Essential Factors for a Healthy Microbiome: A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction Process
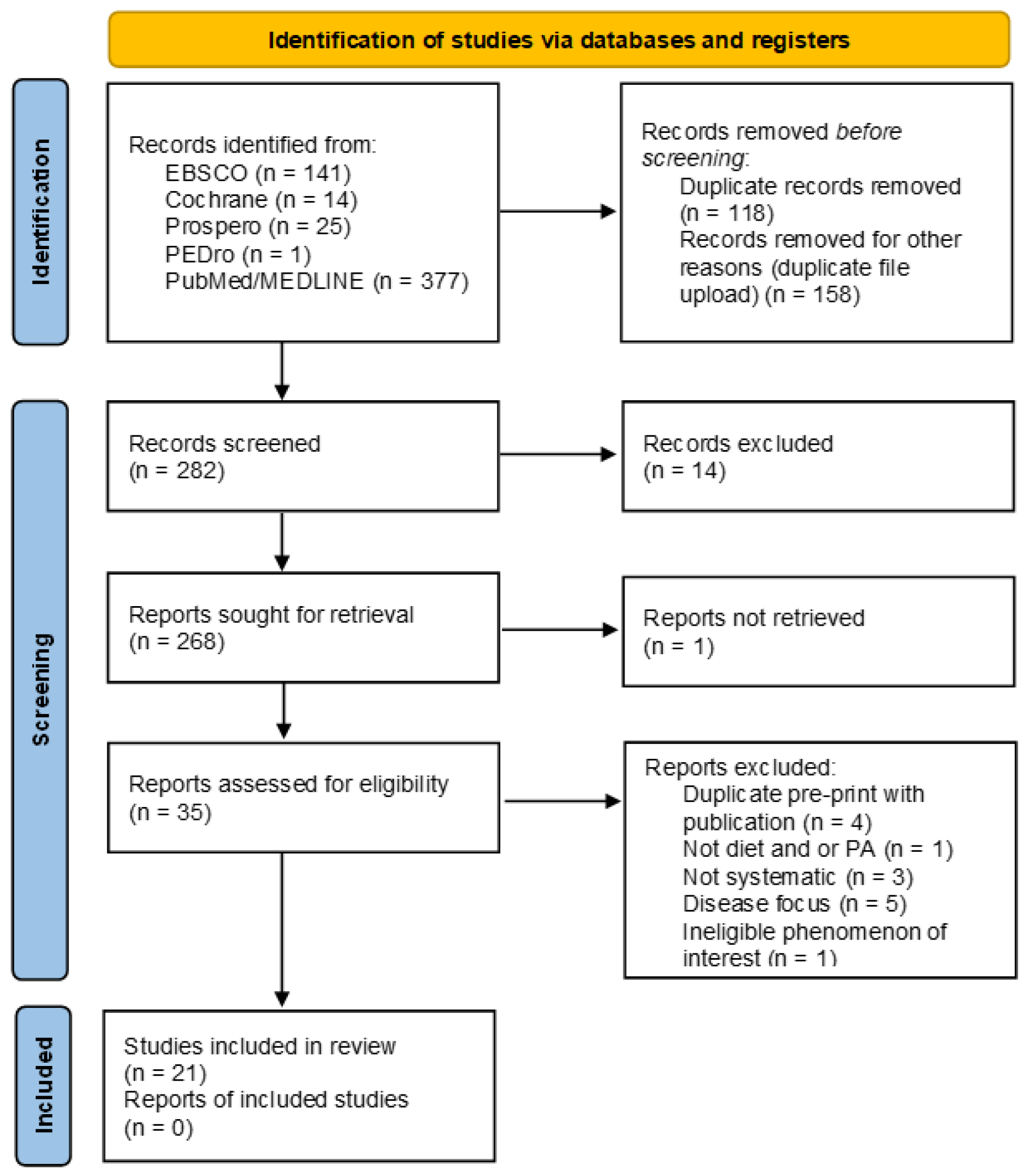
2.4. Results
3. Microbiome
4. Diet
4.1. Western Diet
4.1.1. Ultra-Processed Foods
4.1.2. Protein
4.1.3. Fats
4.1.4. Carbohydrates and Fiber
4.1.5. Diet Supplementation
4.1.6. Water
4.1.7. Plant-Based Diets: Vegetarian and Vegan
4.1.8. Mediterranean
5. Physical Activity
6. Discussion
6.1. Introduction
6.2. Limitations and Strengths of This Review
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Williams, A. Shining a Light on the Resource Curse: An Empirical Analysis of the Relationship Between Natural Resources, Transparency, and Economic Growth. World Dev. 2011, 39, 490–505. [Google Scholar] [CrossRef]
- Swain Ewald, H.A.; Ewald, P.W. Natural Selection, The Microbiome, and Public Health. Yale J. Biol. Med. 2018, 91, 445–455. [Google Scholar]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Jollet, M.; Nay, K.; Chopard, A.; Bareille, M.P.; Beck, A.; Ollendorff, V.; Vernus, B.; Bonnieu, A.; Mariadassou, M.; Rué, O.; et al. Does Physical Inactivity Induce Significant Changes in Human Gut Microbiota? New Answers Using the Dry Immersion Hypoactivity Model. Nutrients 2021, 13, 3865. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailen-Andrino, M.; Perez-Santiago, J.; Gonzalez-Soltero, R.; Perez, M.; Montalvo-Lominchar, M.G.; Mate-Munoz, J.L.; Dominguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef] [Green Version]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mirzayi, C.; Renson, A.; Genomic Standards, C.; Massive, A.; Quality Control, S.; Zohra, F.; Elsafoury, S.; Geistlinger, L.; Kasselman, L.J.; Eckenrode, K.; et al. Reporting guidelines for human microbiome research: The STORMS checklist. Nat. Med. 2021, 27, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- White, R.G.; Hakim, A.J.; Salganik, M.J.; Spiller, M.W.; Johnston, L.G.; Kerr, L.; Kendall, C.; Drake, A.; Wilson, D.; Orroth, K.; et al. Strengthening the Reporting of Observational Studies in Epidemiology for respondent-driven sampling studies: "STROBE-RDS" statement. J. Clin. Epidemiol. 2015, 68, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Little, J.; Higgins, J.P.; Ioannidis, J.P.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. Strengthening the reporting of genetic association studies (STREGA): An extension of the STROBE statement. Eur. J. Epidemiol. 2009, 24, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telle-Hansen, V.H.; Holven, K.B.; Ulven, S.M. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018, 10, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trefflich, I.; Jabakhanji, A.; Menzel, J.; Blaut, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food. Sci. Nutr. 2019, 60, 2990–3004. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [Green Version]
- Wolters, M.; Ahrens, J.; Romani-Perez, M.; Watkins, C.; Sanz, Y.; Benitez-Paez, A.; Stanton, C.; Gunther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, A.; Adolphus, K. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef] [PubMed]
- Tzemah Shahar, R.; Koren, O.; Matarasso, S.; Shochat, T.; Magzal, F.; Agmon, M. Attributes of Physical Activity and Gut Microbiome in Adults: A Systematic Review. Int. J. Sports Med. 2020, 41, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and their Effect on Gut Microbiota, Gut Function and Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.F.; Harding, S.V.; Zafron, M.; Rideout, T.C. A systematic review of the effect of dietary pulses on microbial populations inhabiting the human gut. Benef. Microbes 2020, 11, 457–468. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Lambert, K.; Stanford, J.; Neale, E.P. The effect of nut consumption (tree nuts and peanuts) on the gut microbiota of humans: A systematic review. Br. J. Nutr. 2021, 125, 508–520. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef] [PubMed]
- Dorelli, B.; Gallè, F.; De Vito, C.; Duranti, G.; Iachini, M.; Zaccarin, M.; Preziosi Standoli, J.; Ceci, R.; Romano, F.; Liguori, G.; et al. Can Physical Activity Influence Human Gut Microbiota Composition Independently of Diet? A Systematic Review. Nutrients 2021, 13, 1890. [Google Scholar] [CrossRef]
- Gibiino, G.; De Siena, M.; Sbrancia, M.; Binda, C.; Sambri, V.; Gasbarrini, A.; Fabbri, C. Dietary Habits and Gut Microbiota in Healthy Adults: Focusing on the Right Diet. A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6728. [Google Scholar] [CrossRef] [PubMed]
- Losno, E.A.; Sieferle, K.; Perez-Cueto, F.J.A.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Dotsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Gibson, G.R.; Walton, G.E.; Magistro, D.; Kinnear, W.; Hunter, K. Systematic Review of the Effects of Exercise and Physical Activity on the Gut Microbiome of Older Adults. Nutrients 2022, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef]
- Kimble, R.; Gouinguenet, P.; Ashor, A.; Stewart, C.; Deighton, K.; Matu, J.; Griffiths, A.; Malcomson, F.C.; Joel, A.; Houghton, D.; et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: A systematic review of randomized controlled trials and observational studies. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Clark, H.D.; Wells, G.A.; Huet, C.; McAlister, F.A.; Salmi, L.R.; Fergusson, D.; Laupacis, A. Assessing the quality of randomized trials: Reliability of the Jadad scale. Control Clin. Trials 1999, 20, 448–452. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party 2014. Joanna Briggs Institute Levels of Evidence and Grades of Recommendation; The Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Handu, D.; Moloney, L.; Wolfram, T.; Ziegler, P.; Acosta, A.; Steiber, A. Academy of Nutrition and Dietetics Methodology for Conducting Systematic Reviews for the Evidence Analysis Library. J. Acad. Nutr. Diet. 2016, 116, 311–318. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, J. Food Habit Associated Mycobiota Composition and Their Impact on Human Health. Front. Nutr. 2021, 8, 773577. [Google Scholar] [CrossRef]
- Henderickx, J.G.E.; de Weerd, H.; Groot Jebbink, L.J.; van Zoeren-Grobben, D.; Hemels, M.A.C.; van Lingen, R.A.; Knol, J.; Belzer, C. The first fungi: Mode of delivery determines early life fungal colonization in the intestine of preterm infants. Microbiome Res. Rep. 2022, 1, 7. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Gribaudo, G. Retroviruses of the Human Virobiota: The Recycling of Viral Genes and the Resulting Advantages for Human Hosts During Evolution. Front. Microbiol. 2020, 11, 1140. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Liu, T.; Xing, Y.; Fan, X.; Chen, Z.; Zhao, C.; Liu, L.; Zhao, M.; Hu, X.; Dong, B.; Wang, J.; et al. Fasting and overfeeding affect the expression of the immunity- or inflammation-related genes in the liver of poultry via endogenous retrovirus. Poult. Sci. 2021, 100, 973–981. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Arora, K.; Ameur, H.; Di Cagno, R.; De Angelis, M.; Gobbetti, M. Gluten-free diet and gut microbiome. J. Cereal Sci. 2020, 95, 103058. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef] [PubMed]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef]
- Cotillard, A.; Cartier-Meheust, A.; Litwin, N.S.; Chaumont, S.; Saccareau, M.; Lejzerowicz, F.; Tap, J.; Koutnikova, H.; Lopez, D.G.; McDonald, D.; et al. A posteriori dietary patterns better explain variations of the gut microbiome than individual markers in the American Gut Project. Am. J. Clin. Nutr. 2022, 115, 432–443. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Perri, M.R.; Romano, C.; Marrelli, M.; Zicarelli, L.; Toma, C.C.; Basta, D.; Conforti, F.; Statti, G. Beneficial Role of Fruits, Their Juices, and Freeze-Dried Powders on Inflammatory Bowel Disease and Related Dysbiosis. Plants 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.D.; Cecatti, C.; Fidelix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.R.; Chrismas, B.C.R. Thirty Days of Montmorency Tart Cherry Supplementation Has No Effect on Gut Microbiome Composition, Inflammation, or Glycemic Control in Healthy Adults. Front. Nutr. 2021, 8, 733057. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- McCormick, B.J.J.; Murray-Kolb, L.E.; Lee, G.O.; Schulze, K.J.; Ross, A.C.; Bauck, A.; Lima, A.A.M.; Maciel, B.L.L.; Kosek, M.N.; Seidman, J.C.; et al. Intestinal permeability and inflammation mediate the association between nutrient density of complementary foods and biochemical measures of micronutrient status in young children: Results from the MAL-ED study. Am. J. Clin. Nutr. 2019, 110, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Open Food Facts. Nova Groups for Food Processing. Available online: https://world.openfoodfacts.org/nova (accessed on 17 November 2019).
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamler, J.; Chan, Q.; Daviglus, M.L.; Dyer, A.R.; Van Horn, L.; Garside, D.B.; Miura, K.; Wu, Y.; Ueshima, H.; Zhao, L.; et al. Relation of Dietary Sodium (Salt) to Blood Pressure and Its Possible Modulation by Other Dietary Factors: The INTERMAP Study. Hypertension 2018, 71, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, F.J.; Dong, Y.; Huang, Y.; Wang, C.; Harshfield, G.A.; Zhu, H. Modest Sodium Reduction Increases Circulating Short-Chain Fatty Acids in Untreated Hypertensives: A Randomized, Double-Blind, Placebo-Controlled Trial. Hypertension 2020, 76, 73–79. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simmons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019, 5, e126241. [Google Scholar] [CrossRef] [Green Version]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium Dioxide Nanoparticle Ingestion Alters Nutrient Absorption in an In Vitro Model of the Small Intestine. Nano Impact 2017, 5, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Everard, A. Keeping gut lining at bay: Impact of emulsifiers. Trends Endocrinol. Metab. 2015, 26, 273–274. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Wheildon, N.; Ishikawa, S. Food Additive P-80 Impacts Mouse Gut Microbiota Promoting Intestinal Inflammation, Obesity and Liver Dysfunction. SOJ Microbiol. Infect. Dis. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lock, J.Y.; Carlson, T.L.; Wang, C.M.; Chen, A.; Carrier, R.L. Acute Exposure to Commonly Ingested Emulsifiers Alters Intestinal Mucus Structure and Transport Properties. Sci. Rep. 2018, 8, 10008. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; Vidal, L.; Allegue, G.; Gimenez, A.; Bandeira, E.; Moratorio, X.; Molina, V.; Curutchet, M.R. Consumers’ conceptualization of ultra-processed foods. Appetite 2016, 105, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hawk, T.; Aggarwal, A.; Drewnowski, A. Characterizing Ultra-Processed Foods by Energy Density, Nutrient Density, and Cost. Front. Nutr. 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Hamer, H.M.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1-9. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Marzorati, M.; Vilchez-Vargas, R.; Bussche, J.V.; Truchado, P.; Jauregui, R.; El Hage, R.A.; Pieper, D.H.; Vanhaecke, L.; Van de Wiele, T. High-fiber and high-protein diets shape different gut microbial communities, which ecologically behave similarly under stress conditions, as shown in a gastrointestinal simulator. Mol. Nutr. Food Res. 2017, 61, 1600150. [Google Scholar] [CrossRef] [Green Version]
- Kiilerich, P.; Myrmel, L.S.; Fjaere, E.; Hao, Q.; Hugenholtz, F.; Sonne, S.B.; Derrien, M.; Pedersen, L.M.; Petersen, R.K.; Mortensen, A.; et al. Effect of a long-term high-protein diet on survival, obesity development, and gut microbiota in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E886–E899. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2019, 58, 2625–2638. [Google Scholar] [CrossRef]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans Fat Intake and Its Dietary Sources in General Populations Worldwide: A Systematic Review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufian-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: Implications for host-microbe interactions contributing to obesity. Obes. Rev. 2012, 13, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Nitert, M.D. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2017, 9, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, A.; Quek, S.-Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Perkins, T.D.; van den Berg, A.K. Maple syrup-production, composition, chemistry, and sensory characteristics. Adv. Food Nutr. Res. 2009, 56, 101–143. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Seeram, N.P. Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. J. Agric. Food Chem. 2011, 59, 7708–7716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, M.; St-Pierre, P.; Pilon, G.; Anhê, F.F.; Varin, T.; Marette, A. Effects of various natural sweeteners on insulin resistance, inflammation and liver steatosis in a rat model of diet-induced obesity. FASEB J. 2016, 30, lb650. [Google Scholar] [CrossRef]
- Marette, A. Impact of Free Sugar Replacement by Maple Syrup on Prevention of Metabolic Disorders Associated With Overweight in Humans: Role of Gut Microbiota; ClinicalTrials.gov: Washington, DC, USA, 2019.
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, V.; Ferrao, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [Green Version]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boling, L.; Cuevas, D.A.; Grasis, J.A.; Kang, H.S.; Knowles, B.; Levi, K.; Maughan, H.; McNair, K.; Rojas, M.I.; Sanchez, S.E.; et al. Dietary prophage inducers and antimicrobials: Toward landscaping the human gut microbiome. Gut Microbes 2020, 11, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibberd, M.C.; Wu, M.; Rodionov, D.A.; Li, X.; Cheng, J.; Griffin, N.W.; Barratt, M.J.; Giannone, R.J.; Hettich, R.L.; Osterman, A.L.; et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci. Transl. Med. 2017, 9, eaal4069. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Juste Contin Gomes, M.; Stampini Duarte Martino, H.; Tako, E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients 2021, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, T.; Bretin, O.; Poirel, M.; Tap, J. Drinking Water Source and Intake Are Associated with Distinct Gut Microbiota Signatures in US and UK Populations. J. Nutr. 2022, 152, 171–182. [Google Scholar] [CrossRef]
- Dai, Z.; Sevillano-Rivera, M.C.; Calus, S.T.; Bautista-de Los Santos, Q.M.; Eren, A.M.; van der Wielen, P.; Ijaz, U.Z.; Pinto, A.J. Disinfection exhibits systematic impacts on the drinking water microbiome. Microbiome 2020, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.W.; Fung, T.T.; McEvoy, C.T.; Lin, J.; Epel, E.S. Diet Quality Indices and Leukocyte Telomere Length Among Healthy US Adults: Data From the National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2018, 187, 2192–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crous-Bou, M.; Molinuevo, J.L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10, S296–S303. [Google Scholar] [CrossRef]
- Cassidy, A.; De Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef] [Green Version]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: A review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Milk Fat Intake and Telomere Length in U.S. Women and Men: The Role of the Milk Fat Fraction. Oxidative Med. Cell Longev. 2019, 2019, 1574021. [Google Scholar] [CrossRef]
- Barone, M.; Turroni, S.; Rampelli, S.; Soverini, M.; D’Amico, F.; Biagi, E.; Brigidi, P.; Troiani, E.; Candela, M. Gut microbiome response to a modern Paleolithic diet in a Western lifestyle context. PLoS ONE 2019, 14, e0220619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult humans. Gut Microbes 2010, 1, 135–137. [Google Scholar] [CrossRef] [Green Version]
- Muralidharan, J.; Moreno-Indias, I.; Bullo, M.; Lopez, J.V.; Corella, D.; Castaner, O.; Vidal, J.; Atzeni, A.; Fernandez-Garcia, J.C.; Torres-Collado, L.; et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, M.; Brejnrod, A.D.; Aadland, E.K.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, O.; Holthe, A.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fecal Metabolites and Gut Microbiome: Results from a Randomized Crossover Intervention Study. Mol. Nutr. Food Res. 2019, 63, e1700976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61, 1700363. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jager, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- O’Donovan, C.M.; Madigan, S.M.; Garcia-Perez, I.; Rankin, A.; O’Sullivan, O.; Cotter, P.D. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J. Sci. Med. Sport 2020, 23, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Buan, N.R. Methanogens: Pushing the boundaries of biology. Emerg. Top Life Sci. 2018, 2, 629–646. [Google Scholar] [CrossRef] [Green Version]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisseau, N.; Barnich, N.; Koechlin-Ramonatxo, C. The Nutrition-Microbiota-Physical Activity Triad: An Inspiring New Concept for Health and Sports Performance. Nutrients 2022, 14, 924. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 2018, 44, 41–49. [Google Scholar] [CrossRef]
- Stegen, J.C.; Bottos, E.M.; Jansson, J.K. A unified conceptual framework for prediction and control of microbiomes. Curr. Opin. Microbiol. 2018, 44, 20–27. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Diaz, J.; Reese, A.T. Possibilities and limits for using the gut microbiome to improve captive animal health. Anim. Microbiome 2021, 3, 89. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020.
| PICOS Format | Description |
|---|---|
| Population | Healthy subjects Adult humans aged 18 years or older |
| Intervention or exposure | Diet (Western-style, plant-based, vegan); and/or Physical activity or exercise |
| Comparisons | Diet (omnivore, Western-type, vegetarians, vegans) and/or Physical Activity level; reviews of interventions examining probiotics solely and non-interventional reviews were excluded |
| Outcome | Gut microbiota composition through fecal samples; abundance composition or abundance of specific intestinal bacteria |
| Study design | Systematic reviews of cross-sectional, prospective cohort studies, randomized-controlled trials of either parallel or crossover design; and reviews or studies for background information on food or physical activity |
| Aim and Design of Studies | Number of Studies | Quality | Effect on Microbiome | First Author/Study Name |
|---|---|---|---|---|
| Influence of a healthy diet pattern on the microbiome and inflammatory markers Interventional human trials | 18 | Critical appraisal not reported | Due to heterogeneity in study design and type of subjects, no conclusions could be made | Telle-Hansen (2018) [17] |
| Vegan and vegetarian diet association with gut microbiota composition Cross-sectional/cohort/RCT | 37 | a Newcastle–Ottawa scale NOS: 4.6 out of 10 points | No consistent association between a vegan or vegetarian diet and microbiota composition | Trefflich (2019) [18] |
| Effect of wine and grape polyphenols on human gut microbiota. Meta-analysis RCTs | 7 | b Cochrane Risk of Bias Low (5) (1) unclear (1) high risk | Increased Proteobacteria, Fusobacteria, Firmicutes, Bacteroidetes, and B. uniformis after red wine intake; Decrease in dysbiosis-associated species: Clostridum, Eubacterium, and Bacteroides | Nash (2018) [19] |
| Dietary fiber intervention on microbiome Meta-analysis of RCTs | 64-reviewed 58-retained for meta-analysis 12 studies focused on whole-grain diet versus a low-fiber diet | b Cochrane Risk of Bias Low to moderate risk of bias (n = 64) | Dietary fiber intervention compared to placebo/low fiber diet did not significantly increase α-diversity, but increased abundance of Bifidobacterium spp. No difference in Lactobacillus spp. Abundance with food intervention, but significant in fiber supplement group | So (2018) [20] |
| Dietary fat and gut microbiota Cross-sectional, cohort; interventional studies and randomized controlled trials | 16 | b Cochrane Risk of Bias 14 RCT-low risk; 2 RCT-high-risk a Newcastle-Ottawa NOS: 3 = 8, 3 = 7, 1 + 6, and 2 = 5 | n3, n6 PUFA increase beneficial bacteria; high fat/saturated fat diets reduced richness and diversity and had negative metabolic health outcomes; observational studies show an association between fat and health outcomes | Wolters (2019) [21] |
| Association between exercise and gut microbial composition in mammals RCT, cross-sectional, and cohort studies | Human—20 Animal—5 | b Cochrane Risk of Bias–unclear Low Quality Lack of appraisal tools for heterogeneous models | Exercise was associated with changes in gut microbial composition, an increase in butyrate-producing bacteria, and fecal butyrate | Mitchell (2019) [22] |
| Effects of intact cereal grain fibers on microbiome RCT, RCT crossover, non-randomized | 40 | Critical appraisal not provided | Cereal fiber (6–8 g) increases diversity and abundance; increase in bacterial metabolites | Jefferson (2019) [23] |
| Influence of exercise on the human gut microbiota in healthy adults Observational and case-control | 18 | d PEDro 18—Medium | 4/9 observational studies showed higher levels of physical activity or cardiorespiratory fitness were positively associated with α-diversity | Ortez-Alvarez (2020) [24] |
| Influence of endurance training intervention and gut microbiome Interventional studies > 4 weeks duration | 5 | d PEDro 4 studies score ≥ 4—Fair quality 1 study was rated Poor | PA significantly lowers abundance of Bacteroidetes and increases Firmicutes and β diversity in some studies | Shahar (2020) [25] |
| Effects of dairy and dairy derivatives on the gut microbiota (Bovine, yogurt, soy) | 8 | b Cochrane risk-of-bias 2—low 5—some concerns 1—high risk | Richness and diversity declined in all types of milk, Lactobacillus increased in bovine milk; fermented yogurt and kefir increased Lactobacillus and Bifidobacterium | Aslam (2020) [26] |
| Effect of nut consumption on gut microbiome and gut function RCTs | 8 | b Cochrane risk-of-bias No studies were low risk of bias; variable across categories of analysis | Meta-analysis found no effect on β-diversity; no effect of nut type, dose, duration of intervention; increased abundances of Clostridium, Lachnospira and Roseburia | Creedon (2020) [27] |
| Effect of dietary pulses on microbial populations RCT-C, (cross-over); Interventions with control or placebo group | 5 | Critical appraisal not provided | Bacteroides fragilis OUT↓ i for navy bean pulse flour; No difference in Shannon index for diet with chickpeas; lupin fiber consumption decreased abundance of Bacteroides-Prevotella | Marinangeli (2020) [28] |
| Effect of nut consumption on gut microbiome RCT-C, (cross-over); RCT parallel design, and pre/post-test studies | 8 | f Quality Criteria Checklist and g Risk of Bias Assessment Tool 6/8 positive quality 2 neutral | Nuts in general, but especially walnuts, had an impact on gut microbial composition | Fitzgerald (2021) [29] |
| Association between physical activity and changes in intestinal microbiota composition Cross-sectional and longitudinal studies | 17 | h ROBINS-I 6—low 7—moderate 1—serious 3—not reported | Increase in SCFAs concentration after the training period in lean athletes only; composition and diversity differ by sport | Aya (2021) [30] |
| Physical activity influences on human gut microbiota independent of diet observational | 10 4/10 studies controlled for diet | e JBI Critical Appraisal Checklist-criteria met b Cochrane Risk of Bias −2/20 some concerns | Variability is affected by dietary factors and physical characteristics; use of high protein diets contributes to greater variability among athletes | Dorelli (2021) [31] |
| Dietary habits and gut microbiota in healthy adults cross-sectional and RCT Diet regimen studies | 16 | a Newcastle-Ottawa scale Mean score for cross-sectional studies was 5/10; b Cochrane Collaboration tool risk of bias-Low | Significant impact on some bacterial genera from a rich and varied omnivore diet, such as Mediterranean | Gibiino (2021) [32] |
| Vegan diet and gut microbiota Cross-sectional studies | 9 | a Newcastle-Ottawa scale Most studies scored “medium” quality | Firmicutes/Bacteroidetes ratio is lower in vegans compared to omnivores; Abundance of Bacteriodetes and Prevotella in vegans | Losno (2021) [33] |
| a- and β-diversity in obese and non-obese adults Intervention studies and RCT | 32 22 reported Shannon Index (diversity) 25 studies investigated diversity; 2 did not; 5 did not stratify by BMI | h Adapted ROBINS-I Serious risk in one domain: 22 Moderate: 10 | Higher levels of PA and cardiorespiratory fitness are associated with greater α-diversity and increases in some phyla and certain short-chain fatty acids | Pinart (2021) [34] |
| Effects of exercise and physical activity on the gut microbiome in older adults Observational and interventional studies | 7 | Critical appraisal not provided | PA had beneficial impact on the gut microbial composition of older adults | Ramos (2022) [35] |
| Physical activity and human gut microbiota in healthy and unhealthy subjects Observational and interventional studies | 25 | h ROBINS-I 8 studies scored 4–5 c Jadad Scale 4/5 studies: Moderate e JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies 12/12 Included | No significant change in richness and diversity in gut microbiota for minimum PA recommendations Microbial diversity is associated with aerobic exercise | Cataldi (2022) [36] |
| Effect of MedDiet on microbiota and metabolites RCT and Observational studies | 34 17-RCT 17-Observ | b Cochrane (RCT) Mixed Quality a Newcastle-Ottawa scale (Observational) Prospective Studies: High 2 Moderate 1 Low 2 Cross-sectional High 6 Moderate 6 Low 2 | Overall positive impact of Mediterranean diet on Firmicutes/Bacteroidetes ratio. but effects are not consistent between studies due to adherence differences and fewer species that utilize oligosaccharides and simple sugars | Kimble (2022) [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grace-Farfaglia, P.; Frazier, H.; Iversen, M.D. Essential Factors for a Healthy Microbiome: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8361. https://doi.org/10.3390/ijerph19148361
Grace-Farfaglia P, Frazier H, Iversen MD. Essential Factors for a Healthy Microbiome: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(14):8361. https://doi.org/10.3390/ijerph19148361
Chicago/Turabian StyleGrace-Farfaglia, Patricia, Heather Frazier, and Maura Daly Iversen. 2022. "Essential Factors for a Healthy Microbiome: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 14: 8361. https://doi.org/10.3390/ijerph19148361






