Occupational Exposure among Electronic Repair Workers in Ghana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site
2.2. Design
2.3. Sample Collection
2.4. Air Filter Dissolution and Measurement of Elements
2.5. Statistics
3. Results
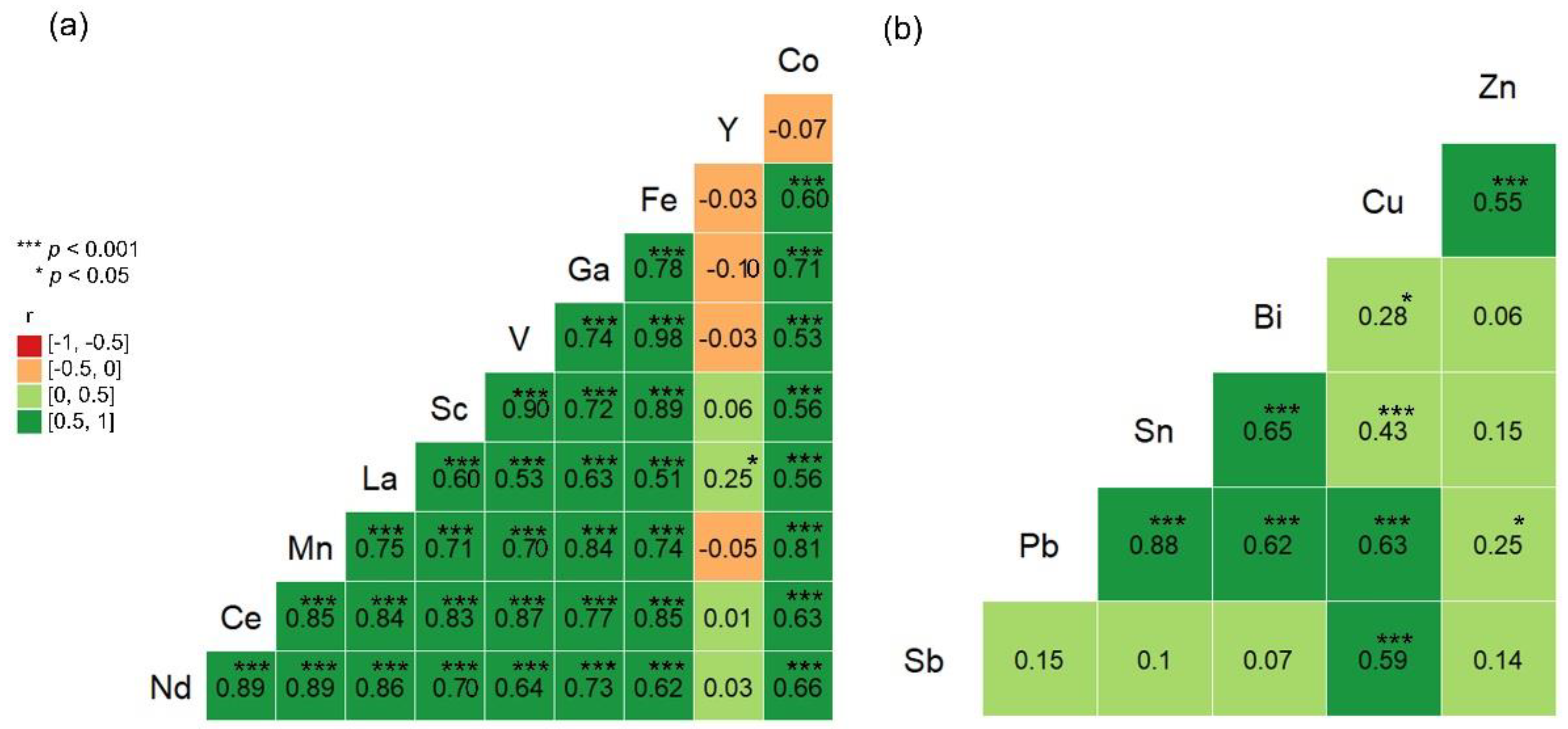
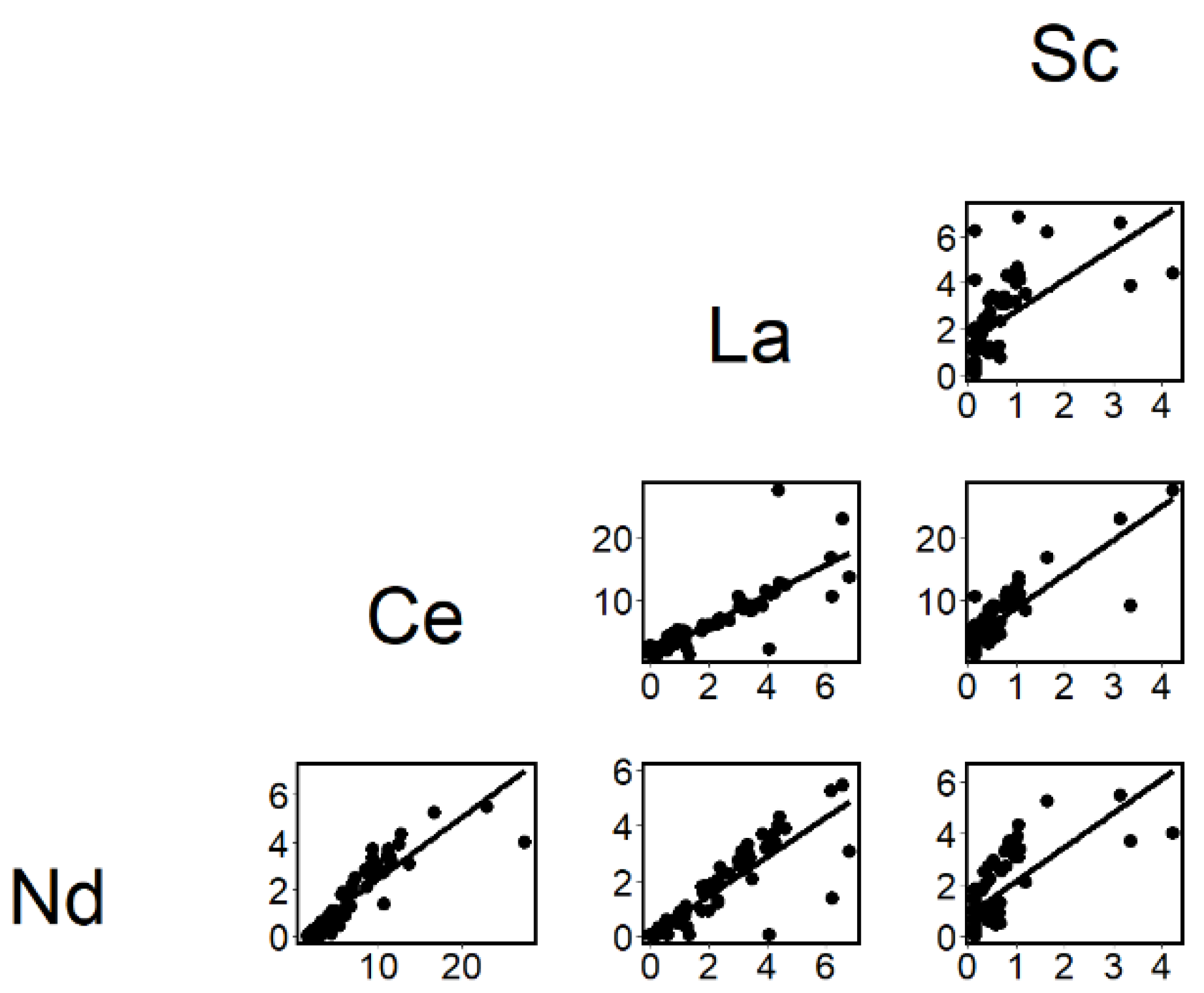
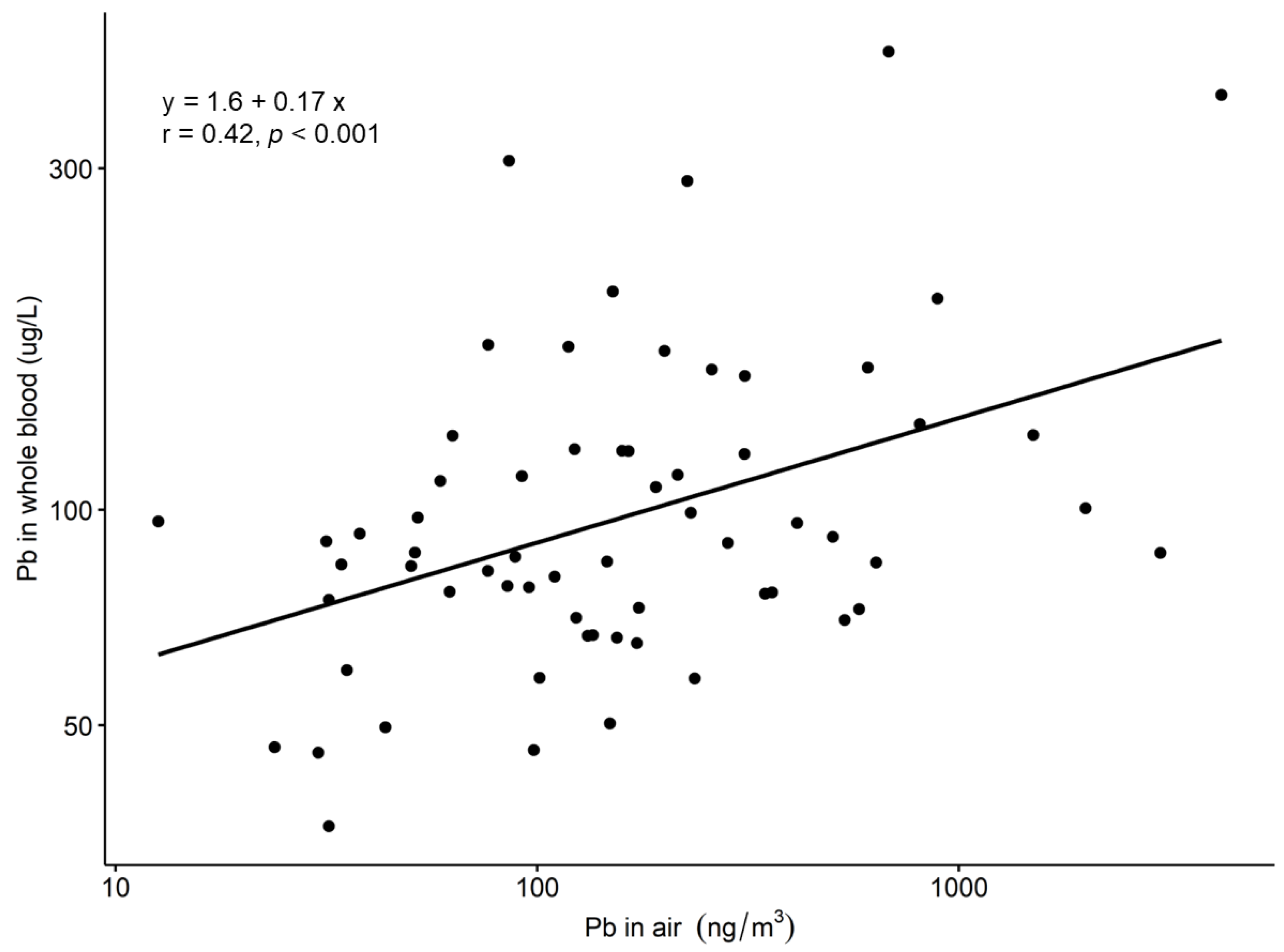
4. Discussion
4.1. Workroom Air Concentrations
4.2. Ranked Principal Component Analysis
4.3. Associations between Workroom Air and Biological Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahan, M.; Kucuker, M.A.; Demirel, B.; Kuchta, K.; Hursthouse, A. Determination of Metal Content of Waste Mobile Phones and Estimation of Their Recovery Potential in Turkey. Int. J. Environ. Res. Public Health 2019, 16, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, A.K.; Li, J.; Koh, L.; Ogunseitan, O.A. Circular economy and electronic waste. Nat. Electron. 2019, 2, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Amoyaw-Osei, Y.; Agyekum, O.O.; Pwamang, J.; Thiébaud, E.; Fasko, R.; Schluep, M. Ghana e-waste country assessment. SBC E-Waste Afr. Proj. 2011. Available online: https://www.basel.int/Portals/4/Basel%20Convention/docs/eWaste/E-wasteAssessmentGhana.pdf (accessed on 24 November 2021).
- Zlamparet, G.L.; Ijomah, W.; Miao, Y.; Awasthi, A.K.; Zeng, X.; Li, J. Remanufacturing strategies: A solution for WEEE problem. J. Clean. Prod. 2017, 149, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Okeme, J.O.; Arrandale, V.H. Electronic Waste Recycling: Occupational Exposures and Work-Related Health Effects. Curr. Environ. Health Rep. 2019, 6, 256–268. [Google Scholar] [CrossRef]
- Lawal, M.; Uzairu, A.; Sallau, M.; Salihu, A.; Tukur, M. Accumulation of Trace Metals in Artisans in Nigeria; a pilot case study of Electronic Repair Technicians, Auto Mechanics and Painters. Int. J. Sci. Glob. Sustain. 2017, 4, 7. [Google Scholar]
- Dartey, E.; Berlinger, B.; Weinbruch, S.; Thomassen, Y.; Odland, J.; Brox, J.; Nartey, V.K.; Yeboah, F.A.; Ellingsen, D.G. Essential and non-essential trace elements among working populations in Ghana. J. Trace Elem. Med. Biol. 2017, 44, 279–287. [Google Scholar] [CrossRef]
- Thongsringklee, M.; Robson, M.G.; Siriwong, W. Health effects of low level exposure to lead among communication radio repair workers at Samutsakhon province, Thailand. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 344–351. [Google Scholar] [CrossRef]
- Arain, A.L.; Neitzel, R.L. A Review of Biomarkers Used for Assessing Human Exposure to Metals from E-Waste. Int. J. Environ. Res. Public Health. 2019, 16, 1802. [Google Scholar] [CrossRef] [Green Version]
- Srigboh, R.K.; Basu, N.; Stephens, J.; Asampong, E.; Perkins, M.; Neitzel, R.L.; Fobil, J. Multiple elemental exposures amongst workers at the Agbogbloshie electronic waste (e-waste) site in Ghana. Chemosphere 2016, 164, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amankwaa, E.F.; Adovor Tsikudo, K.A.; Bowman, J.A. ‘Away’ is a place: The impact of electronic waste recycling on blood lead levels in Ghana. Sci. Total Environ. 2017, 601, 1566–1574. [Google Scholar] [CrossRef]
- Julander, A.; Lundgren, L.; Skare, L.; Grandér, M.; Palm, B.; Vahter, M.; Lidén, C. Formal recycling of e-waste leads to increased exposure to toxic metals: An occupational exposure study from Sweden. Environ. Int. 2014, 73, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerding, J.; Peters, C.; Wegscheider, W.; Stranzinger, J.; Lessmann, F.; Pitzke, K.; Harth, V.; Eickmann, U.; Nienhaus, A. Metal exposure of workers during recycling of electronic waste: A cross-sectional study in sheltered workshops in Germany. Int. Arch. Occup. Environ. 2021, 94, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, E. History of Rare Earth Applications, Rare Earth Market Today. In Industrial Applications of Rare Earth Elements; ACS Symposium Series: Washington, DC, USA, 1981; Volume 164, pp. 3–17. [Google Scholar]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Env. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef] [Green Version]
- Anshu, P.; Hait, S. Comprehensive characterization of printed circuit boards of various end-of-life electrical and electronic equipment for beneficiation investigation. Waste Manag. 2018, 75, 103–123. [Google Scholar] [CrossRef]
- Zhang, H.X.; Buddhudu, S.; Kam, C.H.; Zhou, Y.; Lam, Y.L.; Wong, K.S.; Ooi, B.S.; Ng, S.L.; Que, W.X. Luminescence of Eu3+ and Tb3+ doped Zn2SiO4 nanometer powder phosphors. Mater. Chem. Phys. 2001, 68, 31–35. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Vegliò, F. Design and construction of an industrial mobile plant for WEEE treatment: Investigation on the treatment of fluorescent powders and economic evaluation compared to other e-wastes. J. Taiwan Inst. Chem. Eng. 2017, 80, 769–778. [Google Scholar] [CrossRef]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 2015, 142, 215–220. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kim, H.-O.; Rim, K.-T. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf. Health Work 2019, 10, 409–419. [Google Scholar] [CrossRef]
- Sabbioni, E.; Pietra, R.; Gaglione, P.; Vocaturo, G.; Colombo, F.; Zanoni, M.; Rodi, F. Long-term occupational risk of rare-earth pneumoconiosis A case report as investigated by neutron activation analysis. Sci. Total Environ. 1982, 26, 19–32. [Google Scholar] [CrossRef]
- Waring, P.M.; Watling, R.J. Rare earth deposits in a deceased movie projectionist: A new case of rare earth pneumoconiosis? Med. J. Aust. 1990, 153, 726–730. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Ghio, A.J.; Sheehan, C.E.; Bernhardt, P.F.; Roggli, V.L. Rare earth (cerium oxide) pneumoconiosis: Analytical scanning electron microscopy and literature review. Mod. Pathol. 1995, 8, 859–865. [Google Scholar] [PubMed]
- Pairon, J.-C.; Roos, F.; Sébastien, P.; Chamak, B.; Abd-Alsamad, I.; Bernaudin, J.-F.; Bignon, J.; Brochard, P. Biopersistence of cerium in the human respiratory tract and ultrastructural findings. Am. J. Ind. Med. 1995, 27, 349–358. [Google Scholar] [CrossRef]
- Ghana Districts. A Repository of All Districts in the Republic of Ghana. Available online: https://www.ghanadistricts.com/ (accessed on 6 December 2021).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, version 1.9.12; CRAN; 2019. Available online: https://rdrr.io/cran/psych/ (accessed on 12 June 2022).
- Helsel, D.R. Statistics for Censored Environmental Data Using Minitab and R, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- RStudio. RStudio: Integrated Development for R. RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, version 3.3.2.; CRAN; Springer: New York, NY, USA, 2016. [Google Scholar]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’; CRAN. 2021. Available online: https://ggobi.github.io/ggally/ (accessed on 12 June 2022).
- Kassambara, A. ‘ggplot2’ Based Publication Ready Plots, version 0.4.0.999; CRAN; 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 12 June 2022).
- United Stated Department of Labor. OSHA Annotated Table Z-1. 2021. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 5 July 2022).
- Kerger, B.D.; Loccisano, A.E.; Gerads, R.; Glassman, M.J. Small chamber study of lead exposures from manual soldering of microelectronics. Hum. Ecol. Risk Assess. 2020, 27, 451–464. [Google Scholar] [CrossRef]
- Kulkarni, P.; Chellam, S.; Flanagan, J.B.; Jayanty, R.K.M. Microwave digestion—ICP-MS for elemental analysis in ambient airborne fine particulate matter: Rare earth elements and validation using a filter borne fine particle certified reference material. Anal. Chim. Acta 2007, 599, 170–176. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, J.-H.; Lim, J.-M. Toxic Trace and Earth Crustal Elements of Ambient PM2.5 Using CCT-ICP-MS in an Urban Area of Korea. Environ. Eng. Res. 2013, 18, 3–8. [Google Scholar] [CrossRef]
- Malandrino, M.; Di Martino, M.; Giacomino, A.; Geobaldo, F.; Berto, S.; Grosa, M.M.; Abollino, O. Temporal trends of elements in Turin (Italy) atmospheric particulate matter from 1976 to 2001. Chemosphere 2013, 90, 2578–2588. [Google Scholar] [CrossRef]
- Samek, L.; Stegowski, Z.; Furman, L.; Fiedor, J. Chemical content and estimated sources of fine fraction of particulate matter collected in Krakow. Air. Qual. Atmos. Health 2017, 10, 47–52. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Wang, Z.; Guicherit, R. Rare Earth Elements and Other Metals in Atmospheric Particulate Matter in the Western Part of The Netherlands. Water Air Soil Pollut. 2000, 121, 109–118. [Google Scholar] [CrossRef]
- Meshram, P.; Somani, H.; Pandey, B.D.; Mankhand, T.R.; Deveci, H.; Abhilash. Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J. Clean. Prod. 2017, 157, 322–332. [Google Scholar] [CrossRef]
- Lixandru, A.; Venkatesan, P.; Jönsson, C.; Poenaru, I.; Hall, B.; Yang, Y.; Walton, A.; Güth, K.; Gauß, R.; Gutfleisch, O. Identification and recovery of rare-earth permanent magnets from waste electrical and electronic equipment. Waste Manag. 2017, 68, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Graedel, T.E. Uncovering the end uses of the rare earth elements. Sci. Total Environ. 2013, 461, 781–784. [Google Scholar] [CrossRef]
- Szałatkiewicz, J. Metals content in printed circuit board waste. Pol. J. Environ. Stud. 2014, 23, 2365–2369. [Google Scholar]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical Recovery of Metals from Large Printed Circuit Board Pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, A.E.; Olasehinde, F.E.; Adebayo, O.A.; Ajayi, O.J.; Ghosh, M.K.; Basu, S. Extraction of Copper and Zinc from Waste Printed Circuit Boards. Recycling 2019, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Mudge, S.M.; Pfaffhuber, K.A.; Fobil, J.N.; Bouman, E.A.; Uggerud, H.T.; Thorne, R.J. Using elemental analyses and multivariate statistics to identify the off-site dispersion from informal e-waste processing. Environ. Sci. Process. Impacts 2019, 21, 2042–2057. [Google Scholar] [CrossRef]
- Skerfving, S.; Bergdahl, I.A. Lead. In Handbook on the Toxicology of Metals; Gunnar, F., Nordberg Bruce, A., Fowler Nordberg, M., Eds.; Elsevier: Burlington, MA, USA, 2007. [Google Scholar]
- Ostrakhovitch, E.A. Chapter 56—Tin. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1241–1285. [Google Scholar]
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Chapter 32—Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 667–716. [Google Scholar]
- Niemeier, R.T.; Maier, A.; Reichard, J.F. Rapid Review of Dermal Penetration and Absorption of Inorganic Lead Compounds for Occupational Risk Assessment. Ann. Work Expo. Health 2021, 66, 291–311. [Google Scholar] [CrossRef]
- Mohammadyan, M.; Moosazadeh, M.; Borji, A.; Khanjani, N.; Rahimi Moghadam, S. Investigation of occupational exposure to lead and its relation with blood lead levels in electrical solderers. Environ. Monit. Assess. 2019, 191, 126. [Google Scholar] [CrossRef] [PubMed]
- Arasaratnam, M.; Hashim, Z.; Shamsudin, S.B. Occupational lead exposure of soldering workers in an electronic factory. J. Occup. Saf. Health 2004, 95, 49. [Google Scholar]
- Shamsul, B.; Zailina, H.; Jamal, H.; Ruzita, M.; Arumugam, L.; Daud, A.; Anthony, L.; Rozlan, I.; Asmah, Z.; Roslinah, A. Occupational lead exposure on electronic soldering workers in Malaysia. In Proceedings of the 1st Asia-PAcific Conference on Public Health, Kuala Lumpur, Malaysia, 30 September–3 October 2004. [Google Scholar]
- Pengelly, I.; Groves, J.; Simpson, A.; Northage, C. Workplace Exposure to Rosin-based Solder Flux Fume During Hand Soldering. Ann. Occup. Hyg. 1998, 42, 295–302. [Google Scholar] [CrossRef]
- Smith, P.A.; Son, P.S.; Callaghan, P.M.; Jederberg, W.W.; Kuhlmann, K.; Still, K.R. Sampling and analysis of airborne resin acids and solvent-soluble material derived from heated colophony (rosin) flux: A method to quantify exposure to sensitizing compounds liberated during electronics soldering. Toxicology 1996, 111, 225–238. [Google Scholar] [CrossRef]
- Bierkens, J.; Smolders, R.; Van Holderbeke, M.; Cornelis, C. Predicting blood lead levels from current and past environmental data in Europe. Sci. Total Environ. 2011, 409, 5101–5110. [Google Scholar] [CrossRef]
- Petit, D.; Véron, A.; Flament, P.; Deboudt, K.; Poirier, A. Review of pollutant lead decline in urban air and human blood: A case study from northwestern Europe. C R Geosci. 2015, 347, 247–256. [Google Scholar] [CrossRef]
- Oh, S.-E.; Kim, G.B.; Hwang, S.H.; Ha, M.; Lee, K.-M. Longitudinal trends of blood lead levels before and after leaded gasoline regulation in Korea. Environ Health Toxicol. 2017, 32, e2017019. [Google Scholar] [CrossRef] [Green Version]
- Todd, D.; Todd, H. Outcome and Influence Evaluation of the UNEP Based Partnership for Clean Fuels and Vehicles (PCFV). 2017. Available online: https://www.unep.org/resources/other-evaluation-reportsdocuments/outcome-and-influence-evaluation-unep-based-partnership (accessed on 10 January 2022).
- Hackman, H.K.; Gati, L.; Gordon, A.; Arhin, R.E.; Ofori-Yeboah, B.; Crabbe, G. Possible Health Risk due to the Environmental Exposure of High Levels of Lead in Exhaust Soot of Automobiles in Parts of Accra, Ghana. J. Nat. Sci. Res. 2017, 7, 62–66. [Google Scholar]
- Antwi-Baffour, S.; Darko, E.; Adjei, D.; Kyeremeh, R. Effects of Blood Lead Levels on Haematological Indices of Fuel Station Attendants. ABIO 2015, 17, 230. [Google Scholar]
- Boateng, T.K.; Opoku, F.; Akoto, O. Heavy metal contamination assessment of groundwater quality: A case study of Oti landfill site, Kumasi. Appl. Water Sci. 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Kodom, K.; Preko, K.; Boamah, D. X-ray Fluorescence (XRF) Analysis of Soil Heavy Metal Pollution from an Industrial Area in Kumasi, Ghana. Soil Sediment. Contam. 2012, 21, 1006–1021. [Google Scholar] [CrossRef]
| Element | Median | GM ± GSD | Minimum | Maximum | LOD a | % < LOD |
|---|---|---|---|---|---|---|
| Ag | 1.0 | 1 ± 3 | 0.2 | 76 | 0.04 | 0 |
| As | 1.9 | 2 ± 2 | 0.3 | 14 | 0.2 | 0 |
| Bi | 0.4 | 0.3 ± 4 | <LOD | 29 | 0.05 | 13 |
| Cd | 0.3 | 0.4 ± 3 | <LOD | 13.4 | 0.02 | 36 |
| Ce | 5.4 | 5 ± 2 | 1.4 | 28 | 0.07 | 0 |
| Co | 1.4 | 1 ± 2 | <LOD | 4.8 | 0.2 | 3 |
| Cu | 78 | 81 ± 2 | <LOD | 583 | 31 | 9 |
| Fe b | 6.5 | 6.3 ± 0.001 | 1.9 | 41 | 0.3 | 0 |
| Ga | 3.1 | 3 ± 2 | 0.8 | 8.2 | 0.08 | 0 |
| Hg c | 0.2 | 0.2 ± 3 | <LOD | 1.4 | 0.1 | 30 |
| La | 1.8 | 1 ± 3 | <LOD | 6.8 | 0.05 | 3 |
| Mn | 94 | 90 ± 2 | 23 | 269 | 3.7 | 0 |
| Nd | 1.1 | 1 ± 5 | <LOD | 5.5 | 0.04 | 11 |
| Ni | <LOD | 27 ± 2 | <LOD | 272 | 27 | 55 |
| Pb | 147 | 157 ± 3 | 13 | 4.2 × 103 | 0.9 | 0 |
| Sb | 8.0 | 9 ± 3 | 1.0 | 764 | 0.1 | 0 |
| Sc | 0.4 | 0.4 ± 2 | <LOD | 4.2 | 0.3 | 41 |
| Sn | 107 | 116 ± 4 | 2.4 | 4.3 × 103 | 1.3 | 0 |
| Tl | 0.06 | 0.05 ± 2 | <LOD | 0.4 | 0.03 | 17 |
| V | 13 | 13 ± 2 | 3 | 92 | 0.3 | 0 |
| Y | 0.9 | 0.5 ± 7 | <LOD | 23 | 0.05 | 25 |
| Zn | 271 | 340 ± 2 | 67 | 4.3 × 103 | 23 | 0 |
| Rotated Component | Proportion Variance | Elements | Possible Work Sources |
|---|---|---|---|
| RC1 | 0.38 | Nd, Ce, Mn, La, Sc, V, Ga, Fe, Y, Co | Batteries, magnets, and steel |
| RC2 | 0.25 | Sb, Pb, Sn, Bi, Cu, Zn | Soldering, printed circuit boards |
| RC3 | 0.06 | Cd | |
| RC4 | 0.10 | Ni |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksen Hammer, S.; Dorn, S.L.; Dartey, E.; Berlinger, B.; Thomassen, Y.; Ellingsen, D.G. Occupational Exposure among Electronic Repair Workers in Ghana. Int. J. Environ. Res. Public Health 2022, 19, 8477. https://doi.org/10.3390/ijerph19148477
Eriksen Hammer S, Dorn SL, Dartey E, Berlinger B, Thomassen Y, Ellingsen DG. Occupational Exposure among Electronic Repair Workers in Ghana. International Journal of Environmental Research and Public Health. 2022; 19(14):8477. https://doi.org/10.3390/ijerph19148477
Chicago/Turabian StyleEriksen Hammer, Stine, Stephen L. Dorn, Emmanuel Dartey, Balázs Berlinger, Yngvar Thomassen, and Dag G. Ellingsen. 2022. "Occupational Exposure among Electronic Repair Workers in Ghana" International Journal of Environmental Research and Public Health 19, no. 14: 8477. https://doi.org/10.3390/ijerph19148477
APA StyleEriksen Hammer, S., Dorn, S. L., Dartey, E., Berlinger, B., Thomassen, Y., & Ellingsen, D. G. (2022). Occupational Exposure among Electronic Repair Workers in Ghana. International Journal of Environmental Research and Public Health, 19(14), 8477. https://doi.org/10.3390/ijerph19148477






