3390/ijerph19127494







