Synthesis of CoFe2O4/Peanut Shell Powder Composites and the Associated Magnetic Solid Phase Extraction of Phenoxy Carboxylic Acid Herbicides in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CoFe2O4 Nanoparticles
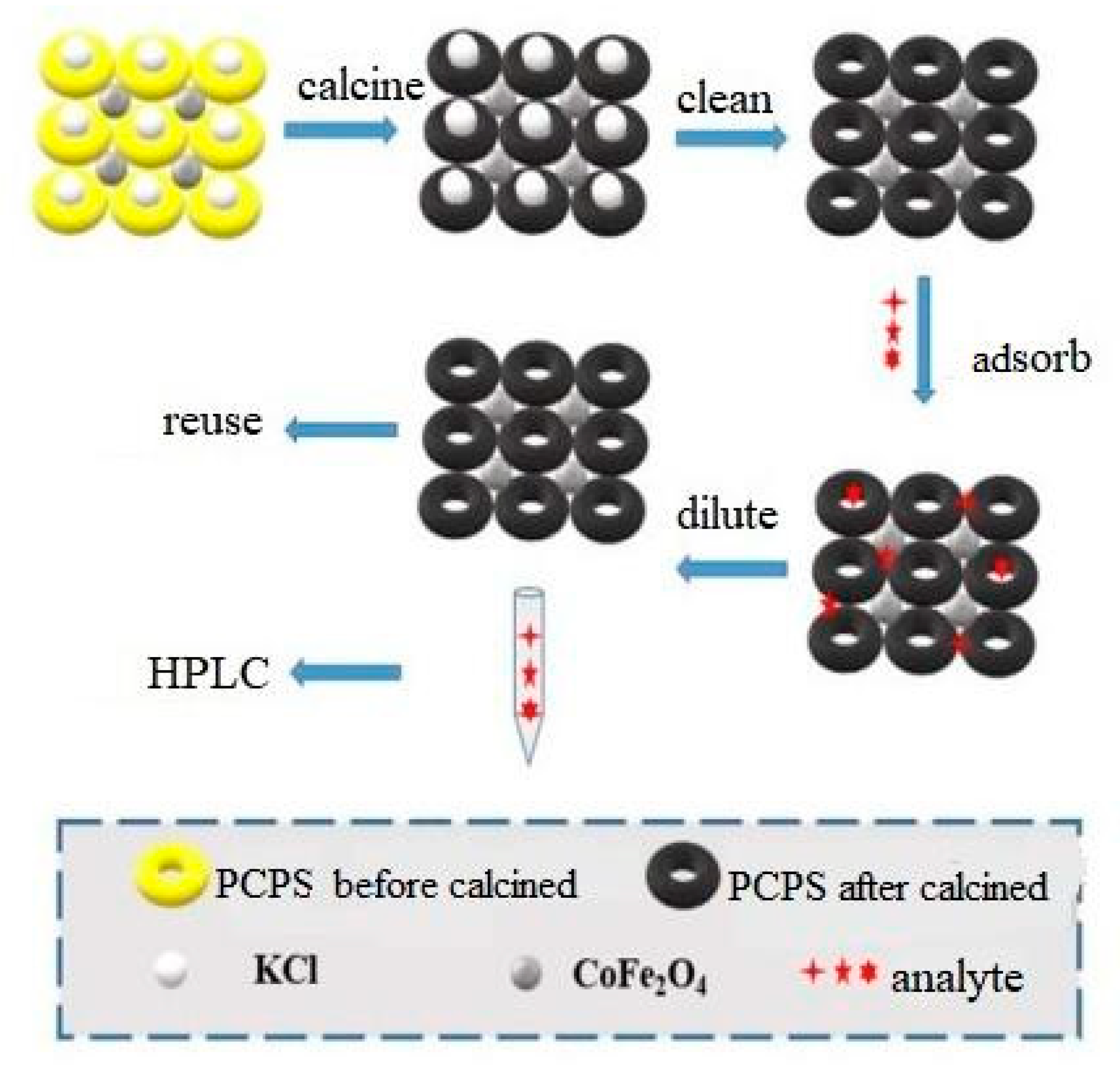
2.2. Preparation of CoFe2O4/PCPS Composites
2.3. Collection of Environmental Water
2.4. Magnetic Solid Phase Extraction Experiment
2.5. Analysis Methods
2.6. The Determination of Zete Potential
3. Results
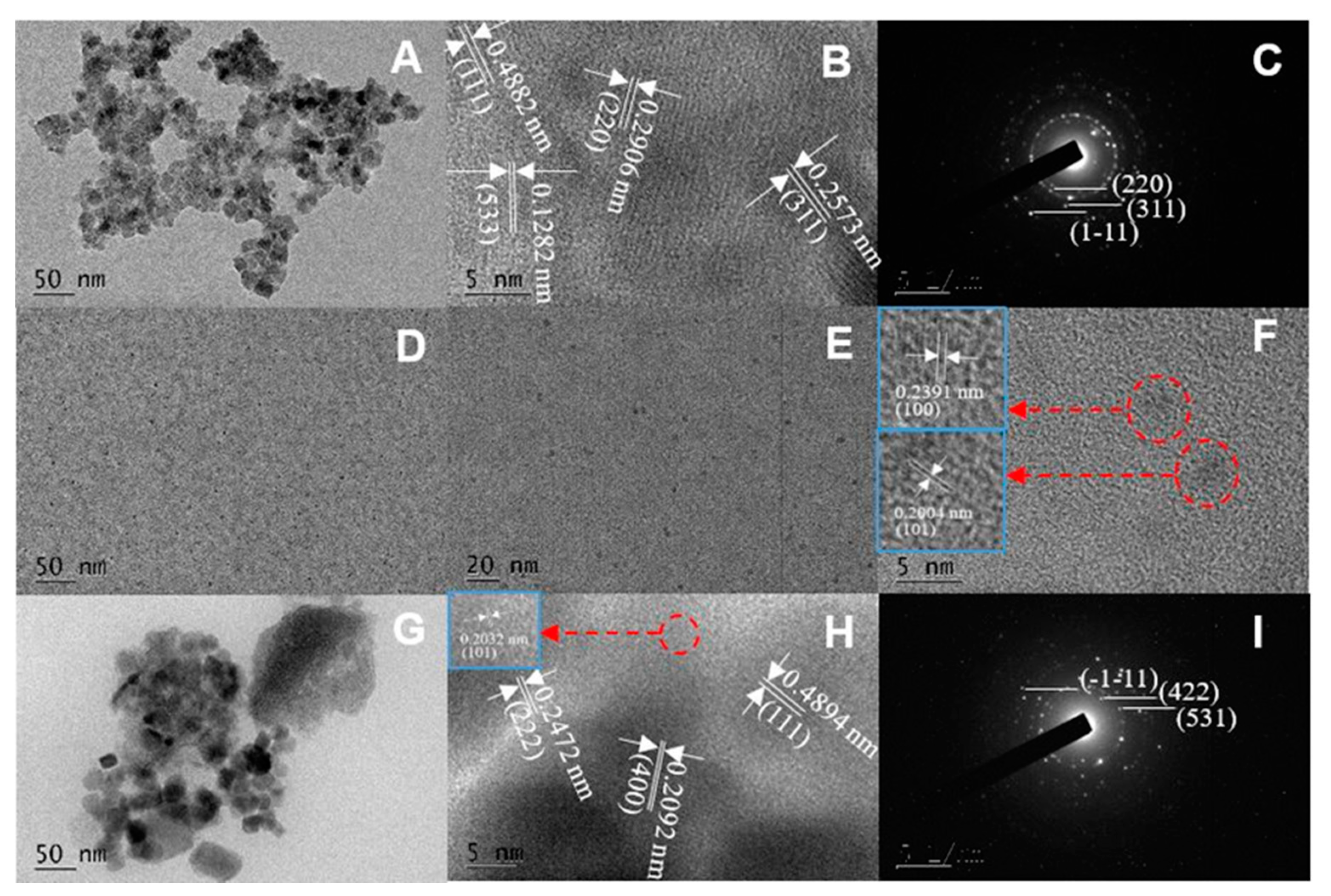
3.1. Morphology Analysis
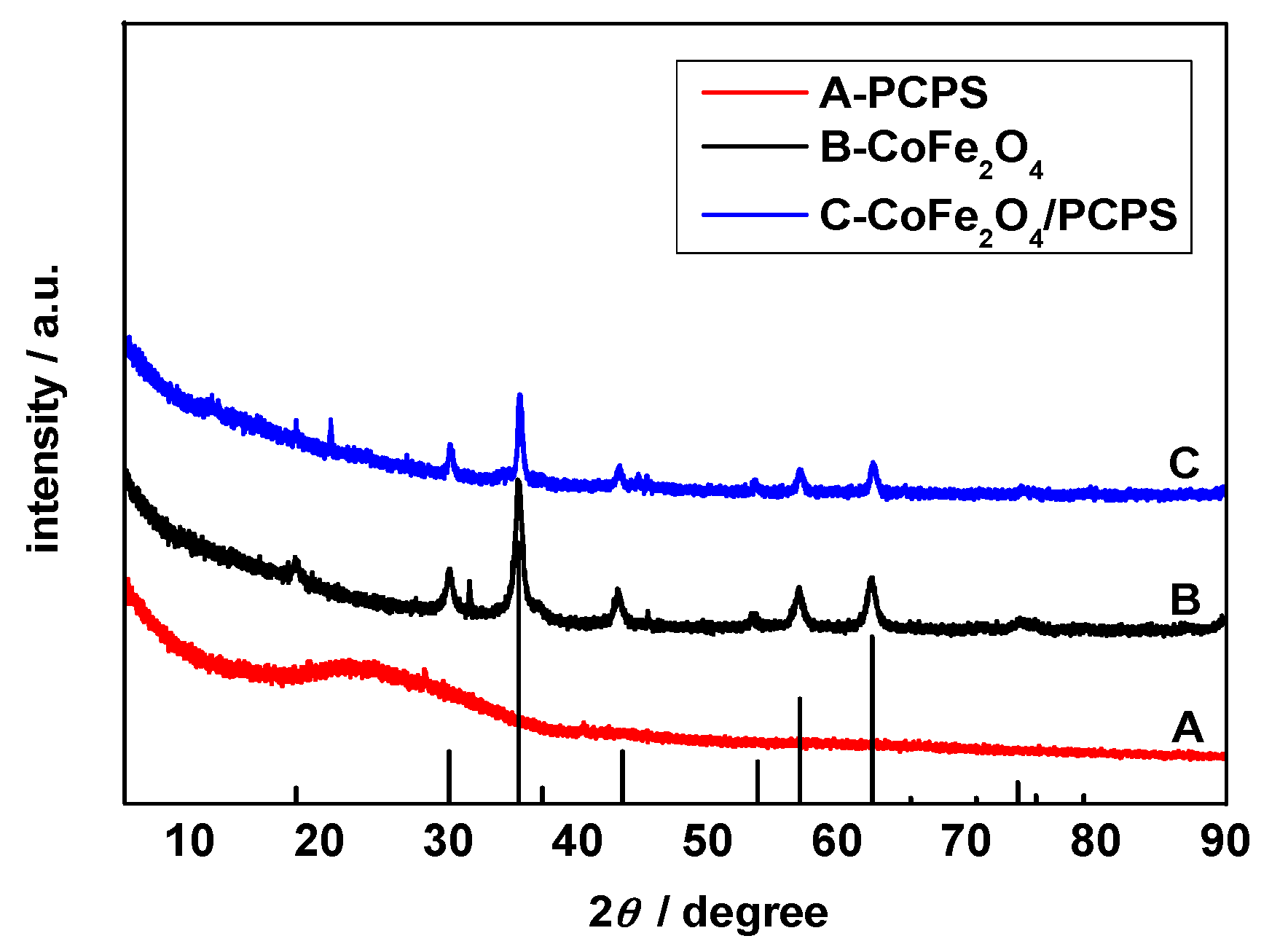
3.2. XRD Analysis
3.3. FTIR Analysis
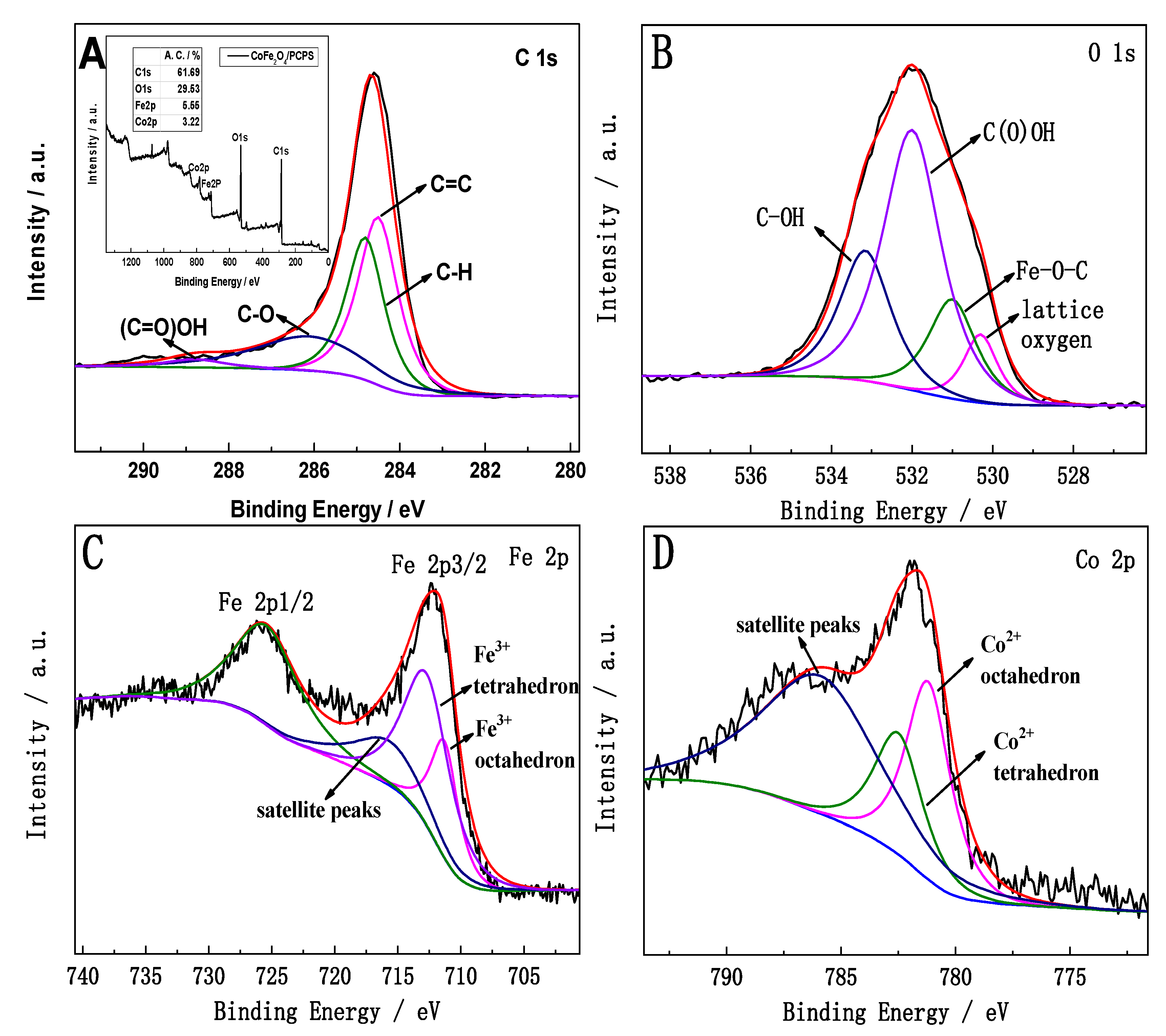
3.4. XPS Analysis
3.5. N2 Adsorption-Desorption Analysis
3.6. MSPE Condition Optimizing
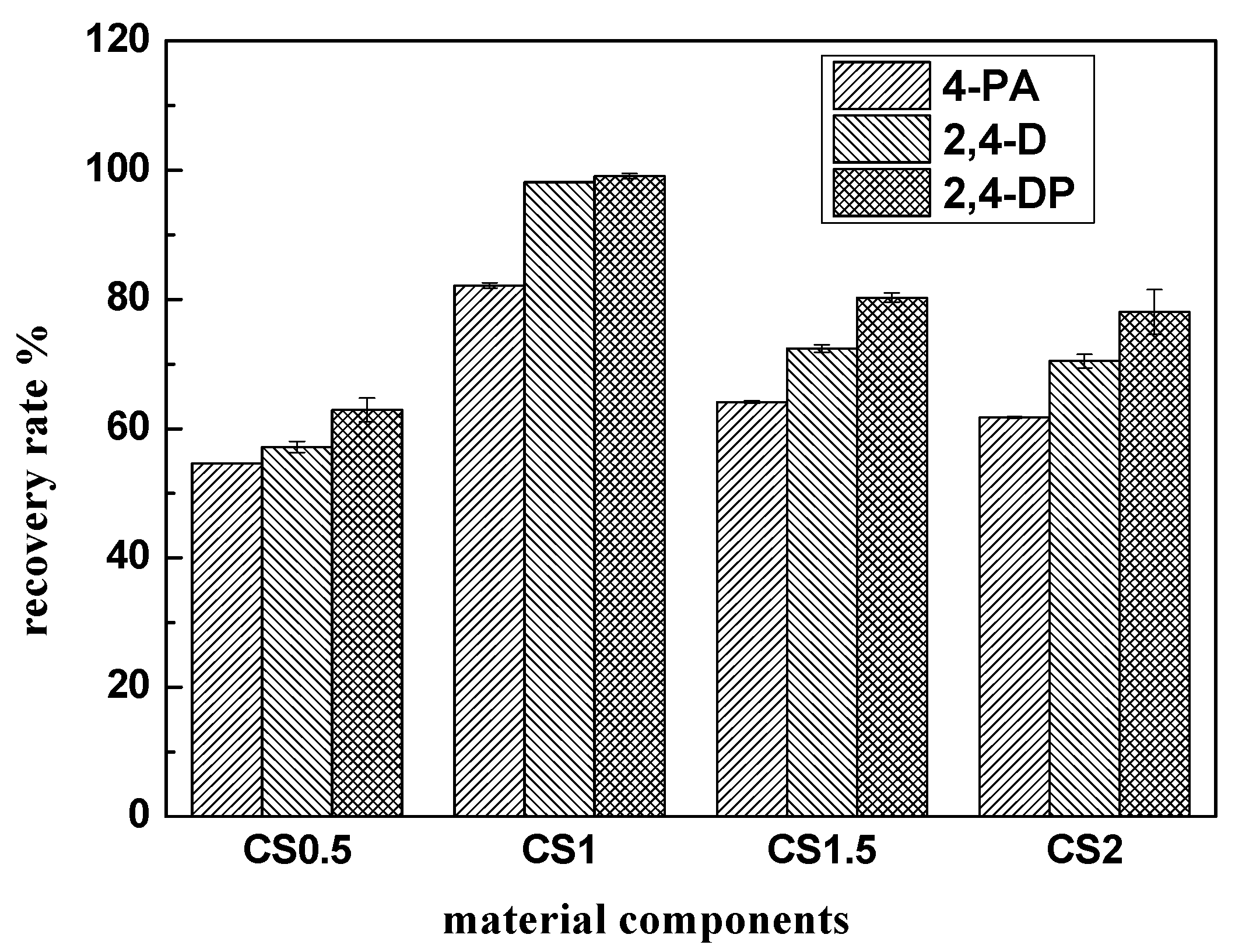
3.6.1. Material Components
3.6.2. Conditional Experiment
3.7. Analysis of Environmental Water Samples
4. Conclusions
- (1)
- The characterizations such as TEM, XRD, FTIR, and BET demonstrated that the CoFe2O4/PCPS composite was successfully synthesized. The material maintained the original structure of PCPS and had a large specific surface area and pore volume.
- (2)
- In the optimization experiment of magnetic solid phase extraction conditions, phenoxy carboxylic herbicides were extracted using 40 mg of the composite material, and adsorption occurred in water samples with a pH of 8.50 for 10 min. The best magnetic solid phase extraction effect was obtained with methanol as the eluent. The recovery rates of all the analyte were greater 82.17%. The material still maintains high performance after ten cycles.
- (3)
- When the established method was applied to environmental water samples, the recoveries were 75.22–94.66%. The RSD was 0.08–3.70%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhu, J.; Si, L.; Du, Q.; Li, H.; Bi, W.; da Young Chen, D. High throughput screening of phenoxy carboxylic acids with dispersive solid phase extraction followed by direct analysis in real time mass spectrometry. Anal. Chim. Acta 2017, 996, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, C.; Zhao, J.; Zhao, P.; Zhao, L. A novel magnetic multi-walled carbon nanotube-based magnetic solid-phase extraction combined with dispersive liquid-liquid microextraction method for the determination of four phenoxy carboxylic acid herbicides in food crops by using ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2018, 10, 3263–3272. [Google Scholar]
- Malagoli, C.; Costanzini, S.; Heck, J.E.; Malavolti, M.; De Girolamo, G.; Oleari, P.; Palazzi, G.; Teggi, S.; Vinceti, M. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int. J. Hyg. Environ. Health 2016, 219, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Arcaute, C.R.; Soloneski, S.; Larramendy, M.L. Toxic and genotoxic effects of the 2,4-dichlorophenoxyacetic acid (2,4-D)-based herbicide on the Neotropical fish Cnesterodon decemmaculatus. Ecotoxicol. Environ. Saf. 2016, 128, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.; Chen, Y.; Xu, G.; Deng, Z.; Du, H.; Wang, Y.; Zhang, S.; Zhao, W. Simultaneous determination of six plant growth regulators in fruits using high performance liquid chromatography based on solid-phase extraction and cleanup with a novel mixedmode functionalized calixarene sorbent. Anal. Methods 2015, 7, 6365–6371. [Google Scholar] [CrossRef]
- Manna, S.; Saha, P.; Roy, D.; Sen, R.; Adhikari, B. Removal of 2,4-dichlorophenoxyacetic acid from aqueous medium using modified jute. J. Taiwan Inst. Chem. Eng. 2016, 67, 292–299. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, X.; Wang, Z.; Gan, R. Progress in application of magnetic solid phase extraction in detection and analysis. Food Sci. 2011, 32, 317–320. (In Chinese) [Google Scholar]
- Xu, Y.; Jin, J.; Li, X.; Han, Y.; Meng, H.; Wu, J.; Zhang, X. Rapid magnetic solid-phase extraction of Congo Red and Basic Red 2 from aqueous solution by ZIF-8@CoFe2O4 hybrid composites. J. Sep. Sci. 2016, 39, 3647–3654. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, Z.; He, C.; Lyu, W.; Yan, W.; Yang, L. Enhanced antimonate (Sb(V)) removal from aqueous solution by La-doped magnetic biochars. Chem. Eng. J. 2018, 354, 623–632. [Google Scholar] [CrossRef]
- Köseoğlu, Y. Rapid Synthesis of Nanocrystalline NiFe2O4 and CoFe2O4 Powders by a Microwave-Assisted Combustion Method. J. Supercond. Nov. Magn. 2013, 26, 1391–1396. [Google Scholar] [CrossRef]
- Ji, G.B.; Tang, S.L.; Ren, S.K. Simplified synthesis of single-crystalline magnetic CoFe2O4 nanorods by a surfactant-assisted hydrothermal process. J. Cryst. Growth 2004, 270, 156–161. [Google Scholar] [CrossRef]
- Martindale, B.C.; Hutton, G.A.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Hu, L.L.; Sun, Y. One-step synthesis of chiral carbon quantum dots and their enantioselective recognition. RSC Adv. 2016, 6, 59956–59960. [Google Scholar] [CrossRef]
- Kauser, A. Polymer/carbon-based quantum dot nanocomposite: Forthcoming materials for technical application. J. Macromlecular Sci. Part A 2019, 56, 341–356. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.; Wang, H.; Li, Y.; Liu, X.; Zhou, Y. The preparation of porous carbon materials derived from bio-protic ionic liquid with application in flexible solid-state supercapacitors. J. Hazard. Mater. 2021, 402, 124023. [Google Scholar] [CrossRef]
- De Andres, P.L.; Ramírez, R.; Vergés, J.A. Strong covalent bonding between two graphene layers. Phys. Rev. B 2008, 77, 0745403. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.X.; Song, B.; Peng, P. Preparation of three-dimensional honeycomb carbon materials and their adsorption of Cr(VI). Chem. Eng. J. 2019, 367, 9–16. [Google Scholar] [CrossRef]
- Yuan, D.S.; Chen, J.X.; Zeng, J.H.; Tan, S.X. Preparation of monodisperse carbon nanospheres for electrochemical capacitors. Electrochem. Commun. 2008, 10, 1067–1070. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S.H.; Xu, R.; Wang, J.W. Boosting Potassium-Ion Battery Performance by Encapsulating Red Phosphorus in Free-Standing Nitrogen-Doped Porous Hollow Carbon Nanofibers. Nano Lett. 2019, 19, 1351–1358. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Zhou, L.; Bao, C.; Zhu, H.; Zhang, Y. Synthesis and application of rGO/CoFe2O4 composite for catalytic degradation of methylene blue on heterogeneous Fenton-like oxidation. J. Taiwan Inst. Chem. Eng. 2016, 67, 484–494. [Google Scholar] [CrossRef]
- Limaye, M.V.; Singh, S.B.; Das, R.; Poddar, P.; Abyaneh, M.K.; Kulkarni, S.K. Magnetic studies of SiO2 coated CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2017, 441, 683–690. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Li, F.; Khaimanov, S.; Tsidaeva, N.; Lahoubi, M. PEG-assisted hydrothermal synthesis of CoFe2O4 nanoparticles with enhanced selective adsorption properties for different dyes. Appl. Surf. Sci. 2016, 389, 1003–1011. [Google Scholar] [CrossRef]
- Yang, J.; Qiao, J.Q.; Cui, S.H.; Li, J.Y.; Zhu, J.J.; Yin, H.X.; Zhan, C.Y.; Lian, H.Z. Magnetic solid-phase extraction of brominated flame retardants from environmental waters with graphene-doped Fe3O4 nanocomposites. J. Sep. Sci. 2015, 38, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Bar, N.; Basu, R.K.; Das, S.K. Comparative study of adsorptive removal of Cr(VI) ion from aqueous solution in fixed bed column by peanut shell and almond shell using empirical models and ANN. Environ. Sci. Pollut. Res. 2017, 24, 10604–10620. [Google Scholar] [CrossRef]
- Geng, X.; Lv, S.; Yang, J.; Cui, S.; Zhao, Z. Carboxyl-functionalized biochar derived from walnut shells with enhanced aqueous adsorption of sulfonamide antibiotics. J. Environ. Manag. 2021, 280, 111749. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zhang, Y.D.; Jiang, Z.W.; Wang, D.M.; Huang, C.Z.; Li, Y.F. Fe3O4 and metal-organic framework MIL-101(Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Wang, W.P.; Yang, H.; Xian, T.; Jiang, J.L. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar] [CrossRef] [Green Version]
- Valimaña-Traverso, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; García, M.Á.; Sierra, I.; Marina, M.L. Cationic amine-bridged periodic mesoporous organosilica materialsfor off-line solid-phase extraction of phenoxy acid herbicides fromwater samples prior to their simultaneous enantiomeric determination by capillary electrophoresis. J. Chromatogr. A 2018, 1566, 146–157. [Google Scholar] [CrossRef]
- Thorstensen, C.W.; Lode, O.; Christiansen, A.L. Development of a solid-phase extraction method for phenoxy acids and bentazone in water and comparison to a liquid-liquid extraction method. J. Agric. Food Chem. 2000, 48, 5829–5833. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Contini, S. Occurrence of polar organic contaminants in the dissolved water phase of the Danube River and its major tributaries using SPE-LC-MS2 analysis. Water Res. 2010, 44, 2325–2335. [Google Scholar] [CrossRef]
- Dong, S.Y.; Huang, G.Q.; Su, M.L.; Huang, T. Environmentally friendly method: Development and application to carbon aerogel as sorbent for solid-phase extraction. ACS Appl. Mater. Interfaces 2015, 7, 22256–22263. [Google Scholar] [CrossRef] [PubMed]



| Item | Parameter |
|---|---|
| Instruments | Waters 2489 |
| Detector | An UV/Visible detector |
| Chromatographic column | A Lichrospher C18 column (150 mm × 4.6 mm, 5 μm) |
| Detection wavelength | 230 nm |
| Mobile phase (volume ratio) | Methanol to water to acetic acid = 50:50:0.2 |
| Flow rate | 1.0 mL·min−1 |
| Injection volume | 20.0 µL |
| Column temperature | 30 °C |
| Analyte | Standard Curve | Linear Range (μg·L−1) | Correlation Coefficient (r2) | LOD (μg·L−1) | LOQ (μg·L−1) |
|---|---|---|---|---|---|
| 4-PA | y = 60.4608x + 240.4391 | 1.0–1000 | 0.9996 | 0.30 | 0.98 |
| 2,4-D | y = 37.6800x + 984.8045 | 2.0–1000 | 0.9978 | 0.58 | 1.94 |
| 2,4-DP | y = 35.9193x − 832.7198 | 2.0–1000 | 0.9995 | 0.59 | 1.96 |
| Water Samples | Standard Addition Concentration (mg·L−1) | Detection of Concentration (mg·L−1) | Recovery Rate b/% (RSD/%) | ||||
|---|---|---|---|---|---|---|---|
| 4-PA | 2,4-D | 2,4-DP | 4-PA | 2,4-D | 2,4-DP | ||
| Yellow River | 0 | —— | —— | —— | |||
| 0.02 | 0.0164 | 0.0175 | 0.0189 | 81.75 (1.66) | 87.45 (2.06) | 94.66 (3.15) | |
| 0.04 | 0.0333 | 0.0346 | 0.0355 | 83.27 (2.39) | 86.41 (1.52) | 88.63 (1.99) | |
| Xuanwu Lake | 0 | —— | —— | —— | |||
| 0.02 | 0.0150 | 0.0159 | 0.0177 | 75.22 (1.25) | 79.66 (3.35) | 88.29 (3.70) | |
| 0.04 | 0.0335 | 0.0333 | 0.0347 | 83.82 (0.85) | 83.13 (3.15) | 86.70 (2.97) | |
| Yangtze River | 0 | —— | —— | —— | |||
| 0.02 | 0.0161 | 0.0163 | 0.0163 | 80.27 (0.61) | 81.65 (1.89) | 81.50 (1.25) | |
| 0.04 | 0.0336 | 0.0353 | 0.0370 | 84.04 (2.27) | 88.15 (1.62) | 92.61 (0.08) | |
| Snow melt | 0 | —— | —— | —— | |||
| 0.02 | 0.0155 | 0.0161 | 0.0162 | 77.30 (1.48) | 80.36 (1.38) | 81.08 (0.56) | |
| 0.04 | 0.0328 | 0.0339 | 0.0358 | 81.88 (2.62) | 84.86 (1.73) | 89.54 (1.46) | |
| Method | Analytical Sample | Sample Volume /mL | Pre-Conditioning Time/min | LODs (μg·L−1) | RSD/% | Recovery Rate/% | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| 4-PA | 2,4-D | 2,4-DP | |||||||
| DSPE a | tap water and lake water | 50 | 13 | 0.2–0.3 | 1.4–8.6 | 83.7–114.4 | [1] | ||
| SPE | river water and waste water | 750 | 11 | 0.3–6.3 | 1.1–11.4 | 95–104 | [28] | ||
| SPE | distilled water, stream and well water | 200 | 100 | 0.02 | 0.2–4.0 | 80.0–110.0 | [29] | ||
| SPE | river water | 400 | 90 | 0.01 | 50.0–80.0 | [30] | |||
| μ-SPE b-MSPE | Reservoir raw water | 10 | 45 | 0.2 | 1.7–5.1 | 89.0–103.0 | [31] | ||
| MSPE | river water, lake water and snow water | 100 | 10 | 0.3 | 0.58 | 0.59 | 0.08–3.70 | 75.22–94.66 | this text |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, D.; Huang, Z.; Du, B. Synthesis of CoFe2O4/Peanut Shell Powder Composites and the Associated Magnetic Solid Phase Extraction of Phenoxy Carboxylic Acid Herbicides in Water. Int. J. Environ. Res. Public Health 2022, 19, 8450. https://doi.org/10.3390/ijerph19148450
Ji D, Huang Z, Du B. Synthesis of CoFe2O4/Peanut Shell Powder Composites and the Associated Magnetic Solid Phase Extraction of Phenoxy Carboxylic Acid Herbicides in Water. International Journal of Environmental Research and Public Health. 2022; 19(14):8450. https://doi.org/10.3390/ijerph19148450
Chicago/Turabian StyleJi, Dongliang, Zhaoqin Huang, and Buyun Du. 2022. "Synthesis of CoFe2O4/Peanut Shell Powder Composites and the Associated Magnetic Solid Phase Extraction of Phenoxy Carboxylic Acid Herbicides in Water" International Journal of Environmental Research and Public Health 19, no. 14: 8450. https://doi.org/10.3390/ijerph19148450






