Application of High-Performance Liquid Chromatography with Diode Array Detection to Simultaneous Analysis of Reference Antioxidants and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) in Free Radical Scavenging Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. HPLC-DAD Conditions
2.3. DPPH Free Radical Scavenging Activity Assay
2.4. Statistical Analysis
3. Results and Discussion
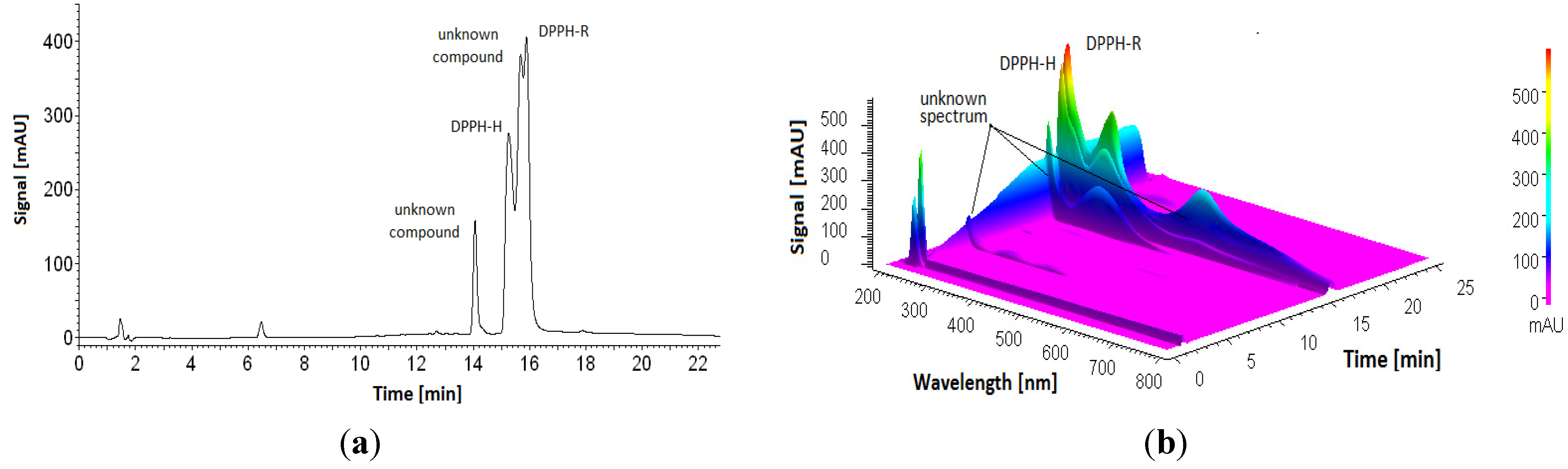
3.1. Stability of DPPH-R in Gradient Elution Mode with Acetonitrile–Water Mobile Phase
3.2. Influence of Analysis Time and UV Radiation Emitted by a Detector on DPPH Stability
3.3. Influence of DPPH-R Concentration on the DPPH-R/DPPH-H Ratio
3.4. Stability of DPPH Free Radical under Acidic Conditions
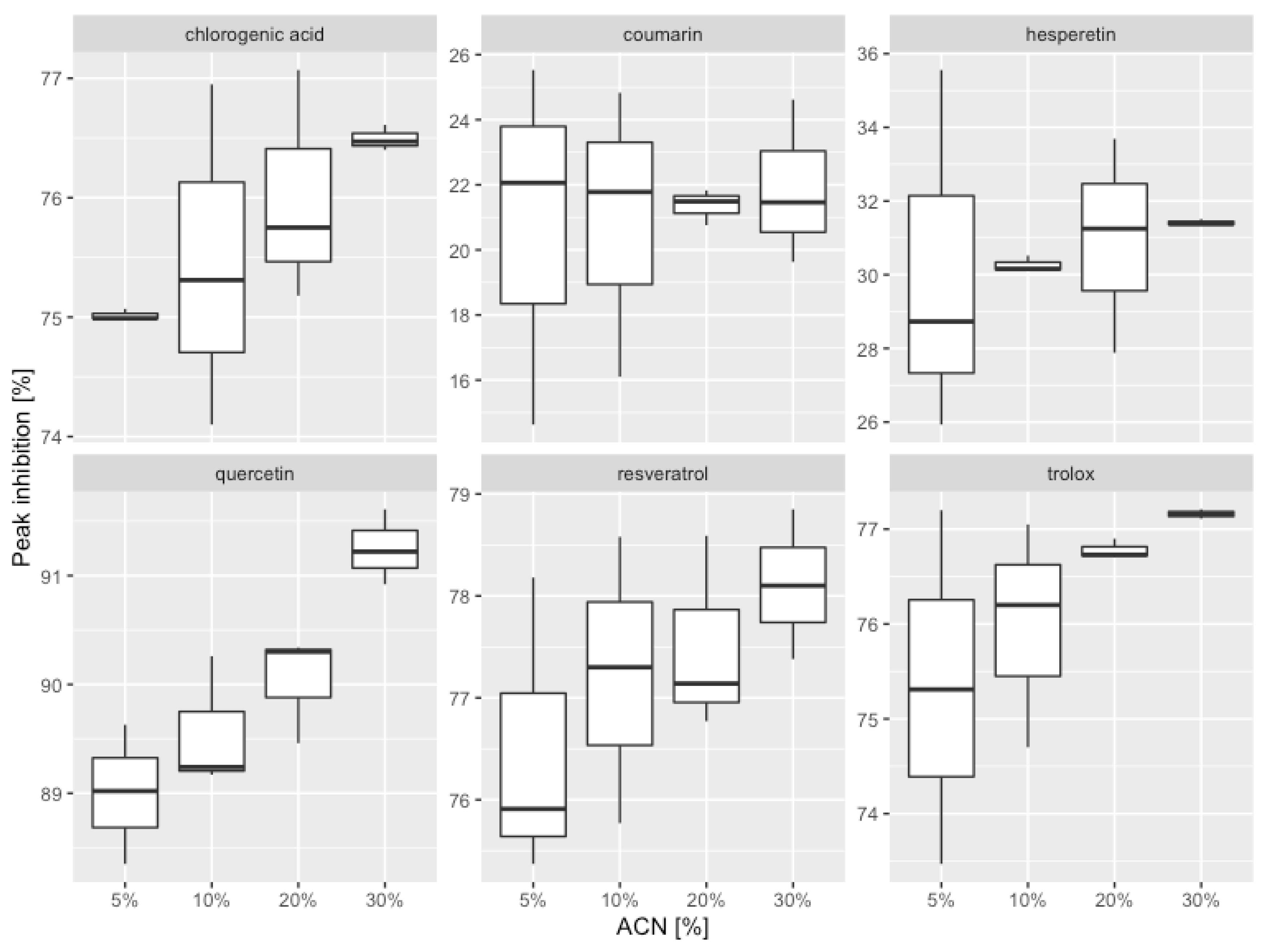
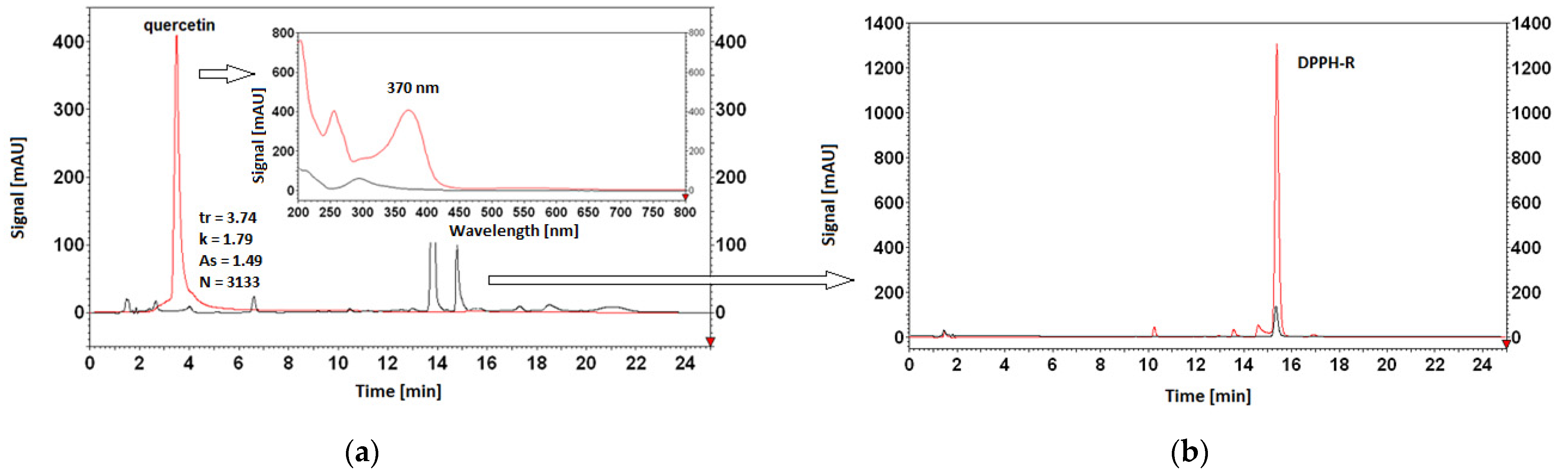
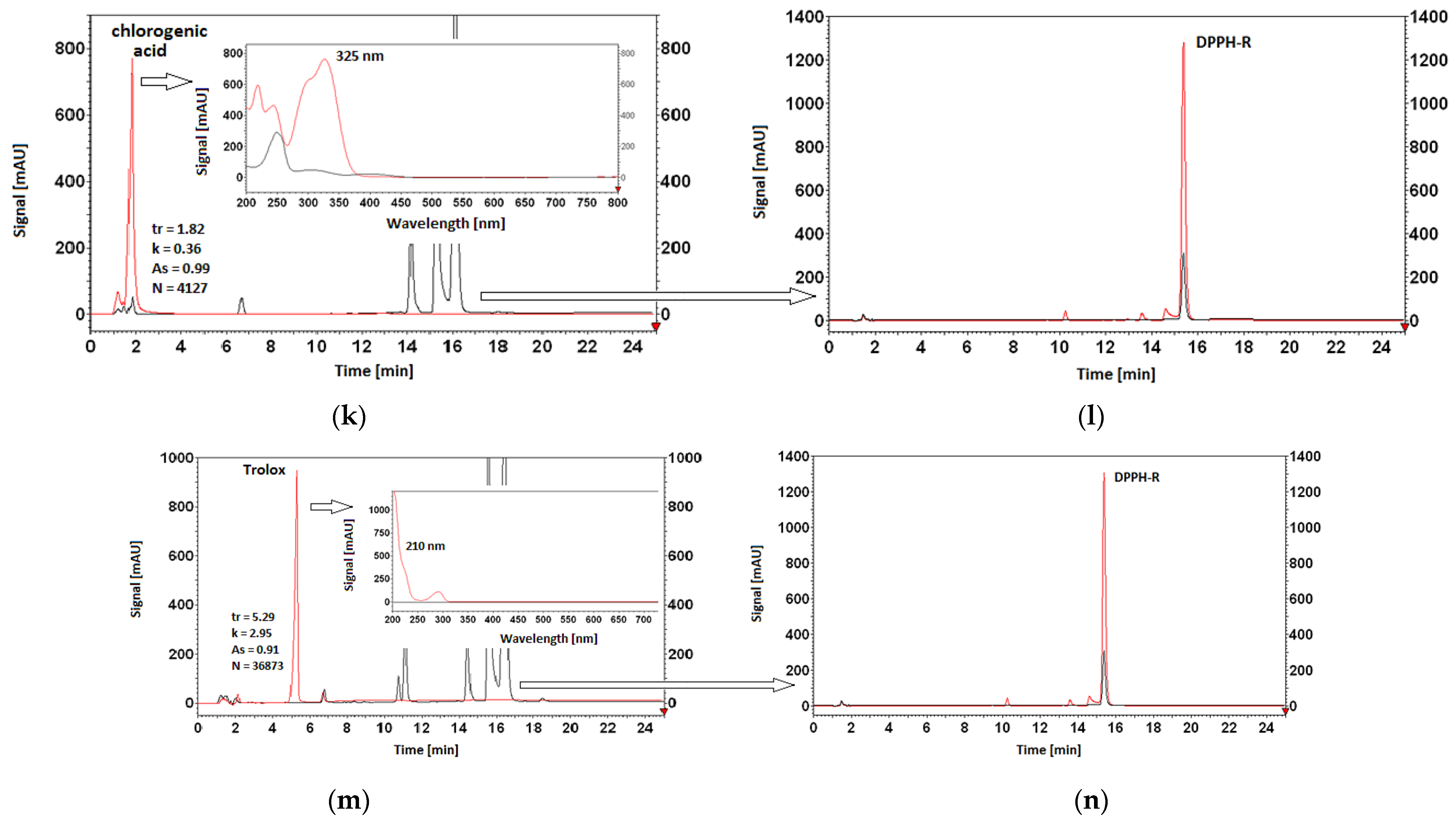
3.5. Antioxidant Properties of AO Standards Measured by DPPH-HPLC-DAD
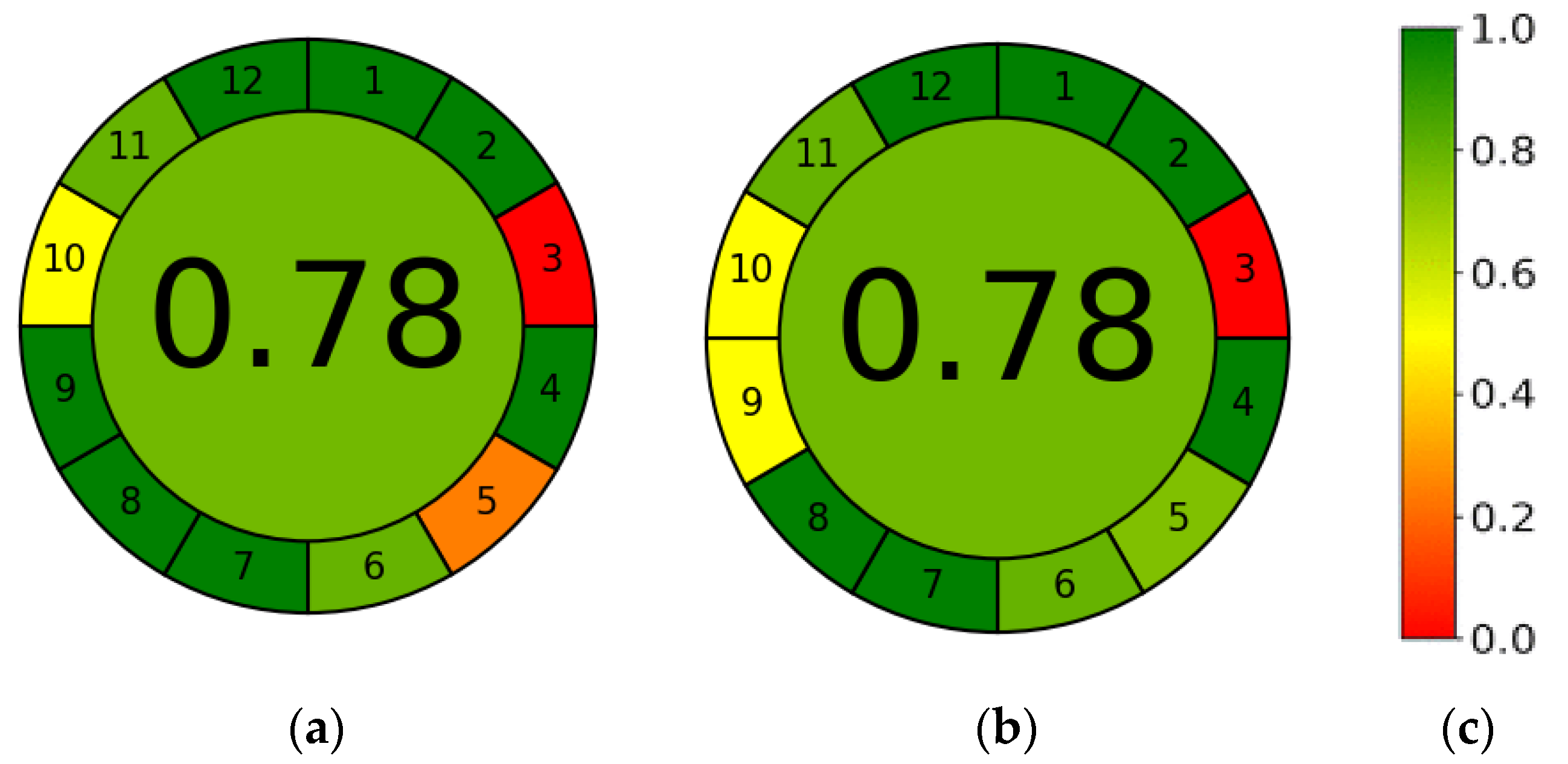
3.6. AGREE—Analytical GREEnness Metric Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Danet, A.F.; Fleschin, Ş.; Aboul-Enein, H.Y. Recent Applications for in Vitro Antioxidant Activity Assay. Crit. Rev. Anal. Chem. 2016, 46, 389–399. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Sawston, UK, 2015; pp. 287–333. [Google Scholar] [CrossRef]
- Barriga-González, G.; Aguilera-Venegas, B.; Folch-Cano, C.; Pérez-Cruz, F.; Olea-Azar, C. Electron spin resonance as a powerful tool for studying antioxidants and radicals. Curr. Med. Chem. 2013, 20, 4731–4743. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Ohba, R.; Ueda, S. Radical scavenging activity of a purple pigment, hordeumin, from uncooked barley bran-fermented broth. J. Agric. Food Chem. 2000, 48, 3198. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical Re-Evaluation of DPPH assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC-DPPH assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening natural antioxidants in peanut shell using DPPH-HPLC-DAD-TOF/MS methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef]
- Chandrasekar, D.; Madhusudhana, K.; Ramakrishna, S.; Diwan, P.V. Determination of DPPH free radical scavenging activity by reversed-phase HPLC: A sensitive screening method for polyherbal formulations. J. Pharm. Biomed. Anal. 2006, 40, 460–464. [Google Scholar] [CrossRef]
- Boudier, A.; Tournebize, J.; Bartosz, G.; El Hani, S.; Bengueddour, R.; Sapin-Minet, A.; Leroy, P. High-performance liquid chromatographic method to evaluate the hydrogen atom transfer during reaction between 1,1-diphenyl-2-picryl-hydrazyl radical and antioxidants. Anal. Chim. Acta 2012, 711, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, M. The [DPPH•/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef]
- Wu, C.; Chen, F.; Wang, X.; Wu, Y.; Dong, M.; He, G.; Galyean, R.D.; He, L.; Huang, G. Tanacetum parthenium by HPLC-ESI-MS/MS and NMR. Phytochem. Anal. 2007, 18, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Nuengchamnong, N.; de Jong, C.F.; Bruyneel, B.; Niessen, W.M.; Irth, H.; Ingkaninan, K. HPLC coupled on-line to ESI-MS and a DPPH-based assay for the rapid identification of anti-oxidants in Butea superba. Phytochem. Anal. 2005, 16, 422–428. [Google Scholar] [CrossRef]
- Tang, D.; Li, H.J.; Chen, J.; Guo, C.W.; Li, P. Rapid and simple method for screening of natural antioxidants from Chinese herb FlosLoniceraeJaponicae by DPPH-HPLC-DAD-TOF/MS. J. Sep. Sci. 2008, 31, 3519–3526. [Google Scholar] [CrossRef]
- Dapkevicius, A.; van Beek, T.A.; Niederländer, H.A.; de Groot, A. On-line detection of antioxidative activity in high-performance liquid chromatography eluates by chemiluminescence. Anal. Chem. 1999, 71, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Arai, H.; Tanizawa, H.; Miyahara, T.; Toyo’oka, T. On-line screening method for antioxidants by liquid chromatography with chemiluminescence detection. Anal. Chim. Acta 1999, 383, 221–230. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.G.; van Beek, T.A. An On-Line HPLC Method for Detection of Radical Scavenging Compounds in Complex Mixtures. Anal. Chem. 2000, 72, 2323–2328. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Evaluation of the anti-radical properties of antioxidants using the DPPH test •: A critical overview and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Karel, M. Lipid Oxidation, Secondary Reactions, and Water Activity of Foods. In Autooxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Liu, H. Comparing Welch’s ANOVA, a Kruskal-Wallis Test and Traditional ANOVA in Case of Heterogeneity of Variance. Ph.D. Thesis, VCU, Richmond, VA, USA, 2015; p. 48. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [Green Version]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In Vitro Phytotoxicity and Antioxidant Activity of Selected Flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef] [PubMed]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compost. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Wu, C.-R.; Huang, M.-Y.; Lin, Y.-T.; Ju, H.-Y.; Ching, H. Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Sak, K. Dependence of DPPH Radical Scavenging Activity of Dietary Flavonoid Quercetin on Reaction Environment. Mini-Rev. Med. Chem. 2014, 14, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.E.; Russo, N.; Grand, A.; Galano, A. A physicochemical examination of the free radical scavenging activity of Trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013, 15, 4642. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 6, 753–758. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Sykula, A.; Kowalska-Baron, A.; Dzeikala, A.; Bodzioch, A.; Lodyga-Chruscinska, E. An experimental and DFT study on free radical scavenging activity of hesperetin Schiff bases. Chem. Phys. 2019, 517, 91–103. [Google Scholar] [CrossRef]
- Furusawa, M.; Tanaka, T.; Ito, T.; Nishikawa, A.; Yamazaki, N.; Nakaya, K.-I.; Matsuura, N.; Tsuchiya, H.; Nagayama, M.; Iinuma, M. Antioxidant activity of hydroflavonoids. J. Health Sci. 2005, 51, 376–378. [Google Scholar] [CrossRef] [Green Version]
- Tobiszewski, M.; Marc, M.; Gałuszka, A.; Namiesnik, J. Green Chemistry Metrics with Special Reference to Green Analytical Chemistry. Molecules 2015, 20, 10928–10946. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
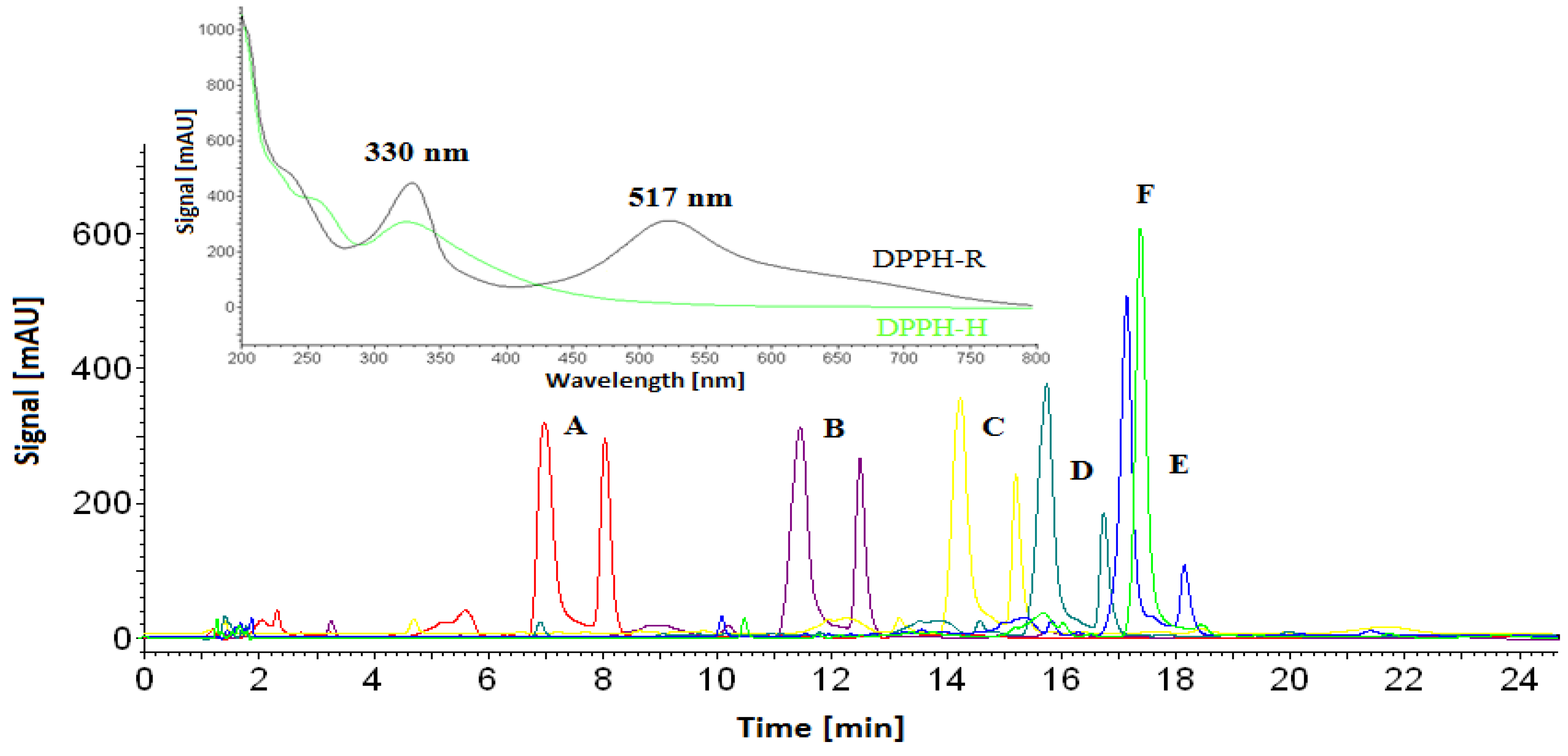
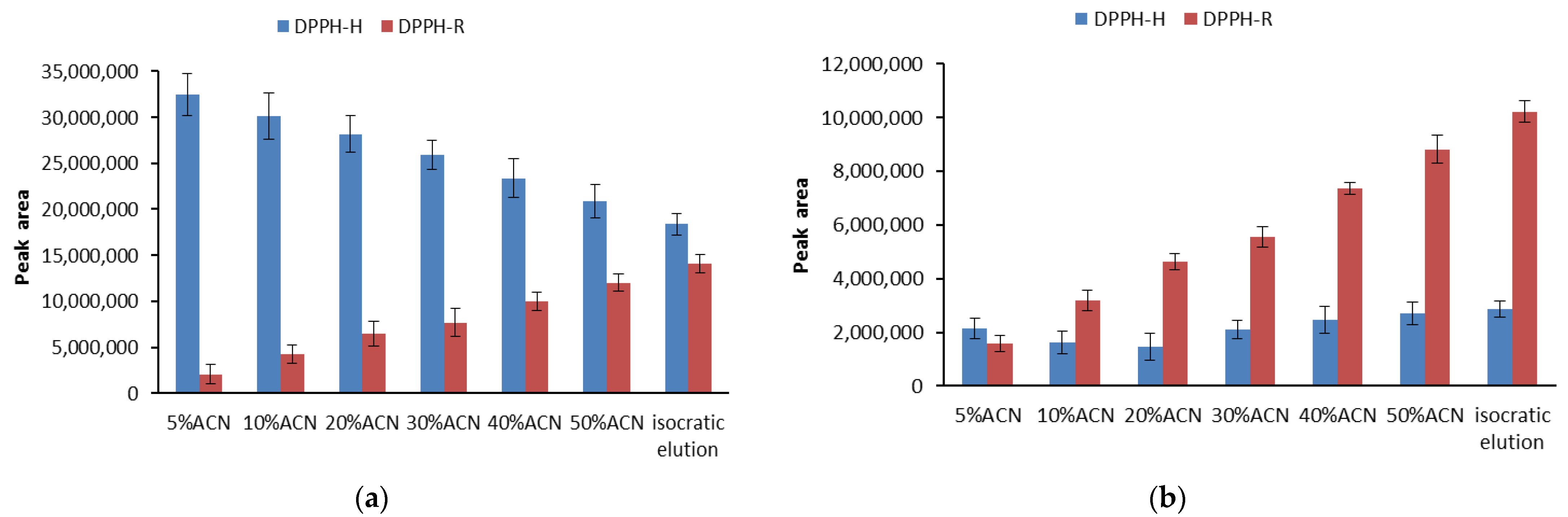
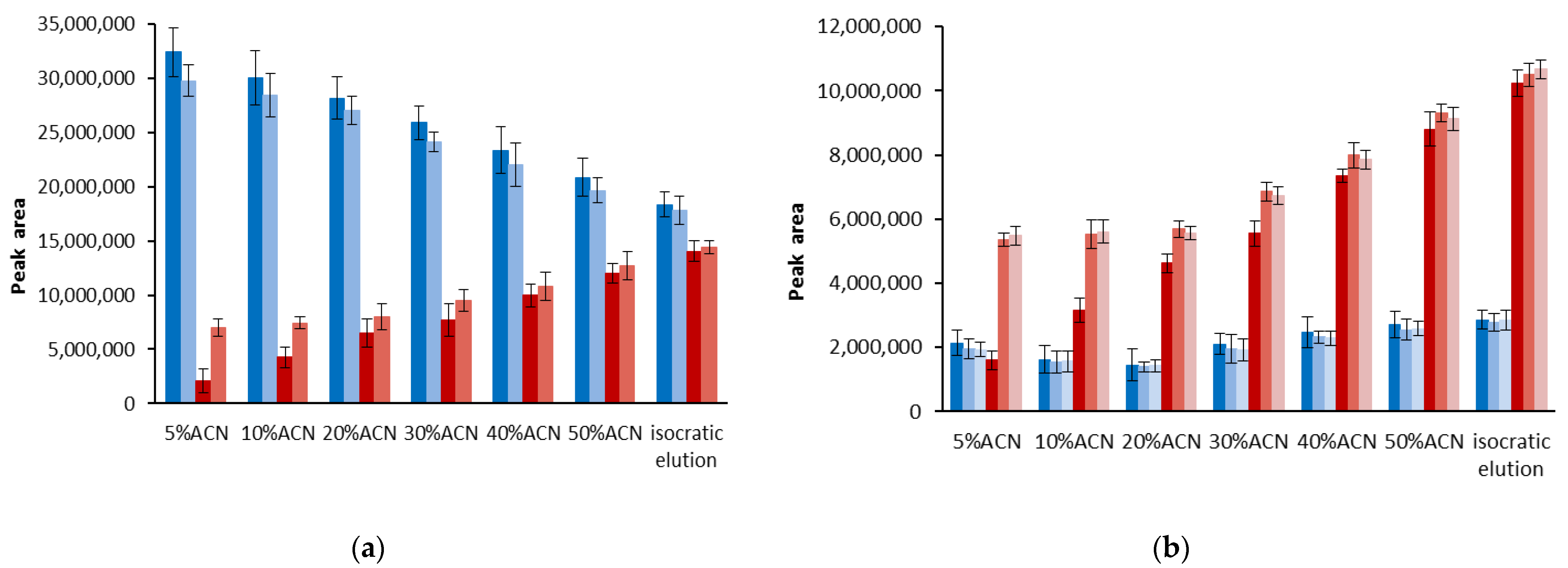
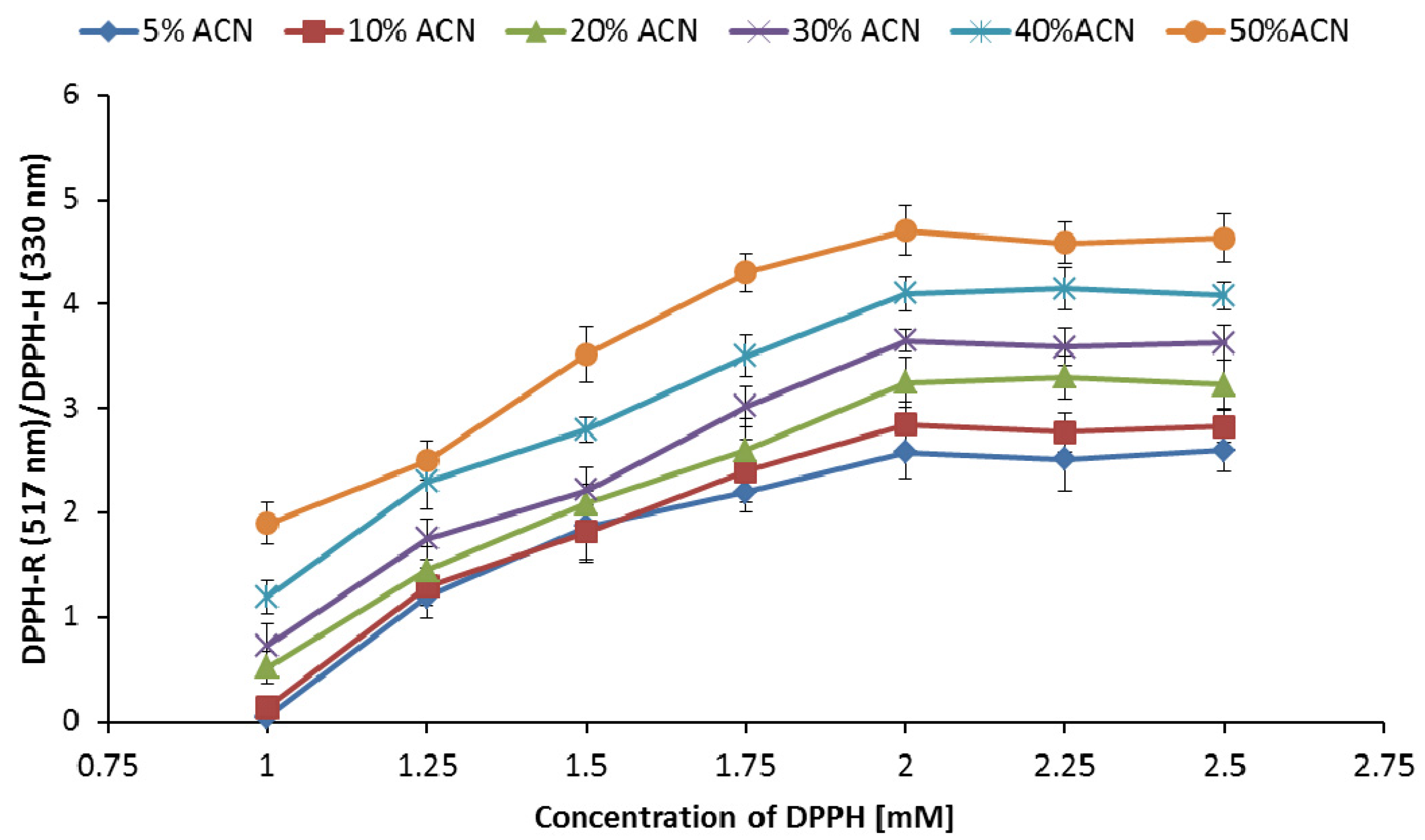


| Gradient Profile | LOD [µmol L−1] | LOQ [µmol L−1] | Linearity Equation: (y = ax + b) | ||||
|---|---|---|---|---|---|---|---|
| a ± SD | b ± SD | R2 | F | se | |||
| λ = 517 nm | |||||||
| 5% ACN | 107.31 | 325.18 | 28,435,512 ± 935,113 | −8,015,561 ± 1,444,263 | 0.9957 | 1,611,206 | 924.68 |
| 10% ACN | 55.23 | 167.35 | 28,440,890 ± 1,303,644 | −8,499,618 ± 2,013,452 | 0.9917 | 2,246,187 | 475.96 |
| 20% ACN | 45.10 | 136.65 | 27,462,657 ± 1,417,624 | −7,311,386 ± 2,189,491 | 0.9895 | 2,442,574 | 375.29 |
| 30% ACN | 31.64 | 95.86 | 27,090,228 ± 1,681,039 | −6,070,836 ± 2,596,330 | 0.9848 | 2,896,440 | 259.70 |
| λ = 330 nm | |||||||
| 5% ACN | 1677.05 | 5081.96 | 3,343,277 ± 81,109 | −744,633 ± 125,272 | 0.9977 | 139,752 | 1699.04 |
| 10% ACN | 642.58 | 1947.20 | 3,421,269 ± 132,552 | −902,170 ± 204,725 | 0.9940 | 228,389 | 666.19 |
| 20% ACN | 200.13 | 606.45 | 3,661,783 ± 245,725 | −1,342,050 ± 379,518 | 0.9823 | 423,386 | 222.07 |
| 30% ACN | 189.41 | 573.97 | 3,246,670 ± 237,833 | −566,917 ± 367,329 | 0.9790 | 409,788 | 186.35 |
| ACN [% (v/v)] | AC (0) 1 | AA (t) 2 | Peak Inhibition | ||
|---|---|---|---|---|---|
| [%] ± SD | Mean [%] | Relative Error [%] | |||
| quercetin | |||||
| 5% | 56,744,541 | 6,236,679 | 89.01 ± 0.64 | 89.96 | 2.45 |
| 10% | 56,920,403 | 5,940,782 | 89.56 ± 0.61 | ||
| 20% | 57,213,505 | 5,701,898 | 90.03 ± 0.50 | ||
| 30% | 58,620,394 | 5,129,109 | 91.25 ± 0.35 | ||
| resveratrol | |||||
| 5% | 56,550,363 | 13,284,246 | 76.51 ± 1.49 | 77.35 | 2.06 |
| 10% | 56,725,622 | 12,896,570 | 77.27 ± 1.41 | ||
| 20% | 57,017,721 | 12,821,575 | 77.51 ± 0.96 | ||
| 30% | 58,419,796 | 12,782,251 | 78.12 ± 0.74 | ||
| Trolox | |||||
| 5% | 56,576,945 | 13,941,691 | 75.36 ± 1.87 | 76.33 | 2.33 |
| 10% | 56,752,287 | 13,618,846 | 76.00 ± 1.19 | ||
| 20% | 57,044,523 | 13,246,879 | 76.78 ± 0.10 | ||
| 30% | 58,447,257 | 13,349,353 | 77.16 ± 0.05 | ||
| chlorogenic acid | |||||
| 5% | 56,608,329 | 14,141,327 | 75.02 ± 0.05 | 75.75 | 1.92 |
| 10% | 56,783,768 | 13,921,676 | 75.48 ± 1.43 | ||
| 20% | 57,076,167 | 13,691,431 | 76.01 ± 0.97 | ||
| 30% | 58,479,679 | 13,748,572 | 76.49 ± 0.11 | ||
| hesperetin | |||||
| 5% | 56,768,577 | 39,634,117 | 30.18 ± 4.95 | 30.71 | 3.92 |
| 10% | 56,944,512 | 39,710,256 | 30.27 ± 0.22 | ||
| 20% | 57,237,739 | 39,506,060 | 30.98 ± 2.92 | ||
| 30% | 58,645,224 | 40,224,759 | 31.41 ± 0.09 | ||
| coumarin | |||||
| 5% | 56,338,341 | 44,578,275 | 20.87 ± 5.57 | 21.29 | 4.83 |
| 10% | 56,512,943 | 44,653,137 | 20.99 ± 4.43 | ||
| 20% | 56,803,947 | 44,675,168 | 21.35 ± 0.55 | ||
| 30% | 58,200,765 | 45,437,337 | 21.93 ± 2.52 | ||
| Reference Antioxidant | n | Statistic | p-Value |
|---|---|---|---|
| chlorogenic acid | 12 | 118.28 | 0.0004 |
| coumarin | 12 | 0.05 | 0.9830 |
| hesperetin | 12 | 17.27 | 0.0130 |
| quercetin | 12 | 10.13 | 0.0210 |
| resveratrol | 12 | 0.85 | 0.5300 |
| Trolox | 12 | 9.22 | 0.0330 |
| Compound | ACN | n | Mean | Homogenic Group | Mean Group 1 | Mean Group 2 |
|---|---|---|---|---|---|---|
| chlorogenic acid | 5% | 3 | 75.017 | a | 75.490 | NA |
| chlorogenic acid | 10% | 3 | 75.453 | ab | 75.490 | 76.493 |
| chlorogenic acid | 20% | 3 | 76.000 | ab | 75.490 | 76.493 |
| chlorogenic acid | 30% | 3 | 76.493 | b | NA | 76.493 |
| coumarin | 5% | 3 | 20.740 | c | 21.227 | NA |
| coumarin | 10% | 3 | 20.903 | c | 21.227 | NA |
| coumarin | 20% | 3 | 21.360 | c | 21.227 | NA |
| coumarin | 30% | 3 | 21.903 | c | 21.227 | NA |
| hesperetin | 5% | 3 | 30.073 | de | 30.427 | 31.413 |
| hesperetin | 10% | 3 | 30.267 | d | 30.427 | NA |
| hesperetin | 20% | 3 | 30.940 | de | 30.427 | 31.413 |
| hesperetin | 30% | 3 | 31.413 | e | NA | 31.413 |
| quercetin | 5% | 3 | 89.000 | f | 89.530 | NA |
| quercetin | 10% | 3 | 89.557 | fg | 89.530 | 91.250 |
| quercetin | 20% | 3 | 90.033 | fg | 89.530 | 91.250 |
| quercetin | 30% | 3 | 91.250 | g | NA | 91.250 |
| resveratrol | 5% | 3 | 76.487 | h | 77.329 | NA |
| resveratrol | 10% | 3 | 77.217 | h | 77.329 | NA |
| resveratrol | 20% | 3 | 77.500 | h | 77.329 | NA |
| resveratrol | 30% | 3 | 78.110 | h | 77.329 | NA |
| trolox | 5% | 3 | 75.327 | ij | 76.030 | 77.160 |
| trolox | 10% | 3 | 75.983 | ij | 76.030 | 77.160 |
| trolox | 20% | 3 | 76.780 | i | 76.030 | NA |
| trolox | 30% | 3 | 77.160 | j | NA | 77.160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarczak-Michalewska, M.; Flieger, J. Application of High-Performance Liquid Chromatography with Diode Array Detection to Simultaneous Analysis of Reference Antioxidants and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) in Free Radical Scavenging Test. Int. J. Environ. Res. Public Health 2022, 19, 8288. https://doi.org/10.3390/ijerph19148288
Tatarczak-Michalewska M, Flieger J. Application of High-Performance Liquid Chromatography with Diode Array Detection to Simultaneous Analysis of Reference Antioxidants and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) in Free Radical Scavenging Test. International Journal of Environmental Research and Public Health. 2022; 19(14):8288. https://doi.org/10.3390/ijerph19148288
Chicago/Turabian StyleTatarczak-Michalewska, Małgorzata, and Jolanta Flieger. 2022. "Application of High-Performance Liquid Chromatography with Diode Array Detection to Simultaneous Analysis of Reference Antioxidants and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) in Free Radical Scavenging Test" International Journal of Environmental Research and Public Health 19, no. 14: 8288. https://doi.org/10.3390/ijerph19148288






