Spatial and Temporal Distribution Characteristics and Potential Risks of Sulfonamides in the Shaanxi Section of the Weihe River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Site Description
2.2. Methods
2.2.1. Reagents and Chemicals
2.2.2. Sample Collection and Pre-Treatment
2.2.3. LC–MS/MS Analysis and Quality Control
2.2.4. Ecological Risk Assessment
2.2.5. Health Risk Assessment
3. Results and Discussion
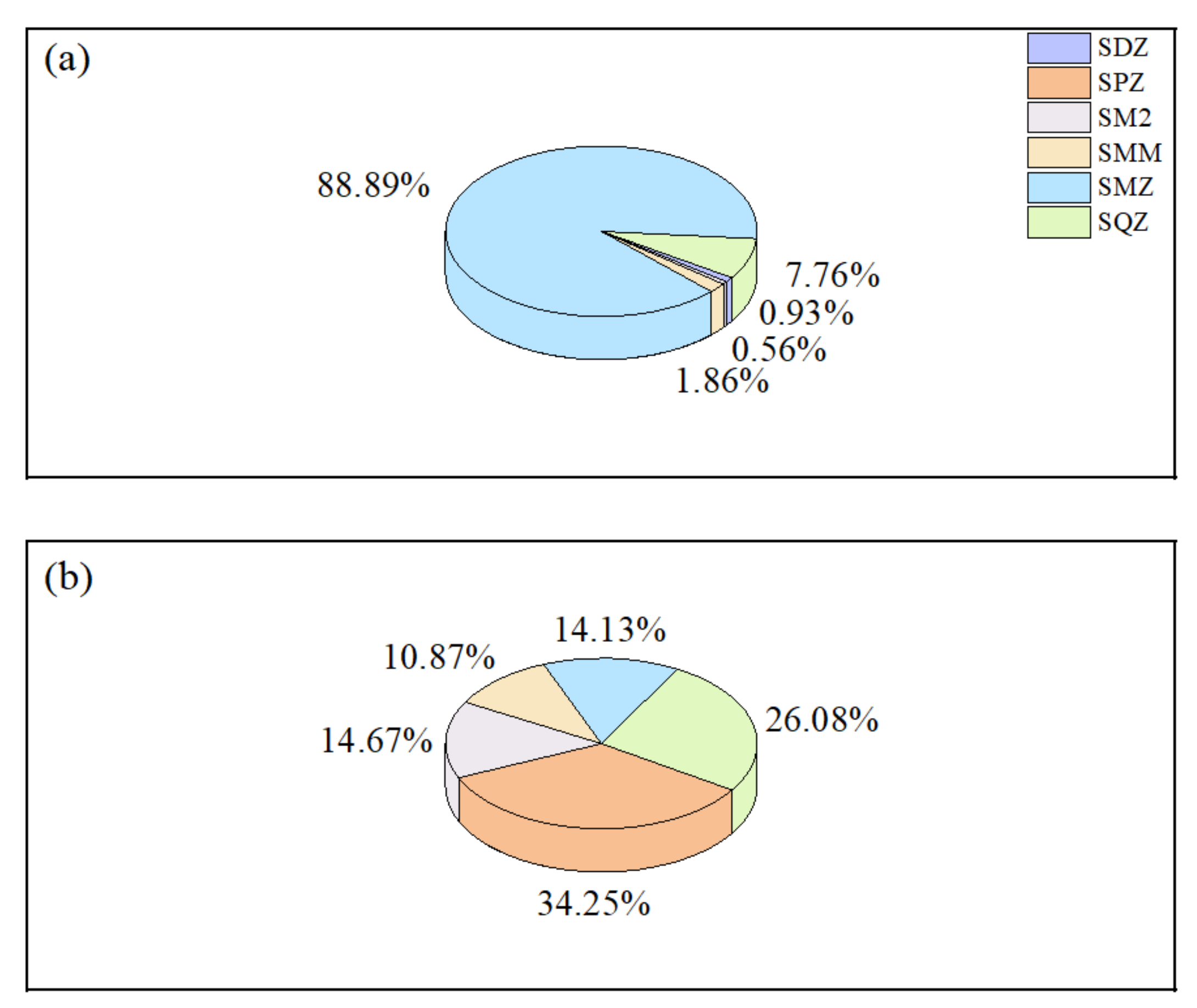
3.1. Concentrations of Sulfonamides in the Shaanxi Section of the Weihe River
3.2. Spatial and Temporal Distribution of Sulfonamides in the Shaanxi Section of the Weihe River
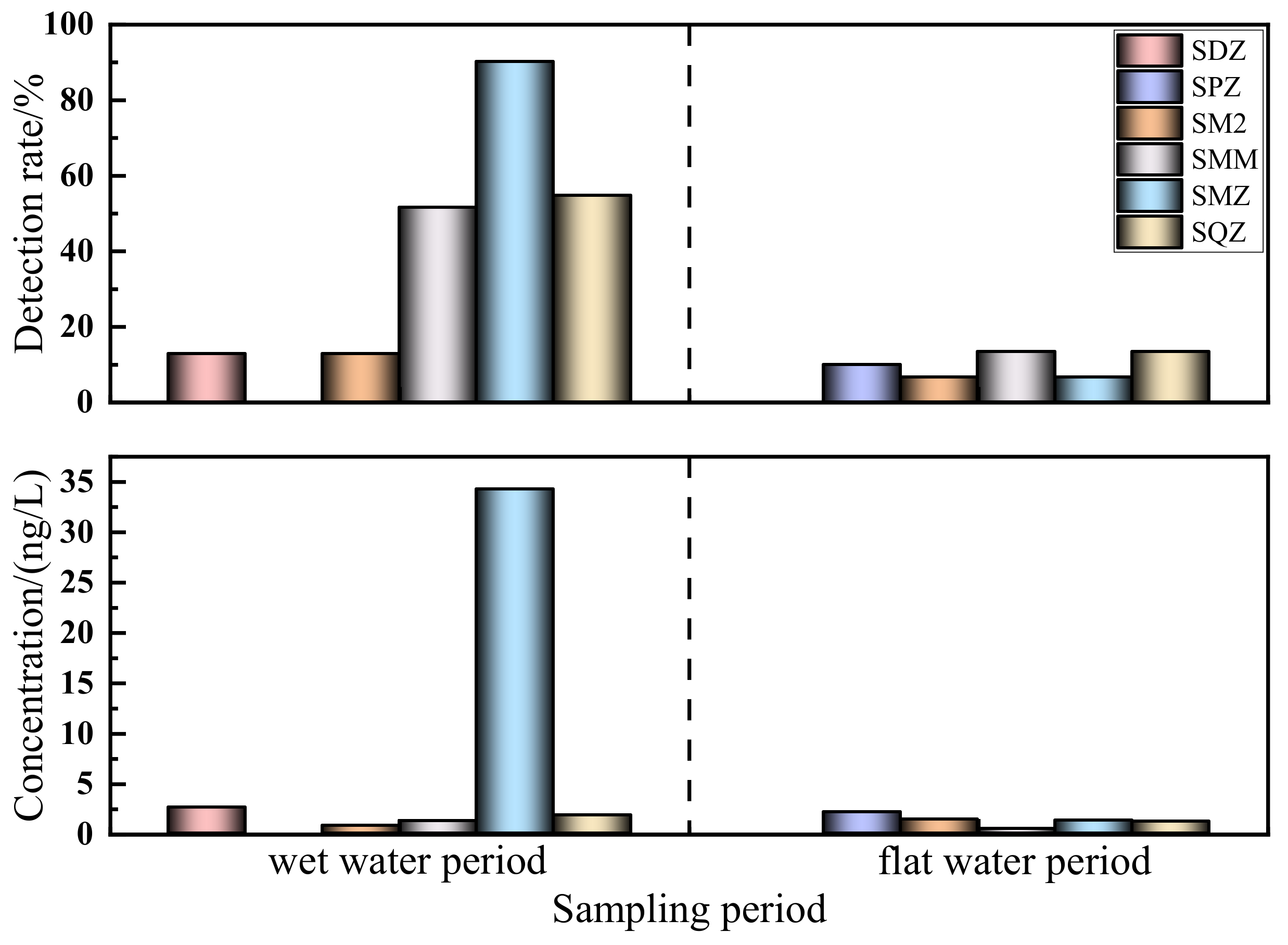
3.2.1. Temporal Distribution of Sulfonamides in the Shaanxi Section of the Weihe River
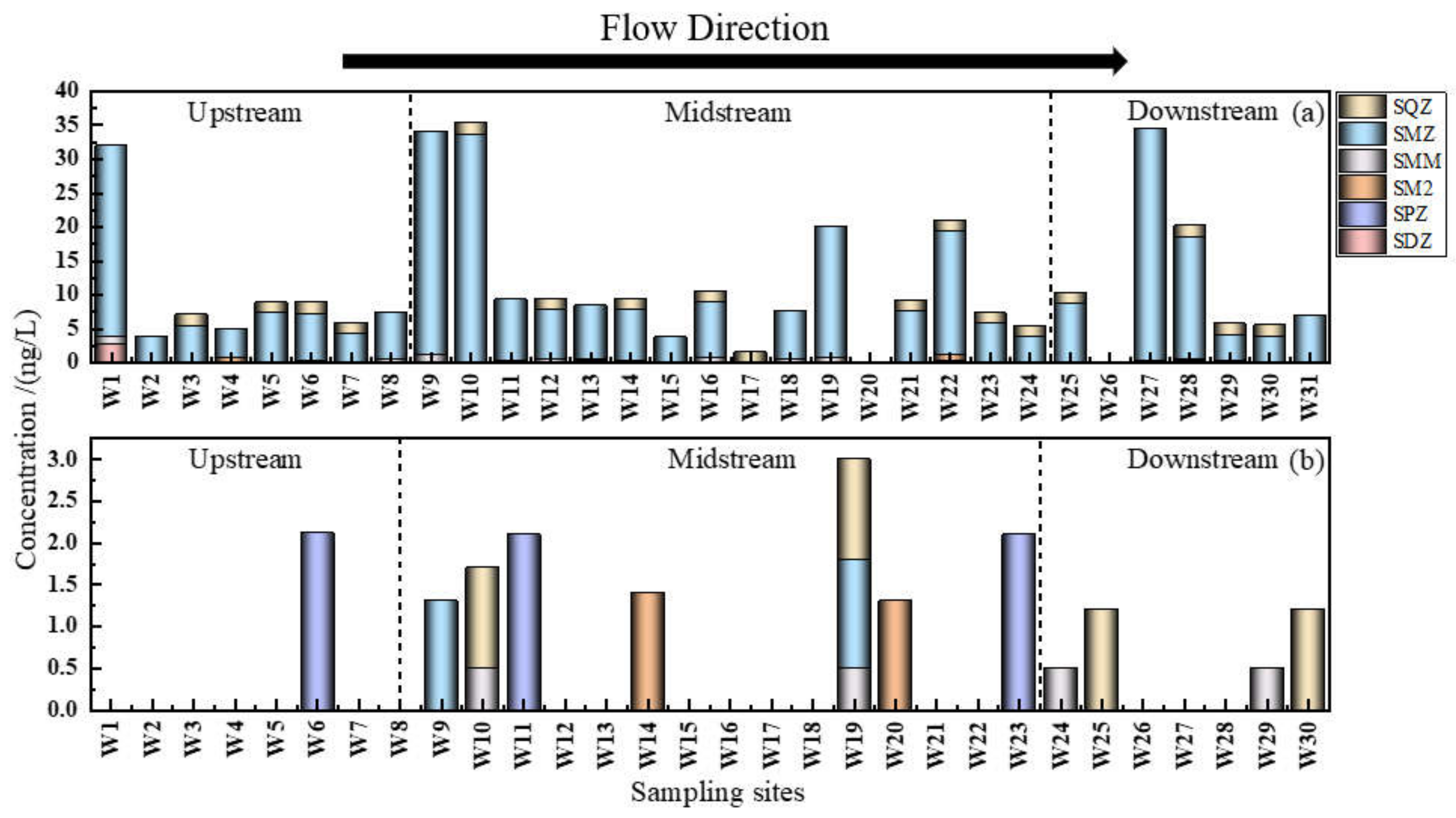
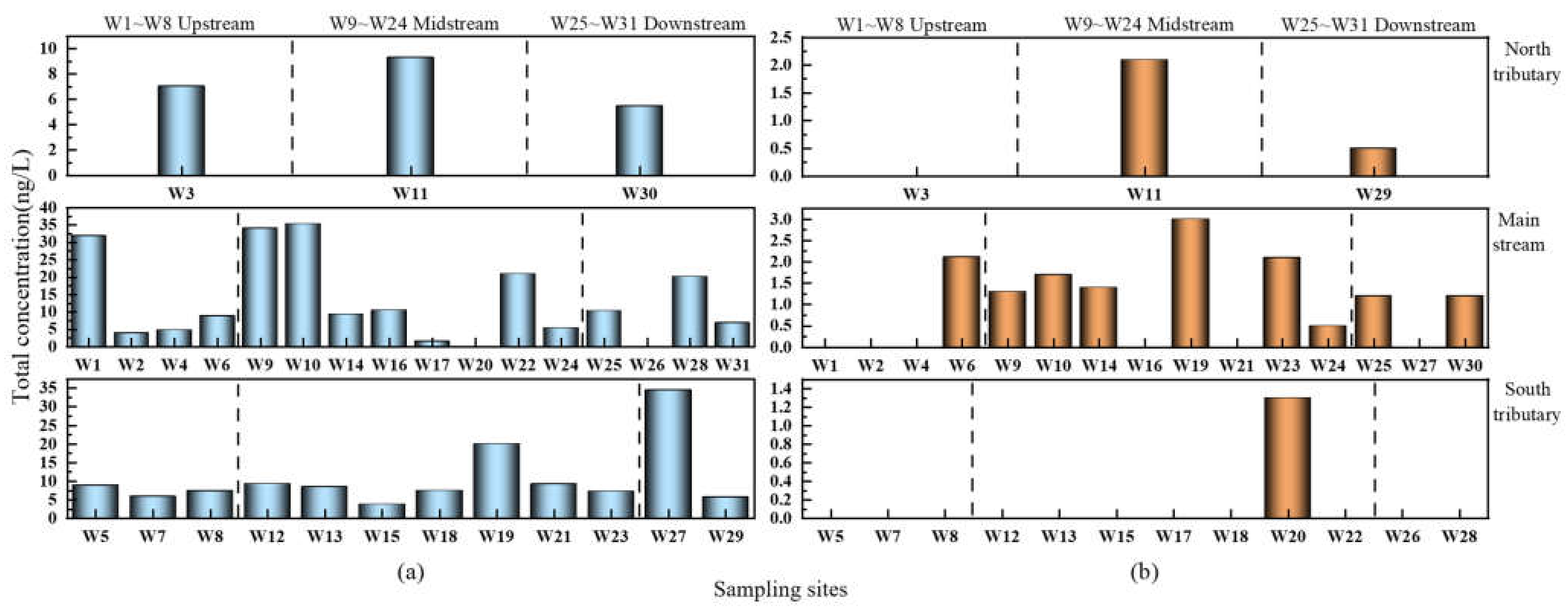
3.2.2. Spatial Distribution Characteristics of Sulfonamides in the Shaanxi Section of the Weihe River
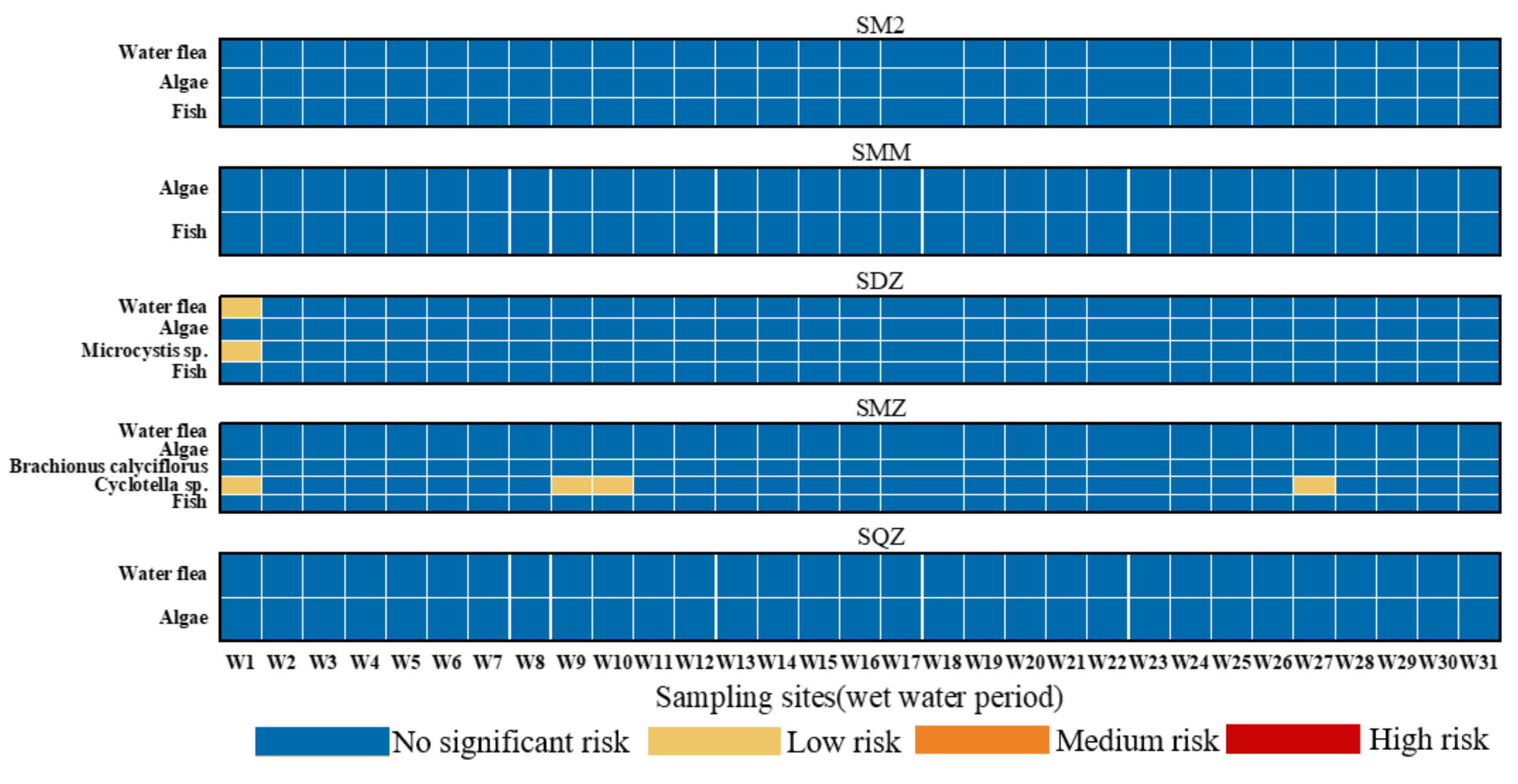
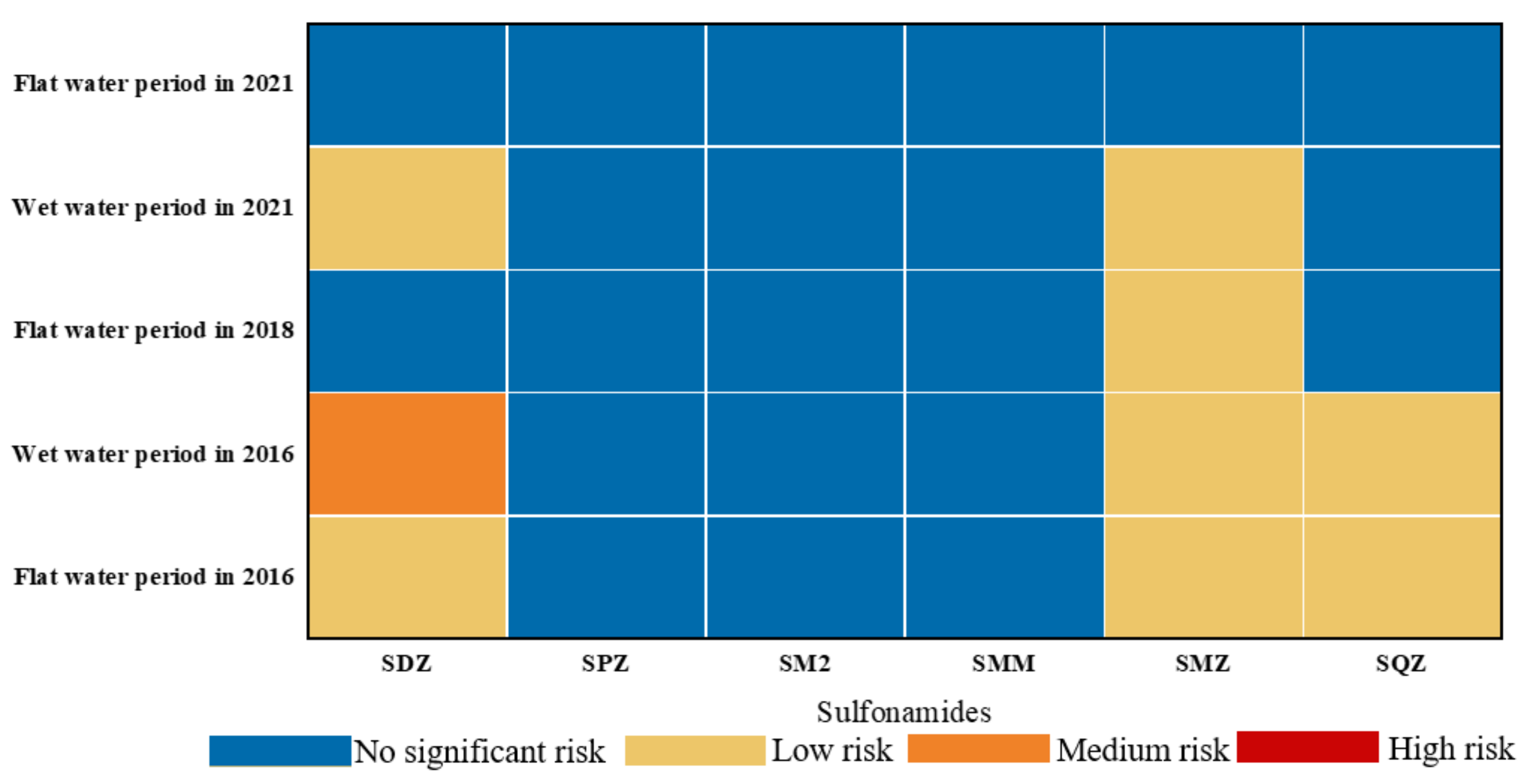
3.3. Ecological Risk Assessment of Sulfonamides in the Shaanxi Section of the Weihe River
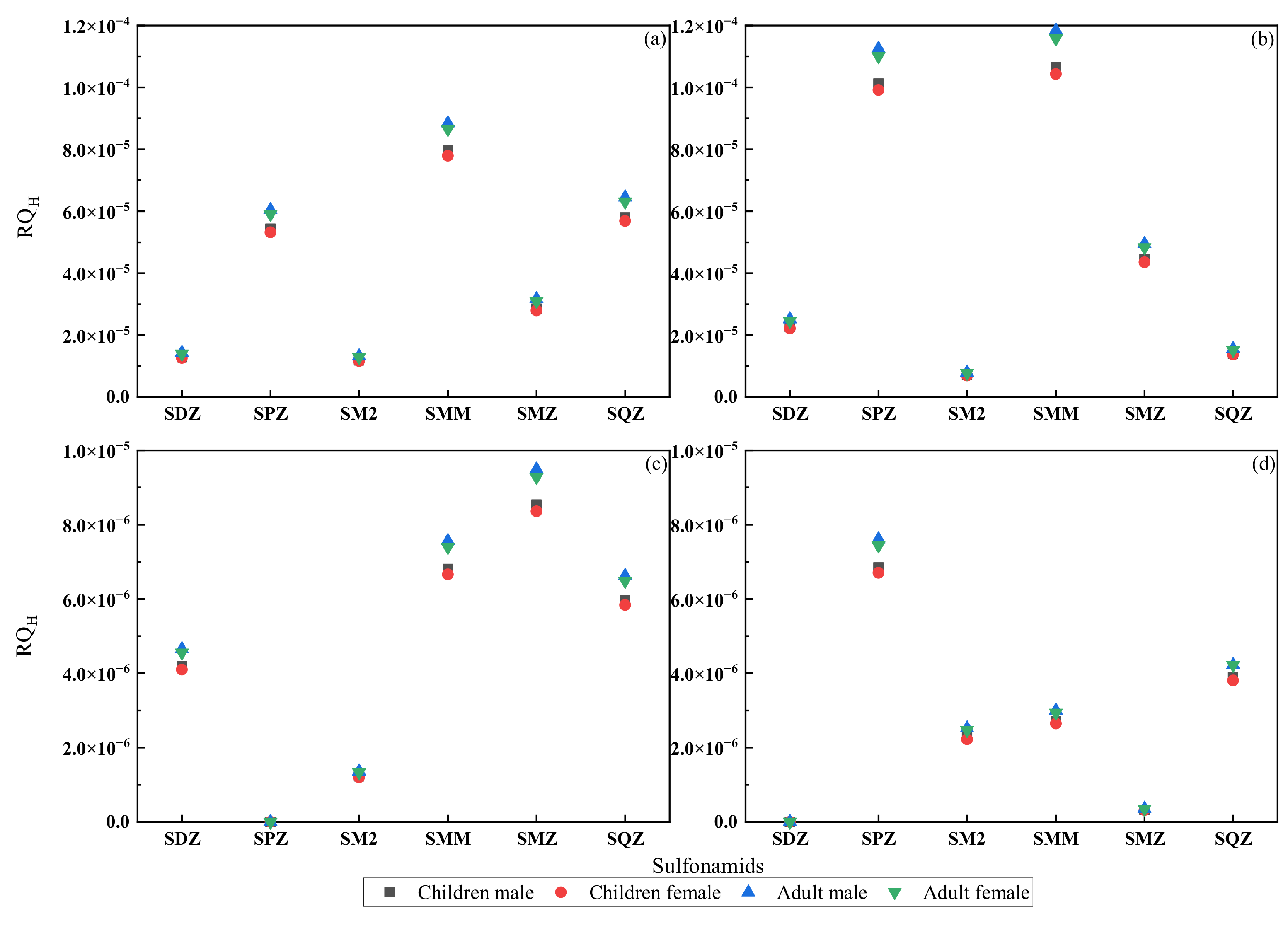
3.4. Health Risk Assessment of Sulfonamides in the Shaanxi Section of the Weihe River
4. Conclusions
- (1)
- In the Shaanxi section of the Weihe River, SAs are abundant. In this basin, six SAs (SDZ, SPZ, SM2, SMM, SMZ, and SQZ) were detected, with five SAs being detected in each of the wet and flat water periods, with detection rates varying from 12.9% to 90.30% and from 8.57% to 13.3%, respectively. In comparison to other domestic and foreign rivers, SA pollution in the Shaanxi section of the Weihe River was rather low, but the risks caused by individual antibiotic usage should still be considered.
- (2)
- In terms of temporal distribution, SAs exhibited seasonal changes associated with rainfall, light intensity, ambient temperature, and biological and microbial activity, with wet water period (ND–34.256 ng/L) > flat water period (ND–2.113 ng/L). In terms of spatial distribution, it was revealed that the order was the main stream (ND–35.296 ng/L) > the south tributary (3.718–34.354 ng/L) > the north tributary (5.476–9.302 ng/L) during the wet water period, and the main stream (ND–3 ng/L) > the north tributary (ND–2.095 ng/L) > the south tributary (ND–1.3 ng/L) during the flat water period, which is directly associated with the enterprises on both sides and the degree of population density, economic development, and agricultural farming.
- (3)
- Except for SDZ and SMZ, which exhibited low risk (0.01 ≤ RQS < 0.1) to water flea and Microcystis sp. in some sampling sites during the wet water period, no other antibiotics showed a substantial ecological risk (RQS < 0.01) to their sensitive species. The risks of six SAs to aquatic organisms have decreased in the last five years, with the order of wet water period > flat water period, which is intrinsically connected to the development of emerging contaminant management in recent years.
- (4)
- In the Shaanxi section of the Weihe River, there was no significant health risk (RQH < 0.01) of SAs in different age groups, and the risks for adults were greater than for children, and in the same age group, males were at higher risk than females. The RQH values in this study declined by an order of one magnitude, compared with those of 2016. However, the risks from antibiotic contaminant interactions and resistance genes were not explored in this study, which solely looked at the effects of the six SAs on diverse aquatic organisms and human health.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lang, H.; Liu, F.; Jin, S.; Yan, T. Presence of Antibiotics in Shallow Groundwater in the Northern and Southwestern Regions of China. Ground Water 2018, 56, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; van der Aa, M.; Wuijts, S.; De Roda Husman, A.M.; Van der Hoek, J.P. Risk governance of potential emerging risks to drinking water quality: Analysing current practices. Environ. Sci. Policy 2018, 84, 97–104. [Google Scholar] [CrossRef]
- Tong, X.; You, L.; Zhang, J.; Chen, H.; Nguyen, V.T.; He, Y.; Gin, K.Y. A comprehensive modelling approach to understanding the fate, transport and potential risks of emerging contaminants in a tropical reservoir. Water Res. 2021, 200, 117298. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, L.; Fan, W.Y.; Yang, C.; Li, E.H.; Du, Y.; Wang, X.L. Inconsistent seasonal variation of antibiotics between surface water and groundwater in the Jianghan Plain: Risks and linkage to land uses. J. Environ. Sci. 2021, 109, 102–113. [Google Scholar] [CrossRef]
- Gothwal, R.; Thatikonda, S. Role of environmental pollution in prevalence of antibiotic resistant bacteria in aquatic environment of river: Case of Musi river, South India. Water Environ. J. 2017, 31, 456–462. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, X.; Zhang, Q.; Wang, J.; Yang, J.; Teng, Y.; Chen, X.; Zhai, Y. Sulfonamide antibiotics in groundwater and their migration in the vadose zone: A case in a drinking water resource. Ecol. Eng. 2021, 162, 106175. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef]
- Yang, W.B.; Zheng, F.F.; Xue, X.X.; Lu, Y.P. Investigation into adsorption mechanisms of sulfonamides onto porous adsorbents. J. Colloid Interf. Sci. 2011, 362, 503–509. [Google Scholar] [CrossRef]
- Nunes, O.C.; Manaia, C.M.; Kolvenbach, B.A.; Corvini, P.F. Living with sulfonamides: A diverse range of mechanisms observed in bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 10389–10408. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhao, J.L.; Wei, X.D.; Liu, M.S. Co-occurrence and ecological risk of antibiotics in surface water of Guangzhou section of Pearl River. Ecol. Environ. Sci. 2017, 26, 1034–1041. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, S.H.; Chen, M.H.; Song, N.H.; Xu, H.Z.; Zhang, Q. Contamination level and risk assessment of 12 sulfonamides in surface water of Nanjing reach of the Yangtze River. Environ. Chem. 2018, 37, 505–512. [Google Scholar] [CrossRef]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Bai, Y.W.; Zhang, Y.; Ma, S.Q.; Guo, C.S.; Zhang, L. Occurrence, distribution, and ecological risk of antibiotics in surface water in the Liaohe River Basin, China. Environ. Sci. 2017, 38, 4553–4561. [Google Scholar] [CrossRef]
- Burke, V.; Richter, D.; Greskowiak, J.; Mehrtens, A.; Schulz, L.; Massmann, G. Occurrence of Antibiotics in Surface and Groundwater of a Drinking Water Catchment Area in Germany. Water Environ. Res. 2016, 88, 652–659. [Google Scholar] [CrossRef]
- Hoa, P.T.; Managaki, S.; Nakada, N.; Takada, H.; Shimizu, A.; Anh, D.H.; Viet, P.H.; Suzuki, S. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 2011, 409, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- Song, J.X.; Cheng, D.D.; Li, Q.; He, X.J.; Long, Y.Q.; Zhang, B. An Evaluation of River Health for the Weihe River in Shaanxi Province, China. Adv. Meteorol. 2015, 2015, 476020. [Google Scholar] [CrossRef] [Green Version]
- Shaanxi Provincial Bulletin of Ecological Environment. Available online: http://sthjt.shaanxi.gov.cn/newstype/hbyw/hjzl/hjzkgb/20190603/40879.html (accessed on 31 May 2022).
- Guerit, I.; Bocquene, G.; James, A.; Thybaud, E.; Minier, C. Environmental risk assessment: A critical approach of the European TGD in an in situ application. Ecotoxicol. Environ. Saf. 2008, 71, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Sanderson, H. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Yu, X.; Zhao, G.; Zhao, Q.; Wang, N.; Jiang, Y.; Jiang, F.; He, G.; Chen, Y.; et al. Exposure of Adults to Antibiotics in a Shanghai Suburban Area and Health Risk Assessment: A Biomonitoring-Based Study. Environ. Sci. Technol. 2018, 52, 13942–13950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, C.P. Acceptable daily intakes for agricultural and veterinary chemicals. Chin. J. Veter. Drug 2005, 39, 39–45. [Google Scholar] [CrossRef]
- Jin, L.; Jiang, L.; Han, Q.; Xue, J.Y.; Ye, H.; Cao, G.M.; Lin, K.F.; Cui, C.Z. Distribution characteristics and health risk assessment of thirteen sulfonamides antibiotics in a drinking water source in East China. Environ. Sci. 2016, 37, 2515–2521. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernandez-Alba, A.R.; Barcelo, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, M.; Zhang, H. Spatial and temporal variations of antibiotics in a tidal river. Environ. Monit. Assess. 2020, 192, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Yin, X.; Song, F.; Gong, Y.; Tu, X.; Wang, Y.; Cao, S.; Liu, J.; Lu, Z. A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 2013, 68, 2445–2452. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef]
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Managaki, S.; Murata, A.; Takada, H.; Tuyen, B.C.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, M.J.; Diaz-Cruz, M.S.; Barcelo, D. Occurrence of sulfonamide residues along the Ebro River basin: Removal in wastewater treatment plants and environmental impact assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, Y.; Li, W.; Liu, J.; Cai, Y. Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J. Environ. Monit. 2012, 14, 1248–1255. [Google Scholar] [CrossRef]
- Yin, Z. Distribution and ecological risk assessment of typical antibiotics in the surface waters of seven major rivers, China. Environ. Sci. Process. Impacts 2021, 23, 1088–1100. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Zou, S.; Ling, Z.; Wang, G.; Yan, W. A preliminary investigation on the occurrence and distribution of antibiotics in the Yellow River and its tributaries, China. Water Environ. Res. 2009, 81, 248–254. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.J.; Zhao, Z.Q.; Yang, B.F.; Rao, J. Distribution characteristics and source analysis of typical antibiotics in some rivers in North China. Admin. Technol. Environ. Monit. 2020, 32, 14–17. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, K.; Huang, Q.; Guo, Z.; Huang, Y.; Yu, K.; He, Y. Comprehensive insights into the occurrence, distribution, risk assessment and indicator screening of antibiotics in a large drinking reservoir system. Sci. Total Environ. 2020, 716, 137060. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicol. Environ. Saf. 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Suthar, S. Occurrence, seasonal variations, and ecological risk of pharmaceuticals and personal care products in River Ganges at two holy cities of India. Chemosphere 2021, 268, 129331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, R.; Li, J.; Cheng, Z.; Luo, C.; Wang, Y.; Yu, K.; Zhang, G. Occurrence and distribution of antibiotics in multiple environmental media of the East River (Dongjiang) catchment, South China. Environ. Sci. Pollut. Res. Int. 2017, 24, 9690–9701. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, J.W.; Yang, X.Y.; Sun, B.C.; Li, K.B.; Zhang, J.T. Contamination characteristic and ecological risk of antibiotics in surface water of the Weihe Guanzhong section. China Environ. Sci. 2017, 37, 2255–2262. [Google Scholar] [CrossRef]
- Wang, J.; Wei, H.; Zhou, X.; Li, K.; Wu, W.; Guo, M. Occurrence and risk assessment of antibiotics in the Xi’an section of the Weihe River, northwestern China. Mar. Pollut. Bull. 2019, 146, 794–800. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, M.; Yang, S.K.; Wang, Z.Z.; Wang, R.Z.; Wang, W.K.; Zhao, Y.Q. Distribution characteristics and ecological risk assessment of antibiotic pollution in the Weihe River Basin. Yellow River 2018, 40, 85–91. [Google Scholar] [CrossRef]
- Li, H.H.; Zhang, Q.; Guo, Y.N. Investigation and review on the water quality of sections of the Weihe River in Baoji. Power Syst. Clean Energy 2009, 25, 81–84. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, W.; Shen, X.; Zhang, F.; Zhou, X.; Shen, J.; Lu, M. Occurrence, Distribution, and Ecological Risk Assessment of Antibiotics in Different Environmental Media in Anqing, Anhui Province, China. Int. J. Environ. Res. Public Health 2021, 18, 8112. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Y.; Lin, Y.; An, D. Occurrence, spatial distribution, and seasonal variation of emerging trace organic pollutants in source water for Shanghai, China. Sci. Total Environ. 2018, 639, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Li, E.; Liu, X.; Wei, H.; Yang, C.; Lu, S.; Ning, K. Distribution of antibiotic resistance genes in an agriculturally disturbed lake in China: Their links with microbial communities, antibiotics, and water quality. J. Hazard. Mater. 2020, 393, 122426. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yuan, S.; Kabba, J.A.; Chen, C.; Liu, W.; Chang, J.; Fang, Y. Analysis of Antibiotic Use Patterns and Trends Based on Procurement Data of Healthcare Institutions in Shaanxi Province, Western China, 2015–2018. Int. J. Environ. Res. Public Health 2020, 17, 7536. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Zhou, S.H.; Han, X.K.; Zhang, L.L.; Ding, S.Y.; Li, Y.; Zhang, D.J.; Zarin, K. Occurrence, distribution, and source track of antibiotics and antibiotic resistance genes in the main rivers of Chongqing city, southwest China. J. Hazard. Mater. 2020, 389, 122110. [Google Scholar] [CrossRef] [PubMed]


| Sulfonamides | Corresponding Sensitive Species | EC50/(mg/L) | Toxicity Type | AF | PNEC/(ng/L) |
|---|---|---|---|---|---|
| SDZ | Fish | 890 | Acute toxicity | 1000 | 890,000 |
| Microcystis sp. | 0.135 | 135 | |||
| Algae | 0.52 | 520 | |||
| Water flea | 0.21 | 210 | |||
| SPZ | Lemna minor | 0.46 | Acute toxicity | 1000 | 460 |
| Chlorella vulgaris | 5.28 | 5280 | |||
| SM2 | Fish | 517 | Acute toxicity | 1000 | 517,000 |
| Algae | 38 | 38,000 | |||
| Water flea | 4 | 4000 | |||
| SMM | Fish | 450 | Acute toxicity | 1000 | 450,000 |
| Algae | 8.56 | 8560 | |||
| SMZ | Fish | 890 | Acute toxicity | 1000 | 89,000 |
| Cyclotella sp. | 2.4 | 2400 | |||
| Brachionus calyciflorus | 26.27 | 26,270 | |||
| Algae | 51 | 51,000 | |||
| Water flea | 4.5 | 4500 | |||
| SQZ | Algae | 20 | Acute toxicity | 50 | 400 |
| Water flea | 84.46 | 1000 | 84,460 | ||
| SCP | Chlorella vulgaris | 32.25 | Acute toxicity | 1000 | 32,250 |
| Research Subjects | Gender | BW/kg | DWI/L·d |
|---|---|---|---|
| Children | Male | 24 | 0.81 |
| Female | 23 | 0.76 | |
| Adults | Male | 66.1 | 2.48 |
| Female | 57.8 | 2.12 |
| Sulfonamides | ADI/(μg·kg−1·d−1) | References |
|---|---|---|
| SDZ | 20 | [24] |
| SPZ | 10 | |
| SM2 | 20 | |
| SMM | 6 | [25] |
| SMZ | 130 | [24] |
| SQZ | 10 | [25] |
| SDM | 10 | |
| SCP | 50 | [26] |
| SMR | 50 | |
| STZ | 50 |
| Sulfonamides | Wet Water Period | Flat Water Period | ||||||
|---|---|---|---|---|---|---|---|---|
| Min/(ng/L) | Max/(ng/L) | Mean/(ng/L) | Detectionrates/% | Min/(ng/L) | Max/(ng/L) | Mean/(ng/L) | Detection Rates/% | |
| SDZ | ND | 2.584 | 0.106 | 12.90 | ND | ND | ND | 0 |
| SPZ | ND | ND | ND | 0 | ND | 2.113 | 0.210 | 10.00 |
| SM2 | ND | 0.756 | 0.064 | 12.90 | ND | 1.4 | 0.090 | 6.70 |
| SMM | ND | 1.260 | 0.212 | 51.60 | ND | 0.500 | 0.067 | 13.30 |
| SMZ | ND | 34.256 | 10.126 | 90.30 | ND | 1.3 | 0.087 | 6.70 |
| SQZ | ND | 1.84 | 0.884 | 54.80 | ND | 1.2 | 0.160 | 13.30 |
| Name | Concentration/(ng/L) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | SDZ | SPZ | SM2 | SMM | SMZ | SQZ | ||
| Shaanxi section of the Weihe River | Max | 2.584 | ND | 0.756 | 1.26 | 34.256 | 1.84 | - |
| Min | ND | ND | ND | ND | ND | ND | ||
| Guangzhou section of the Pearl River | Max | 13.7 | 10.4 | 256 | 56.8 | 210 | 3.02 | [12] |
| Min | 3.5 | ND | 8.2 | 9.14 | 2.66 | ND | ||
| Nanjing section of the Yangtze River | Max | 6.59 | 1.01 | - | ND | 6.76 | - | [13] |
| Min | 2.52 | 0.36 | - | ND | 8.98 | - | ||
| Haihe River | Max | 270 | - | 940 | - | 660 | - | [14] |
| Min | ND | - | ND | - | ND | - | ||
| Liaohe River | Max | - | 0.96 | 15.91 | - | 670.27 | 14.59 | [15] |
| Min | - | ND | ND | - | ND | ND | ||
| Songhua River | Max | 505 | 85.0 | 16.1 | ND | 940 | - | [31] |
| Min | 0.86 | ND | ND | ND | ND | - | ||
| Hongkong River | Max | 14.8 | 3.2 | 580.4 | - | 3.1 | - | [32] |
| Min | 1 | 0.9 | 16.5 | - | 1.1 | - | ||
| Bangladesh River | Max | 0.58 | - | 11.35 | - | 7.24 | - | [33] |
| Min | 0.03 | - | 0.02 | - | 0.03 | - | ||
| Mekong River | Max | - | - | 328 | - | 174 | - | [34] |
| Min | - | - | 15 | - | 20 | - | ||
| Ebro River | Max | 6.4 | - | ND | - | 35.6 | - | [35] |
| Min | 1.3 | - | ND | - | 1.88 | - | ||
| Youngshan River | Max | 20 | - | 20 | - | 110 | - | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Yang, S.; Sun, Y.; Ye, F.; Jiang, J.; Kou, X.; Yang, F. Spatial and Temporal Distribution Characteristics and Potential Risks of Sulfonamides in the Shaanxi Section of the Weihe River. Int. J. Environ. Res. Public Health 2022, 19, 8607. https://doi.org/10.3390/ijerph19148607
Duan L, Yang S, Sun Y, Ye F, Jiang J, Kou X, Yang F. Spatial and Temporal Distribution Characteristics and Potential Risks of Sulfonamides in the Shaanxi Section of the Weihe River. International Journal of Environmental Research and Public Health. 2022; 19(14):8607. https://doi.org/10.3390/ijerph19148607
Chicago/Turabian StyleDuan, Lei, Siyue Yang, Yaqiao Sun, Fei Ye, Jie Jiang, Xiaomei Kou, and Fan Yang. 2022. "Spatial and Temporal Distribution Characteristics and Potential Risks of Sulfonamides in the Shaanxi Section of the Weihe River" International Journal of Environmental Research and Public Health 19, no. 14: 8607. https://doi.org/10.3390/ijerph19148607





