Physical Activity Evaluation Using Activity Trackers for Type 2 Diabetes Prevention in Patients with Prediabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Intervention Tools
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Body Mass Composition
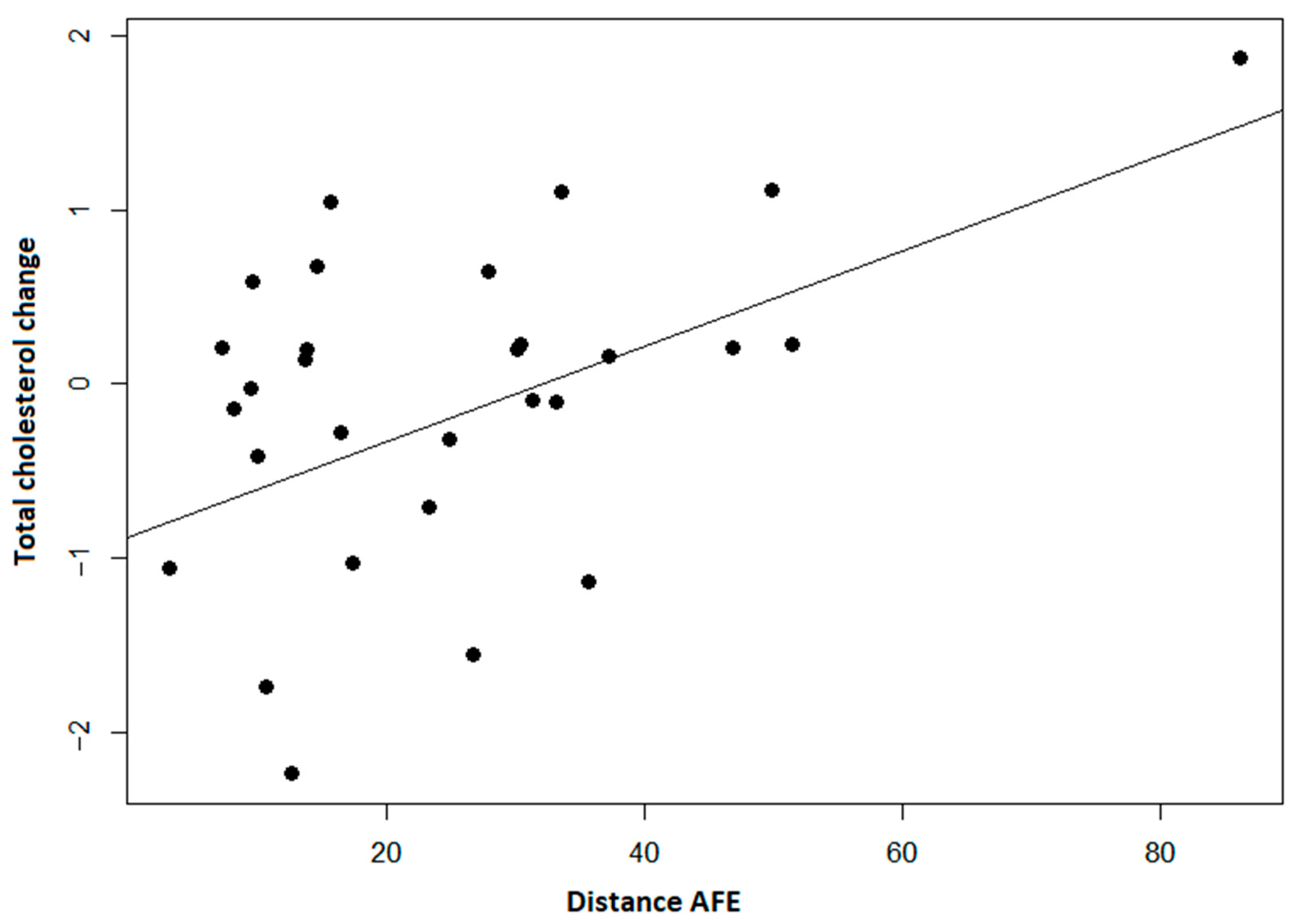
3.3. Blood Tests
3.4. Physical Activity Evaluation Using Fitbit Inspire Physical Activity Tracker Data
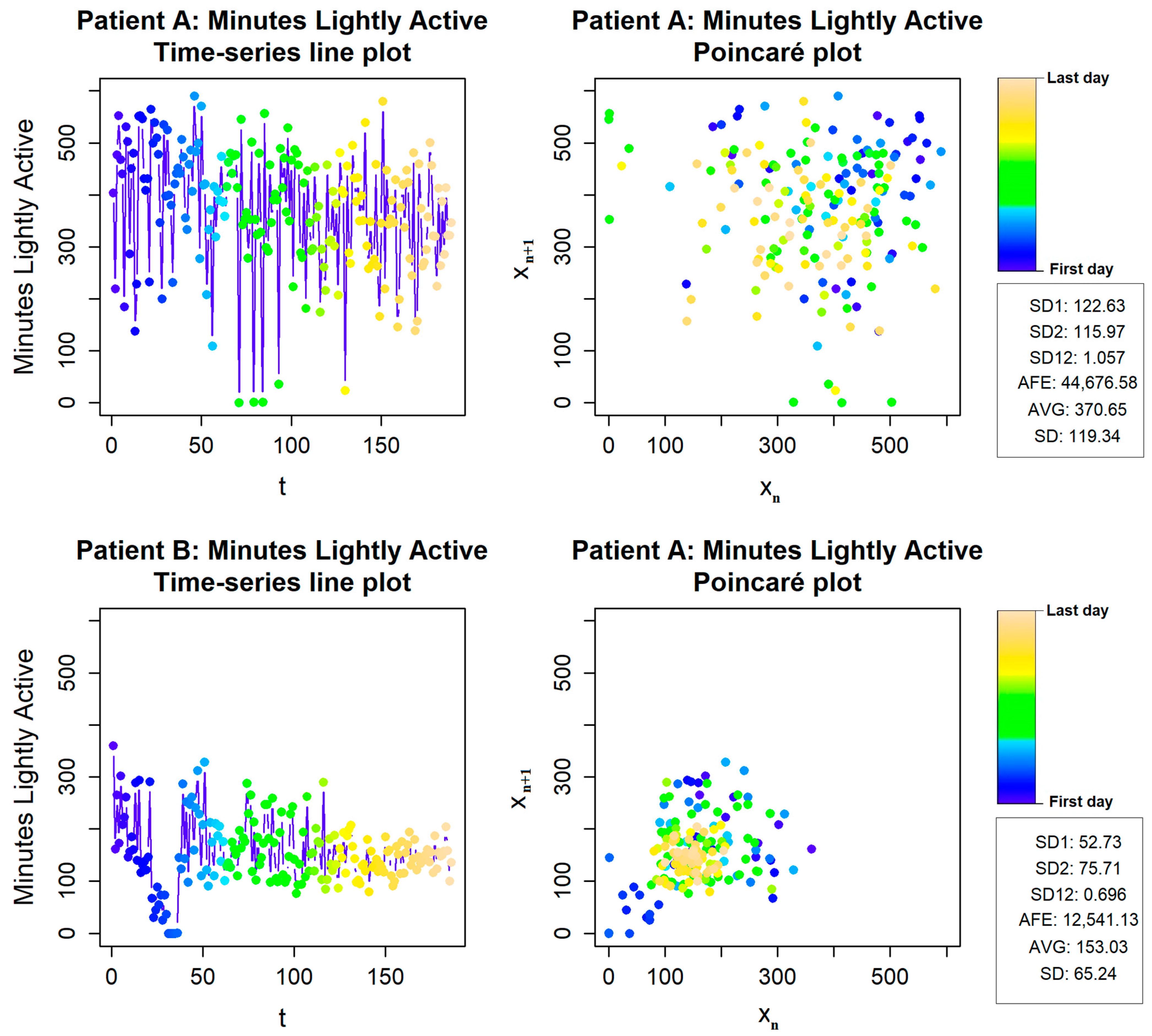
3.5. Physical Activity Variability Evaluation Using Fitbit Inspire Data
4. Discussion
4.1. Principal Result
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFE | Area of fitting ellipse |
| AVG | Average value |
| AT | Activity tracker |
| BMI | Body mass index |
| DPP | Diabetes prevention program |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| PBF | Percent body fat |
| VFL | Visceral fat level |
| VFA | Visceral fat area |
| WHR | Waist to hip ratio |
| AC | Abdominal circumference |
| MBF | Mass of body fat |
| PA | Physical activity |
| SD | Standard deviation |
| SD1 | Short-term variability |
| SD2 | Long-term variability |
| SD12 | The ratio of SD1 and SD2 |
| T2D | Type 2 diabetes |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. Available online: https://www.research.ed.ac.uk/en/publications/idf-diabetes-atlas-global-regional-and-country-level-diabetes-pre (accessed on 13 February 2022). [CrossRef] [PubMed]
- Heianza, Y.; Hara, S.; Arase, Y.; Saito, K.; Fujiwara, K.; Tsuji, H.; Kodama, S.; Hsieh, S.D.; Mori, Y.; Shimano, H.; et al. HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): A longitudinal cohort study. Lancet 2011, 378, 147–155. Available online: https://pubmed.ncbi.nlm.nih.gov/21705064/ (accessed on 13 February 2022). [CrossRef]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 10, CD012661. Available online: https://pubmed.ncbi.nlm.nih.gov/30371961/ (accessed on 13 February 2022). [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. Available online: https://pubmed.ncbi.nlm.nih.gov/22683128/ (accessed on 13 February 2022). [CrossRef] [Green Version]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. Available online: https://pubmed.ncbi.nlm.nih.gov/27881363/ (accessed on 13 February 2022). [CrossRef] [Green Version]
- Yeboah, J.; Bertoni, A.G.; Herrington, D.M.; Post, W.S.; Burke, G.L. Impaired Fasting Glucose and the Risk of Incident Diabetes Mellitus and Cardiovascular Events in an Adult Population: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2011, 58, 140–146. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3146297/ (accessed on 13 February 2022). [CrossRef] [Green Version]
- Risk, C.; Ford, E.S.; Zhao, G.; Li, C. Quarterly Focus Issue: Prevention/Outcomes Pre-Diabetes and the Risk for Cardiovascular Disease A Systematic Review of the Evidence. J. Am. Coll. Cardiol. 2010, 55, 1310–1317. [Google Scholar]
- Diabetes Prevention Program Research Group; Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. Available online: https://pubmed.ncbi.nlm.nih.gov/19878986/ (accessed on 13 February 2022).
- Iabetes, D.; Revention, P.; Rogram, P.; Esearch, R.; Roup, G. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa012512 (accessed on 13 February 2022). [CrossRef]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. Available online: https://pubmed.ncbi.nlm.nih.gov/17098085/ (accessed on 13 February 2022). [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. Available online: https://pubmed.ncbi.nlm.nih.gov/24731674/ (accessed on 13 February 2022). [CrossRef]
- Nathan, D.M.; Bennett, P.H.; Crandall, J.P.; Edelstein, S.L.; Goldberg, R.B.; Kahn, S.E.; Knowler, W.C.; Mather, K.J.; Mudaliar, S.; Orchard, T.J.; et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 2019, 62, 1319–1328. Available online: https://pubmed.ncbi.nlm.nih.gov/31270584/ (accessed on 13 February 2022). [CrossRef] [PubMed] [Green Version]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M.; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health and Cardiovascular, Exercise, Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology, and Council. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart association. Circulation 2013, 128, 2259–2279. Available online: https://www.ahajournals.org/doi/abs/10.1161/01.cir.0000435708.67487.da (accessed on 2 February 2022). [CrossRef] [PubMed]
- Bassett, D.R.; Toth, L.P.; LaMunion, S.R.; Crouter, S.E. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sports Med. 2017, 47, 1303–1315. Available online: https://link.springer.com/article/10.1007/s40279-016-0663-1 (accessed on 2 February 2022). [CrossRef] [PubMed] [Green Version]
- Brickwood, K.J.; Watson, G.; O’brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. Available online: https://mhealth.jmir.org/2019/4/e11819 (accessed on 2 February 2022). [CrossRef] [PubMed]
- Straiton, N.; Alharbi, M.; Bauman, A.; Neubeck, L.; Gullick, J.; Bhindi, R.; Gallagher, R. The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas 2018, 112, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. Available online: https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-015-0314-1 (accessed on 2 February 2022). [CrossRef] [Green Version]
- Sergamumas. Available online: https://hi.lt/php/serg14.php?dat_file=serg14.txt (accessed on 2 February 2022).
- Tang, M.S.S.; Moore, K.; McGavigan, A.; Clark, R.A.; Ganesan, A.N. Effectiveness of Wearable Trackers on Physical Activity in Healthy Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. JMIR Mhealth Uhealth 2020, 8, e15576. Available online: https://mhealth.jmir.org/2020/7/e15576 (accessed on 30 March 2022). [CrossRef]
- Yun, I.; Joo, H.J.; Park, Y.S.; Park, E.C. Association between Physical Exercise and Glycated Hemoglobin Levels in Korean Patients Diagnosed with Diabetes. Int. J. Environ. Res. Public Health 2022, 19, 3280. Available online: https://www.mdpi.com/1660-4601/19/6/3280/htm (accessed on 30 March 2022). [CrossRef]
- Franssen, W.M.A.; Franssen, G.H.L.M.; Spaas, J.; Solmi, F.; Eijnde, B.O. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 57. Available online: https://link.springer.com/articles/10.1186/s12966-020-00955-2 (accessed on 30 March 2022). [CrossRef]
- Chan, A.; Chan, D.; Lee, H.; Ng, C.C.; Yeo, A.H.L. Reporting adherence, validity and physical activity measures of wearable activity trackers in medical research: A systematic review. Int. J. Med. Inform. 2022, 160, 104696. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. Available online: https://diabetesjournals.org/care/article/44/Supplement_1/S15/30859/2-Classification-and-Diagnosis-of-Diabetes (accessed on 22 March 2022). [CrossRef] [PubMed]
- Feehan, L.M.; Geldman, J.; Sayre, E.C.; Park, C.; Ezzat, A.M.; Yoo, J.Y.; Hamilton, C.B.; Li, L.C. Accuracy of Fitbit Devices: Systematic Review and Narrative Syntheses of Quantitative Data. JMIR Mhealth Uhealth 2018, 6, e10527. Available online: https://mhealth.jmir.org/2018/8/e10527 (accessed on 20 June 2022). [CrossRef] [PubMed] [Green Version]
- El Fatouhi, D.; Delrieu, L.; Goetzinger, C.; Malisoux, L.; Affret, A.; Campo, D.; Fagherazzi, G. Associations of Physical Activity Level and Variability with 6-Month Weight Change Among 26,935 Users of Connected Devices: Observational Real-Life Study. JMIR Mhealth Uhealth 2021, 9, e25385. Available online: https://mhealth.jmir.org/2021/4/e25385 (accessed on 2 February 2022). [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; de Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough? For adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. Available online: https://pubmed.ncbi.nlm.nih.gov/21798015/ (accessed on 1 February 2022). [CrossRef] [Green Version]
- Golińska, A.K. Poincaré plots in analysis of selected biomedical signals. Stud. Log. Gramm. Rhetor. 2013, 35, 117–127. [Google Scholar] [CrossRef]
- Satti, R.; Abid, N.U.H.; Bottaro, M.; de Rui, M.; Garrido, M.; Rauofy, M.R.; Montagnese, S.; Mani, A.R. The application of the extended Poincaré plot in the analysis of physiological variabilities. Front. Physiol. 2019, 10, 116. [Google Scholar] [CrossRef]
- Jamovi-Stats. Open. Now. Available online: https://www.jamovi.org/ (accessed on 2 February 2022).
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 2 February 2022).
- CRAN-Package RHRV. Available online: https://cran.r-project.org/web/packages/RHRV/index.html (accessed on 2 February 2022).
- García Martínez, C.A.; Otero Quintana, A.; Vila, X.A.; Lado Touriño, M.J.; Rodríguez-Liñares, L.; Rodríguez Presedo, J.M.; Méndez PenÍn, A.J. Heart Rate Variability Analysis of ECG Data [R package RHRV Version 4.2.6]. Available online: https://CRAN.R-project.org/package=RHRV (accessed on 2 February 2022).
- Ringeval, M.; Wagner, G.; Denford, J.; Paré, G.; Kitsiou, S. Fitbit-Based Interventions for Healthy Lifestyle Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e23954. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7589007/ (accessed on 13 February 2022). [CrossRef]
- da Silva, B.G.C.; da Silva, I.C.M.; Ekelund, U.; Brage, S.; Ong, K.K.; de Lucia Rolfe, E.; Lima, N.P.; da Silva, S.G.; de França, G.V.A.; Horta, B.L. Associations of physical activity and sedentary time with body composition in Brazilian young adults. Sci. Rep. 2019, 9, 5444. Available online: https://www.nature.com/articles/s41598-019-41935-2 (accessed on 21 June 2022). [CrossRef] [Green Version]
- Carnero, E.A.; Dubis, G.S.; Hames, K.C.; Jakicic, J.M.; Houmard, J.A.; Coen, P.M.; Goodpaster, B.H. Randomized Trial Reveals that Physical Activity and Energy Expenditure are associated with Weight and Body Composition after RYGB. Obesity 2017, 25, 1206. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513190/ (accessed on 21 June 2022). [CrossRef]

| Baseline (n = 30) | Follow-Up—Baseline (n = 30) | p Value | Effect Size | SRM | |
|---|---|---|---|---|---|
| Weight [kg] # | 87.6 ± 16.9 | −1.46 ± 3.31 | 0.022 * | −0.4406 | −0.4411 |
| MBF [kg] # | 32.0 ± 9.72 | −1.8 ± 2.36 | <0.001 * | −0.7617 | −0.7627 |
| PBF [%] # | 36.1 ± 6.86 | −1.43 ± 1.59 | <0.001 * | −0.8971 | −0.8994 |
| VFL [Units] $ | 16.0 [13.0–18.0] | −0.867 [0–1.75] | <0.001 * | −0.87619 | −0.8337 |
| VFA [cm2] # | 169 ± 79.1 | −21.9 ± 23.9 | <0.001 * | −0.9162 | −0.9163 |
| WHR # | 0.948 ± 0.0912 | −0.022 ± 0.0282 | <0.001 * | −0.7798 | −0.7801 |
| AC [cm] # | 98.1 ± 13.0 | −2.16 ± 2.84 | <0.001 * | −0.7618 | −0.7606 |
| MBF Lt.ARM [kg] # | 1.93 ± 0.612 | −0.0777 ± 0.149 | 0.008 * | −0.5227 | −0.5215 |
| MBF Rt.ARM [kg] # | 1.91 ± 0.625 | −0.088 ± 0.159 | 0.005 * | −0.5518 | −0.5535 |
| MBF Lt.LEG [kg] # | 5.84 ± 1.75 | −0.345 ± 0.442 | <0.001 * | −0.7810 | −0.7805 |
| MBF Rt.LEG [kg] # | 5.83 ± 1.76 | −0.362 ± 0.444 | <0.001 * | −0.8146 | −0.8153 |
| MBF Trunk [kg] # | 16.5 ± 4.98 | −0.924 ± 1.2 | <0.001 * | −0.7666 | −0.7700 |
| Baseline (n = 30) | Follow-Up—Baseline (n = 30) | p Value | Effect Size | SRM | |
|---|---|---|---|---|---|
| HgbA1c [%] # | 5.61 ± 0.352 | 0.0467 ± 0.2788 | 0.367 | 0.1674 | 0.1675 |
| HgbA1c [mmol/L] # | 37.7 ± 3.74 | 0.5333 ± 2.9564 | 0.331 | 0.1804 | 0.1804 |
| TC [mmol/L] # | 5.70 ± 1.14 | 0.199 ± 1.1188 | 0.338 | 0.1779 | 0.1779 |
| TG [mmol/L] $ | 1.81 [1.19–2.50] | −0.182 [−0.77–0.303] | 0.271 | −0.2344 | −0.1844 |
| HDL [mmol/L] $ | 1.29 [1.12–1.52] | 0.0287 [−0.1–0.13] | 0.593 | 0.1140 | 0.1643 |
| LDL [mmol/L] # | 3.44 ± 1.04 | 0.228 ± 1.0574 | 0.247 | 0.2156 | 0.2156 |
| Steps per Day | |||
| Month | Mean of count | Std. Deviation | Coefficient of variance |
| 1st | 9908 | 3625 | 0.43 |
| 2nd | 9816 | 3834 | 0.41 |
| 3rd | 8881 | 3969 | 0.46 |
| 4th | 8217 | 4117 | 0.53 |
| 5th | 7971 | 3597 | 0.52 |
| 6th | 8038 | 3897 | 0.54 |
| Activity: Sedentary | |||
| Month | Mean of minutes | Std. Deviation | Coefficient of variance |
| 1st | 804 | 274 | 0.24 |
| 2nd | 805 | 287 | 0.19 |
| 3rd | 816 | 275 | 0.18 |
| 4th | 898 | 322 | 0.18 |
| 5th | 927 | 290 | 0.17 |
| 6th | 871 | 266 | 0.25 |
| Activity: Lightly active | |||
| Month | Mean of minutes | Std. Deviation | Coefficient of variance |
| 1st | 281 | 117 | 0.4 |
| 2nd | 270 | 116 | 0.29 |
| 3rd | 263 | 121 | 0.36 |
| 4th | 216 | 132 | 0.54 |
| 5th | 211 | 105 | 0.55 |
| 6th | 233 | 99 | 0.45 |
| Activity: Fairly active | |||
| Month | Mean of minutes | Std. Deviation | Coefficient of variance |
| 1st | 17 | 15 | 1.05 |
| 2nd | 16 | 14 | 1.17 |
| 3rd | 14 | 11 | 1.06 |
| 4th | 10 | 10 | 1.25 |
| 5th | 11 | 10 | 1.49 |
| 6th | 14 | 13 | 1.34 |
| Activity: Very active | |||
| Month | Mean of minutes | Std. Deviation | Coefficient of variance |
| 1st | 8 | 8 | 1.28 |
| 2nd | 10 | 10 | 1.33 |
| 3rd | 9 | 10 | 1.25 |
| 4th | 10 | 15 | 1.43 |
| 5th | 9 | 13 | 1.74 |
| 6th | 11 | 14 | 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bliudzius, A.; Svaikeviciene, K.; Puronaite, R.; Kasiulevicius, V. Physical Activity Evaluation Using Activity Trackers for Type 2 Diabetes Prevention in Patients with Prediabetes. Int. J. Environ. Res. Public Health 2022, 19, 8251. https://doi.org/10.3390/ijerph19148251
Bliudzius A, Svaikeviciene K, Puronaite R, Kasiulevicius V. Physical Activity Evaluation Using Activity Trackers for Type 2 Diabetes Prevention in Patients with Prediabetes. International Journal of Environmental Research and Public Health. 2022; 19(14):8251. https://doi.org/10.3390/ijerph19148251
Chicago/Turabian StyleBliudzius, Antanas, Kristina Svaikeviciene, Roma Puronaite, and Vytautas Kasiulevicius. 2022. "Physical Activity Evaluation Using Activity Trackers for Type 2 Diabetes Prevention in Patients with Prediabetes" International Journal of Environmental Research and Public Health 19, no. 14: 8251. https://doi.org/10.3390/ijerph19148251






