A Modeling Approach for Quantifying Human-Beneficial Terpene Emission in the Forest: A Pilot Study Applying to a Recreational Forest in South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Model of Emissions of Gases and Aerosols from Nature (MEGAN)
2.2.1. Light-Responsive Emission Activity Factor ()
2.2.2. Temperature-Responsive Emission Activity Factor ()
2.2.3. Leaf-Age Emission Activity Factor ()
2.2.4. Soil Moisture and -Responsive Emission Activity Factors ( and )
2.3. MEGAN Model Setup
2.4. MEGAN Model Modification and Implementation
2.5. Analysis of Human-Beneficial Terpene Emission
3. Results
3.1. Temporal Variation of Human-Beneficial Terpene Emission Rate
3.2. Spatial Patterns of Terpene Emission Rate
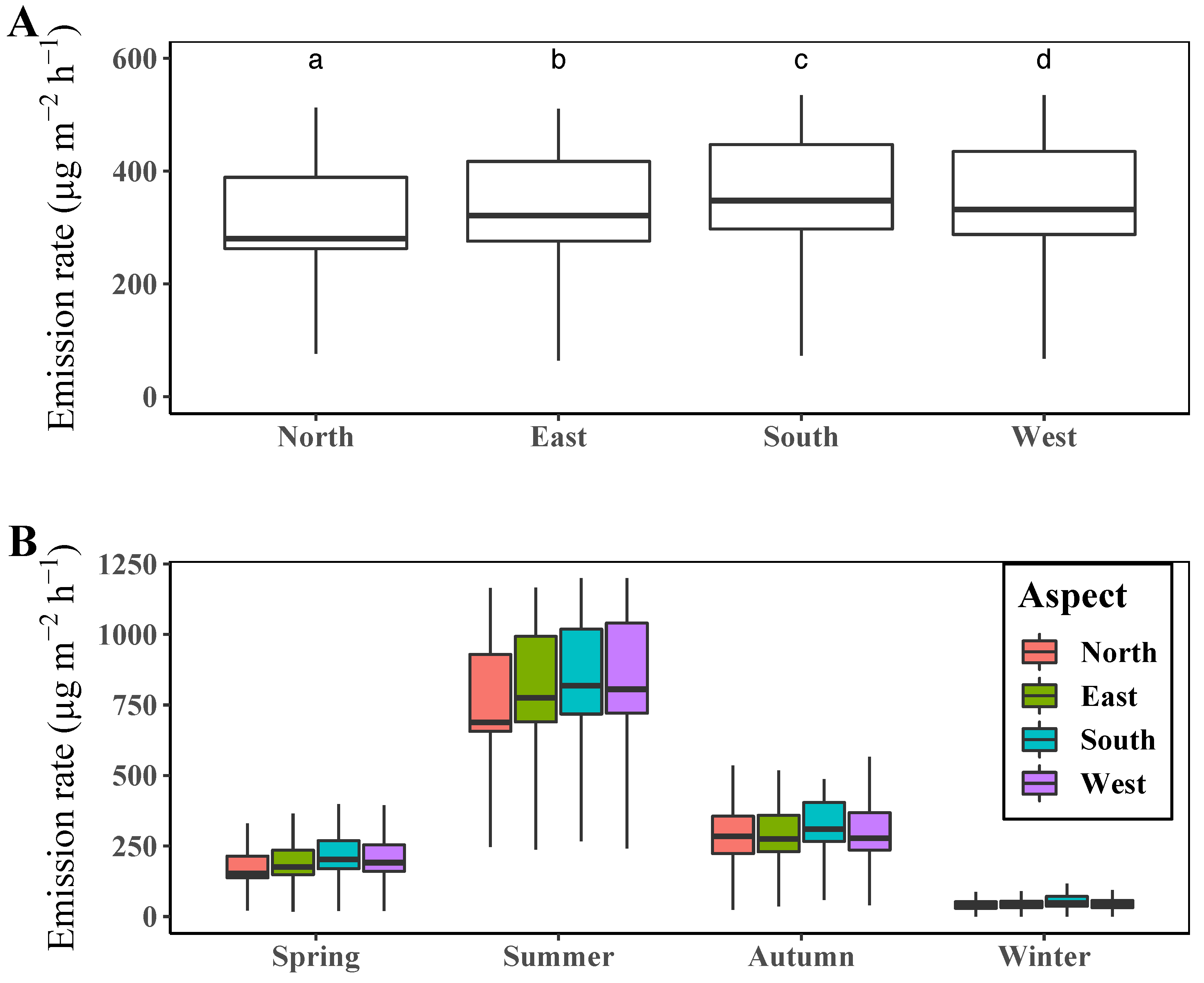
3.3. Comparison of Terpene Emission Rate by Slope Aspect
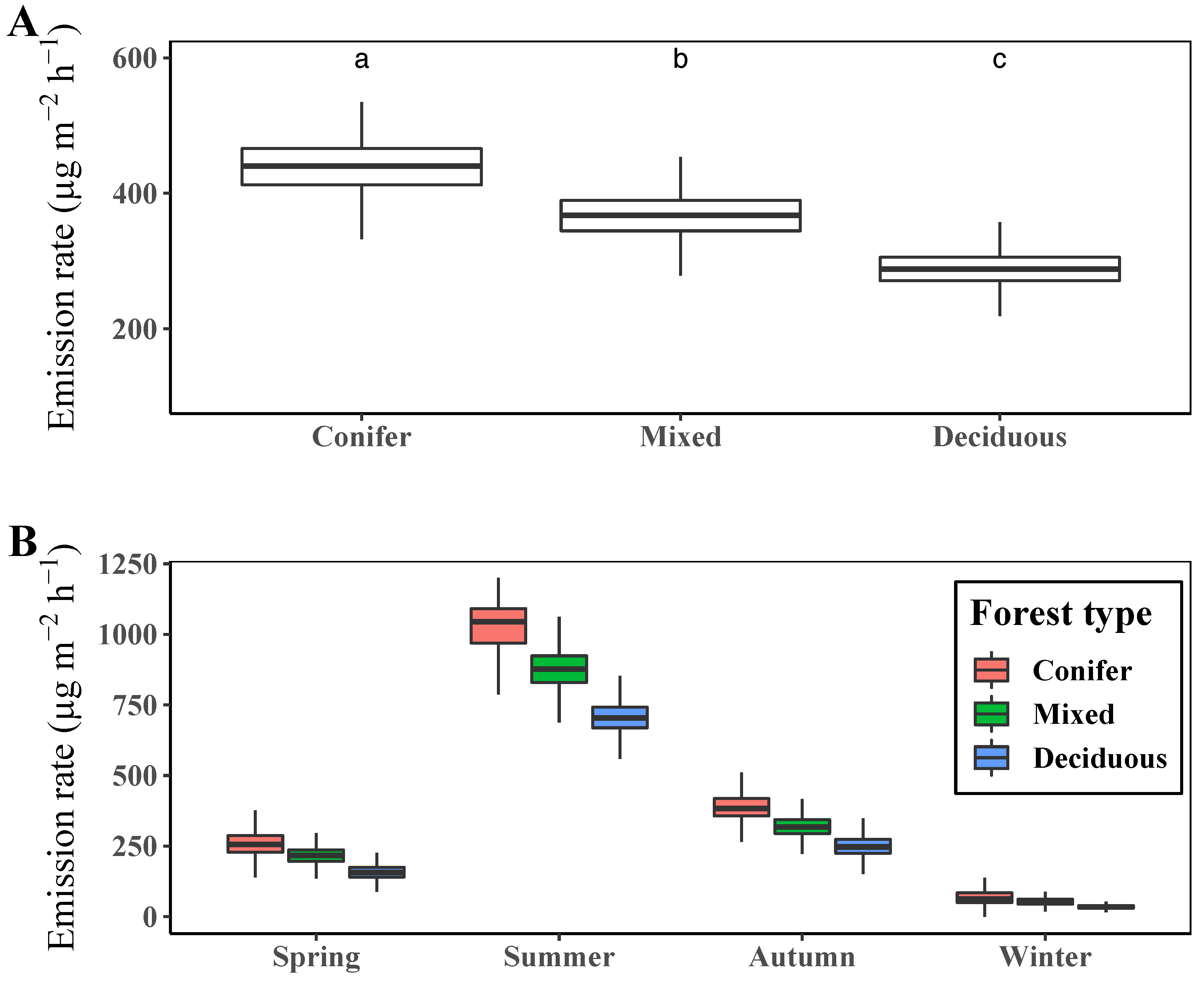
3.4. Comparison of Terpene Emission Rate by Plant Functional Type (PFT)
4. Discussion
4.1. Spatiotemporal Patterns of Terpenes
4.2. Validity of the Model for Estimating Terpenes
4.3. Optimal Strategies for Using Forests for Therapeutic Purposes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korea Forest Service. Statistical Yearbook of Forestry in 2018; Technical Report; Korea Forest Service: Daejeon, Korea, 2018. (In Korean) [Google Scholar]
- Youn, Y.C. Use of forest resources, traditional forest-related knowledge and livelihood of forest dependent communities: Cases in South Korea. For. Ecol. Manag. 2009, 257, 2027–2034. [Google Scholar] [CrossRef]
- Shin, W.S.; Kim, J.J.; Lim, S.S.; Yoo, R.H.; Jeong, M.A.; Lee, J.; Park, S. Paradigm shift on forest utilization: Forest service for health promotion in the Republic of Korea. Net. J. Agric. Sci. 2017, 5, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.; Wakayama, Y.; et al. Visiting a Forest, but Not a City, Increases Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Preventative Med. 2009, 15, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Preventative Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Naturforsch. C 2016, 71, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Kim, J.C.; Dinh, T.V.; Oh, H.K.; Son, Y.S.; Ahn, J.W.; Song, K.Y.; Choi, I.Y.; Park, C.R.; Suzlejko, J.; Kim, K.H. The Potential Benefits of Therapeutic Treatment Using Gaseous Terpenes at Ambient Low Levels. Appl. Sci. 2019, 9, 4507. [Google Scholar] [CrossRef] [Green Version]
- Llusiá, J.; Peñuelas, J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am. J. Bot. 2000, 87, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Holzke, C.; Dindorf, T.; Kesselmeier, J.; Kuhn, U.; Koppmann, R. Terpene emissions from European beech (Fagus sylvatica L.): Pattern and Emission Behaviour Over two Vegetation Periods. J. Atmos. Chem. 2006, 55, 81–102. [Google Scholar] [CrossRef]
- Helmig, D.; Daly, R.W.; Milford, J.; Guenther, A. Seasonal trends of biogenic terpene emissions. Chemosphere 2013, 93, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Lai, J.; Liu, Y.; Zheng, L.; Fang, X.; Song, W.; Yi, Z. Biogenic volatile organic compound emissions from Pinus massoniana and Schima superba seedlings: Their responses to foliar and soil application of nitrogen. Sci. Total Environ. 2020, 705, 135761. [Google Scholar] [CrossRef]
- Hartikainen, K.; Riikonen, J.; Nerg, A.M.; Kivimäenpää, M.; Ahonen, V.; Tervahauta, A.; Kärenlampi, S.; Mäenpää, M.; Rousi, M.; Kontunen-Soppela, S.; et al. Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environ. Exp. Bot. 2012, 84, 33–43. [Google Scholar] [CrossRef]
- Ghimire, R.P.; Kivimäenpää, M.; Kasurinen, A.; Häikiö, E.; Holopainen, T.; Holopainen, J.K. Herbivore-induced BVOC emissions of Scots pine under warming, elevated ozone and increased nitrogen availability in an open-field exposure. Agric. For. Meteorol. 2017, 242, 21–32. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Yu, X. Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ. Pollut. 2020, 259, 113955. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Karl, T.; Guenther, A.; Tyndall, G.; Orlando, J.; Harley, P.; Rasmussen, R.; Apel, E. Emissions and ambient distributions of Biogenic Volatile Organic Compounds (BVOC) in a ponderosa pine ecosystem: Interpretation of PTR-MS mass spectra. Atmos. Chem. Phys. 2010, 10, 1759–1771. [Google Scholar] [CrossRef] [Green Version]
- Sartelet, K.N.; Couvidat, F.; Seigneur, C.; Roustan, Y. Impact of biogenic emissions on air quality over Europe and North America. Atmos. Environ. 2012, 53, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Situ, S.; Guenther, A.; Wang, X.; Jiang, X.; Turnipseed, A.; Wu, Z.; Bai, J.; Wang, X. Impacts of seasonal and regional variability in biogenic VOC emissions on surface ozone in the Pearl River delta region, China. Atmos. Chem. Phys. 2013, 13, 11803–11817. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, K.M.; Cope, M.E.; Galbally, I.E.; Lee, S.; Nelson, P.F. Isoprene and monoterpene emissions in south-east Australia: Comparison of a multi-layer canopy model with MEGAN and with atmospheric observations. Atmos. Chem. Phys. 2018, 18, 7539–7556. [Google Scholar] [CrossRef] [Green Version]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Park, S.H.; Lee, H.J.; Kang, J.W.; Lee, K.M.; Yeon, P.S. Study on NVOCs Concentration Characteristics by Season, Time and Climatic Factors: Focused on Pinus densiflora Forest in National Center for Forest Therapy. J. People Plants Environ. 2018, 21, 403–409. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Bae, Y.T.; Lee, J.K.; Adzic, T.; Lee, S.M.; Lee, K.J.; Shin, M.Y.; Ko, D.W.; Kim, K.W. Analysis of the Phytoncide Emission Trend in Saneum Recreational Forest. J. Korean Inst. For. Recreat. 2017, 21, 65–73. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, G.; Park, S.; Kim, E.; Kim, S. Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests. Forests 2021, 12, 543. [Google Scholar] [CrossRef]
- Lee, Y.K.; Woo, J.S.; Choi, S.R.; Shin, E.S. Comparison of Phytoncide (monoterpene) Concentration by Type of Recreational Forest. J. Environ. Health Sci. 2015, 41, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef] [Green Version]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Cho, K.S.; Jeon, Y.; Kim, J.B.; ran Lim, Y.; Lee, K.; Lee, I.S. Characteristics and distribution of terpenes in South Korean forests. J. Ecol. Environ. 2017, 41, 19. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kim, B.U.; Kim, H.; Kim, S. The Variability of Ozone Sensitivity to Anthropogenic Emissions with Biogenic Emissions Modeled by MEGAN and BEIS3. Atmosphere 2017, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Heald, C.L.; Wilkinson, M.J.; Monson, R.K.; Alo, C.A.; Wang, G.; Guenther, A. Response of isoprene emission to ambient CO2 changes and implications for global budgets. Glob. Chang. Biol. 2009, 15, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Korea Forest Service. Mountain Meteorology Observation System. 2021. Available online: http://mw.nifos.go.kr (accessed on 30 April 2021).
- Korea Meteorological Administration. KMA Weather Data Service: Open Met Data Portal. 2021. Available online: https://data.kma.go.kr (accessed on 30 April 2021).
- Dos Reis, M.G.; Ribeiro, A. Conversion factors and general equations applied in agricultural and forest meteorology. Agrometeoros 2019, 27, 227–258. [Google Scholar]
- Mavi, H.S.; Tupper, G.J. Agrometeorology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Korea Forest Service. Forest map (1:5000) via the Korean National Spatial Data Infrastructure Portal. 2020. Available online: http://data.nsdi.go.kr (accessed on 25 November 2020).
- Jang, Y.; Eo, Y.; Jang, M.; Woo, J.H.; Kim, Y.; Lee, J.B.; Lim, J.H. Impact of Land Cover and Leaf Area Index on BVOC Emissions over the Korean Peninsula. Atmosphere 2020, 11, 806. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Borchers, H.W. pracma: Practical Numerical Math Functions. R Package Version 2.3.3. 2021. Available online: https://CRAN.R-project.org/package=pracma (accessed on 30 April 2021).
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Folker, M.; Richardson, A.D.; Tomelleri, E. phenopix: Process Digital Images of a Vegetation Cover. R Package Version 2.4.2. 2020. Available online: https://CRAN.R-project.org/package=phenopix (accessed on 30 April 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pearson, R.K. Data cleaning for dynamic modeling and control. In Proceedings of the 1999 European Control Conference (ECC), Karlsruhe, Germany, 31 August–3 September 1999; pp. 2584–2589. [Google Scholar] [CrossRef]
- Beck, P.S.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Cai, Z.; Jönsson, P.; Jin, H.; Eklundh, L. Performance of Smoothing Methods for Reconstructing NDVI Time-Series and Estimating Vegetation Phenology from MODIS Data. Remote Sens. 2017, 9, 1271. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Luo, J.; Huang, Q.; Wu, W.; Sun, Y. Weighted Double-Logistic Function Fitting Method for Reconstructing the High-Quality Sentinel-2 NDVI Time Series Data Set. Remote Sens. 2019, 11, 2342. [Google Scholar] [CrossRef] [Green Version]
- Corripio, J.G. Vectorial algebra algorithms for calculating terrain parameters from DEMs and solar radiation modelling in mountainous terrain. Int. J. Geogr. Inf. Sci. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Corripio, J.G. insol: Solar Radiation. R Package Version 1.2.2. 2021. Available online: https://CRAN.R-project.org/package=insol (accessed on 30 April 2021).
- Laffineur, Q.; Aubinet, M.; Schoon, N.; Amelynck, C.; Müller, J.F.; Dewulf, J.; Van Langenhove, H.; Steppe, K.; Šimpraga, M.; Heinesch, B. Isoprene and monoterpene emissions from a mixed temperate forest. Atmos. Environ. 2011, 45, 3157–3168. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, M.; Kim, S.; Guenther, A.; Park, J.; Cho, G.; Kim, H.S. Measurements of Isoprene and Monoterpenes at Mt. Taehwa and Estimation of Their Emissions. Korean J. Agric. For. Meteorol. 2015, 17, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Llusia, J.; Roahtyn, S.; Yakir, D.; Rotenberg, E.; Seco, R.; Guenther, A.; Peñuelas, J. Photosynthesis, stomatal conductance and terpene emission response to water availability in dry and mesic Mediterranean forests. Trees 2016, 30, 749–759. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.C. Characteristic of Biogenic VOCs Emission and the Impact on the Ozone Formation in Jeju Island. Ph.D. Thesis, Department of Environmental Enginering, Jeju National University, Jeju, Korea, 2013. [Google Scholar]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.T.; Kim, J.C.; Hong, J.H. A study on the comparison of Biogenic VOC (BVOC) Emissions Estimates by BEIS and CORINAIR Methodologies. J. Korean Soc. Atmos. 2006, 22, 167–177. [Google Scholar]
- Tani, A.; Nozoe, S.; Aoki, M.; Hewitt, C. Monoterpene fluxes measured above a Japanese red pine forest at Oshiba plateau, Japan. Atmos. Environ. 2002, 36, 3391–3402. [Google Scholar] [CrossRef]
- Sakulyanontvittaya, T.; Duhl, T.; Wiedinmyer, C.; Helmig, D.; Matsunaga, S.; Potosnak, M.; Milford, J.; Guenther, A. Monoterpene and Sesquiterpene Emission Estimates for the United States. Environ. Sci. Technol. 2008, 42, 1623–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagori, J.; Janssen, R.H.H.; Fry, J.L.; Krol, M.; Jimenez, J.L.; Hu, W.; Vilà-Guerau de Arellano, J. Biogenic emissions and land–atmosphere interactions as drivers of the daytime evolution of secondary organic aerosol in the southeastern US. Atmos. Chem. Phys. 2019, 19, 701–729. [Google Scholar] [CrossRef] [Green Version]
- Oh, G.Y.; Park, G.H.; Kim, I.S.; Bae, J.S.; Park, H.Y.; Seo, Y.G.; Yang, S.I.; Lee, J.K.; Jeong, S.H.; Lee, W.J. Comparison of Major Monoterpene Concentrations in the Ambient Air of South Korea Forests. J. Korean. For. Soc. 2010, 99, 698–705. [Google Scholar]
- Kim, M.H.; Park, S.Y.; Kim, I.S.; Oh, G.Y.; Lee, H.H.; Kim, E.I.; An, K.W. A Comparative Study on the Terpene Retention Volume from Chamaecyparis obtusa by Difference of the Sampling Methods. J. Korean Inst. Rec. 2018, 22, 1–9. [Google Scholar]




| Compound Class | Compound Species | Needleleaf Conifer | Broadleaf Deciduous |
|---|---|---|---|
| Isoprene | Isoprene | 600 | 10,000 |
| Monoterpene | Myrcene | 70 | 30 |
| Sabinene | 70 | 50 | |
| Limonene | 100 | 80 | |
| 3-Carene | 160 | 30 | |
| t--Ocimene | 70 | 120 | |
| -Pinene | 300 | 130 | |
| -Pinene | 500 | 400 | |
| Others | 180 | 150 | |
| Sesquiterpene | -Farnesene | 40 | 40 |
| -Caryophyllene | 80 | 40 | |
| Others | 120 | 100 | |
| Other VOCs | 232-MBO | 700 | 0.01 |
| Methanol | 900 | 900 | |
| Acetone | 240 | 240 | |
| CO | 600 | 600 | |
| Bidirectional VOC | 500 | 500 | |
| Stress VOC | 300 | 300 | |
| Others | 140 | 140 |
| Compound Class | Compound Species | LDF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Isoprene | Isoprene | 0.13 | 1.0 | 95 | 2.00 | 0.05 | 0.60 | 1.00 | 0.90 |
| Monoterpene | Myrcene | 0.10 | 0.6 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 |
| Sabinene | 0.10 | 0.6 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| Limonene | 0.10 | 0.2 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| 3-Carene | 0.10 | 0.2 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| t--Ocimene | 0.10 | 0.8 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| -Pinene | 0.10 | 0.2 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| -Pinene | 0.10 | 0.6 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| Others | 0.10 | 0.4 | 80 | 1.83 | 2.00 | 1.80 | 1.00 | 1.05 | |
| Sesquiterpene | -Farnesene | 0.17 | 0.5 | 130 | 2.37 | 0.40 | 0.60 | 1.00 | 0.95 |
| -Caryophyllene | 0.17 | 0.5 | 130 | 2.37 | 0.40 | 0.60 | 1.00 | 0.95 | |
| Others | 0.17 | 0.5 | 130 | 2.37 | 0.40 | 0.60 | 1.00 | 0.95 | |
| Other VOC | 232-MBO | 0.13 | 1.0 | 95 | 2.00 | 0.05 | 0.60 | 1.00 | 0.90 |
| Methanol | 0.08 | 0.8 | 60 | 1.60 | 3.50 | 3.00 | 1.00 | 1.20 | |
| Acetone | 0.10 | 0.2 | 80 | 1.83 | 1.00 | 1.00 | 1.00 | 1.00 | |
| CO | 0.08 | 1.0 | 60 | 1.60 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Bidirectional VOC | 0.13 | 0.8 | 95 | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Stress VOC | 0.10 | 0.8 | 80 | 1.83 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Others | 0.10 | 0.2 | 80 | 1.83 | 1.00 | 1.00 | 1.00 | 1.00 |
| Parameter | Source |
|---|---|
| Air and leaf temperatures | Temperature measured 10 above the ground at Mt. Bongmi from Korea Forest Service [39] |
| PPFD | Average solar radiation from four meteorological stations surrounding the site [40]. |
| Plant functional type (PFT) | Forest type of forest map (1:5000) from Korea Forest Service [43] |
| Leaf area index (LAI) | Estimated from NDVI index derived from Sentinel-2 L2 surface reflectance data with a 10 spatial resolution obtained from Google Earth Engine [45] |
| Compound Class | Compound | Annual Mean Emission Rate (g mh) | Proportion (%) |
|---|---|---|---|
| Monoterpene | -Pinene | 82.8 | 24.6 |
| -Pinene | 73.3 | 21.8 | |
| Limonene | 32.4 | 9.6 | |
| 3-Carene | 30.6 | 9.1 | |
| Sabinene | 10.9 | 3.3 | |
| Myrcene | 8.7 | 2.6 | |
| t--Ocimene | 8.2 | 2.5 | |
| Other Monoterpenes | 46.1 | 13.7 | |
| Total | 292.9 | 87.1 | |
| Sesquiterpene | -Caryophyllene | 12.0 | 3.6 |
| -Farnesene | 8.5 | 2.5 | |
| Other Sesquiterpenes | 23.0 | 6.8 | |
| Total | 43.5 | 12.9 |
| Season | Compound Class | Mean Emission Rate (g mh) | Proportion (%) |
|---|---|---|---|
| Spring | Monoterpene | 175.6 ± 140.6 | 90.6 |
| Sesquiterpene | 18.1 ± 23.9 | 9.4 | |
| Summer | Monoterpene | 688.5 ± 239.7 | 84.8 |
| Sesquiterpene | 123.9 ± 74.0 | 15.2 | |
| Autumn | Monoterpene | 259.3 ± 172.2 | 89.8 |
| Sesquiterpene | 29.5 ± 29.4 | 10.2 | |
| Winter | Monoterpene | 45.5 ± 22.6 | 96.2 |
| Sesquiterpene | 1.8 ± 1.6 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.; Ko, D.W.; Kim, K.W.; Shin, M.Y. A Modeling Approach for Quantifying Human-Beneficial Terpene Emission in the Forest: A Pilot Study Applying to a Recreational Forest in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 8278. https://doi.org/10.3390/ijerph19148278
Choi K, Ko DW, Kim KW, Shin MY. A Modeling Approach for Quantifying Human-Beneficial Terpene Emission in the Forest: A Pilot Study Applying to a Recreational Forest in South Korea. International Journal of Environmental Research and Public Health. 2022; 19(14):8278. https://doi.org/10.3390/ijerph19148278
Chicago/Turabian StyleChoi, Kwanghun, Dongwook W. Ko, Ki Weon Kim, and Man Yong Shin. 2022. "A Modeling Approach for Quantifying Human-Beneficial Terpene Emission in the Forest: A Pilot Study Applying to a Recreational Forest in South Korea" International Journal of Environmental Research and Public Health 19, no. 14: 8278. https://doi.org/10.3390/ijerph19148278






