Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study
Abstract
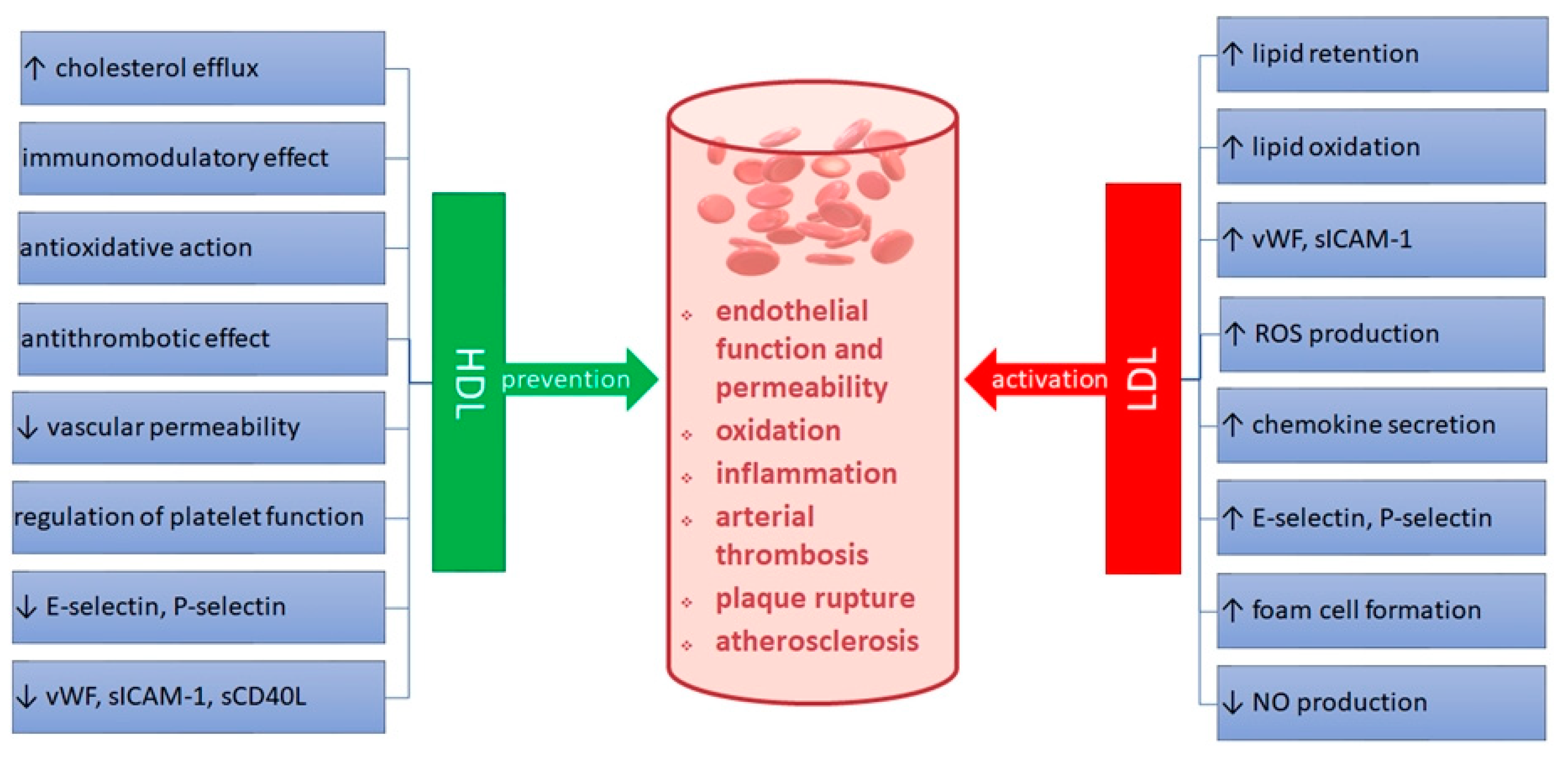
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annema, W.; von Eckardstein, A. High-density lipoproteins—Multifunctional but vulnerable protections from atherosclerosis. Circ. J. 2013, 77, 2432–2448. [Google Scholar] [CrossRef]
- Huma Bindu, G.; Rao, V.S.; Kakkar, V.V. Friends turns foe: Transformation of anti-inflammatory HDL to proinflammatory HDL during acute phase response. Cholesterol 2011, 2011, 274629. [Google Scholar]
- Nofer, J.R.; Brodde, M.; Kehrel, B. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 726–735. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef]
- Yin, K.; Chen, W.J.; Zhou, Z.G.; Zhao, G.J.; Lv, Y.C.; Ouyang, X.P.; Yu, X.H.; Fu, Y.; Jiang, Z.S.; Tang, C.K. Apolipoprotein A-1 inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages. J. Atheroscler. Thromb. 2012, 19, 823–836. [Google Scholar] [CrossRef]
- Constans, J.; Conri, C. Circulating markers of endothelial function in cardiovascular disease. Clin. Chim. Acta 2006, 368, 33–47. [Google Scholar] [CrossRef]
- Tretjakovs, P.; Jurka, A.; Bormane, I.; Mikelsone, I.; Elksne, K.; Krievina, G.; Reihmane, D.; Verbovenko, J.; Bahs, G. Circulating adhesion molecules, matrix metalloproteinase-9, plasminogen activator inhibitor-1, and myeloperoxidase in coronary artery disease patients with stable and unstable angina. Clin. Chim. Acta 2012, 413, 25–29. [Google Scholar] [CrossRef]
- Moreira, M.C.S.; Pinto, I.S.J.; Mourão, A.A.; Fajemiroye, J.O.; Colombari, E.; Reis, Â.A.S.; Freiria-Oliveira, A.H.; Ferreira-Neto, M.L.; Pedrino, G.R. Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front. Physiol. 2015, 6, 234. [Google Scholar] [CrossRef]
- Tasci, I.; Dogru, T.; Sonmez, A.; Genc, H.; Kilic, S.; Olgun, A.; Gok, M.; Erdem, G.; Erikci, S. Soluble CD40 ligand levels in otherwise healthy subject with impaired fasting glucose. Mediat. Inflamm. 2006, 5, 32508. [Google Scholar] [CrossRef]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees, ESC/EAS guidelines for the management for dyslipidaemias, The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), European Association for Cardiovascular Prevention & Rehabilitation. Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.; Dalgadok, V.; Ferencel, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Kei, A.; Tellis, C.; Liberopoulos, E.; Tselepis, A.; Elisaf, M. Effect of switch to the highest dose of rosuvastatin versus add-on-statin fenofibrate versus add-on-statin nicotinic acid/laropiprant on oxidative stress markers in patients with mixed dyslipidemia. Cardiovasc. Ther. 2014, 32, 139–146. [Google Scholar] [CrossRef]
- Hoshiga, M.; Arishiro, K.; Nakakaoji, T.; Myiazaki, N.; Negoro, N.; Okabe, T.; Kohbayashi, E.; Ishihara, T.; Hanafusa, T. Switching to aggressive statin improves vascular endothelial functions in patients with stable coronary artery disease. J. Atheroscler. Thromb. 2010, 17, 705–711. [Google Scholar] [CrossRef][Green Version]
- Blanco-Colio, L.M.; Martin-Ventura, J.L.; de Teresa, E.; Farsang, C.; Gaw, A.; Gensini, G.F.; Leiter, L.A.; Langer, A.; Martineau, P.; Egido, J. Atorvastatin decreases elevated soluble CD40L in subjects at high cardiovascular risk. Atorvastatin on inflammatory markers study: A substudy of ACTFAST. Kidney Int. 2008, 74, 60–63. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Vaisar, T. Inflammation, Remodeling and Other Factors Affecting HDL Cholesterol Efflux. Curr. Opin. Lipidol. 2017, 28, 52–59. [Google Scholar] [CrossRef]
- Eren, E.; Ellidag, H.Y.; Aydin, O.; Yilmaz, N. HDL functionality and crystal-based sterile inflammation in atherosclerosis. Clin. Chim. Acta 2015, 439, 18–23. [Google Scholar] [CrossRef]
- Obradovic, S.; Djukanovic, N.; Todorovic, Z.; Markovic, I.; Zamaklar-Trifunovic, D.; Protic, D.; Ostojic, M. Men with lower HDL cholesterol levels have significant increment of soluble CD40 ligand and high-sensitivity CRP levels following the cessation of long-term clopidogrel therapy. J. Atheroscler. Thromb. 2015, 22, 284–292. [Google Scholar] [CrossRef][Green Version]
- Tabet, F.; Vickers, K.C.; Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef]
- Annema, W.; von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl. Res. 2016, 173, 30–57. [Google Scholar] [CrossRef]
- Ragbir, S.; Farmer, J.A. Dysfunctional high-density lipoprotein and atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 343–348. [Google Scholar] [CrossRef]
- Annema, W.; Willemsen, H.M.; de Boer, J.F.; Dikkers, A.; van der Giet, M.; Nieuwland, W.; Muller Kobold, A.C.; van Pelt, L.J.; Slart, R.H.; van der Horst, I.C.; et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J. Clin. Lipidol. 2016, 10, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Siepi, D.; Bagaglia, F.; Pirro, M.; Ciuffetti, G.; Schillaci, G.; Mannarino, E. Mechanisms of high-density lipoprotein cholesterol effects on the endothelial function in hyperlipidemia. Metabolism 2003, 52, 1191–1195. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Villa, B.; Omoboni, L.; Dmitrieff, C.; Franceschini, G. Elevated soluble cellular adhesion molecules in subjects with low HDL-cholesterol. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 656–661. [Google Scholar] [CrossRef]
- Stanojević, N.B.; Ivanović, Z.J.; Djurović, S.; Kalimanovska, V.S.; Spasić, S.; Oštrić, D.K.; Memon, L. Lack of association between low HDL-cholesterol and elevated circulating cellular adhesion molecules in normolipidemic CAD patients and healthy subjects. Int. Heart J. 2005, 46, 593–600. [Google Scholar] [CrossRef]
- Lee, K.; Lip, G.; Tayebjee, M.; Foster, W.; Blann, A. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood 2005, 105, 526–632. [Google Scholar] [CrossRef] [PubMed]
- Spiel, A.; Gilbert, J.; Jilma, B. Von Willebrand factor in cardiovascular disease, Focus on acute coronary syndrome. Circ. J. 2008, 117, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The role of von Willebrand Factor in vascular inflammation: From pathogenesis to targeted therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.Q.; Zhao, S.P.; Li, Y.F.; Li, J.; Zhou, H.N. Elevated soluble CD40 ligand is related to the endothelial adhesion molecules in patients with acute coronary syndrome. Clin. Chim. Acta 2002, 319, 19–26. [Google Scholar] [CrossRef]
- Genc, H.; Dogru, T.; Tapan, S.; Tasci, I.; Bozoglu, E.; Gok, M.; Aslan, F.; Celebi, G.; Erdem, G.; Avcu, F.; et al. Soluble CD40 ligand, soluble P-selectin, and von Willebrand factor level in subjects with prediabetes: The impact of metabolic syndrome. Clin. Chem. 2012, 45, 92–95. [Google Scholar] [CrossRef]
- Akinci, G.; Coskun, S.; Akinci, B.; Hekimsoy, Z.; Bayindir, B.; Onur, E.; Ozmen, B. Atherosclerosis risk factors in children of parents with metabolic syndrome. Atherosclerosis 2007, 194, e165–e171. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A meta-analysis of low-density lipoproteins cholesterol, non-high density lipoproteins cholesterol, apolipoprotein B as markers of cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Islam, S.; McQueen, M.; Pencina, M.; Furberg, C.D.; Thanassoulis, G.; Yusuf, S. Age and Cardiovascular Risk Attributable to Apolipoprotein B, Low-Density Lipoprotein Cholesterol or Non-High-Density Lipoprotein Cholesterol. J. Am. Heart Assoc. 2016, 5, e003665. [Google Scholar] [CrossRef] [PubMed]
- Vaverkova, H.; Karasek, D.; Navotny, D.; Jackuliakova, D.; Lukes, J.; Halenka, M.; Frohlich, J. Apolipoprotein B versus LDL-cholesterol: Association with other risk factors for atherosclerosis. Clin. Biochem. 2009, 42, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.; Yilmaz, N.; Aydin, O. High density lipoprotein and it’s dysfunction. Open Biochem. J. 2012, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Hafiane, A.; Schwertani, A.; Genest, J. High-Density Lipoproteins: Biology, Epidemiology, and Clinical Management. Can. J. Cardiol. 2017, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A.; Genest, J. HDL, Atherosclerosis, and emerging therapies. Cholesterol 2013, 2013, 891403. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Banegas, J.R.; Roy, C.; Dallongeville, J.; De Backer, G.; Guallar, E.; Perk, J.; Hajage, D.; Henriksson, K.M.; Borghi, C. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc. Disord. 2017, 17, 160. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Mariani, M.V.; Netti, L.; Birtolo, L.B.; De Orchi, P.; Chimenti, C.; Maestrini, V.; et al. Potential Role of eNOS Genetic Variants in Ischemic Heart Disease Susceptibility and Clinical Presentation. About J. Cardiovasc. Dev. Dis. 2021, 8, 116. [Google Scholar] [CrossRef]
- Karmali, K.N.; Lloyd-Jones, D.M.; Berendsen, M.; Goff, D.C.; Sanghavi, D.M.; Brown, N.; Korenovska, L.; Huffman, M.D. Drugs for primary prevention of atherosclerotic cardiovascular disease: An overview of systematic reviews. JAMA Cardiol. 2016, 1, 341–349. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Schneider, W.R. The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2008, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]



| Parameter | CAD (n = 57) | C (n = 23) | p |
|---|---|---|---|
| Gender: women/men | 16 (28%)/41 (72%) | 13 (57%)/10 (43%) | 0.115/0.240 |
| Age (years) | 62.6 ± 10.5 ^ | 54.3 ± 13.2 ^ | 0.003 |
| Age: women/men (years) | 61.1 ± 13.8 ^/63.3 ± 9.1 ^ | 57.3 ± 9.0 ^/45.8 ± 12.9 ^ | 0.235/0.001 |
| BMI (kg/m2) | 26.69 ± 4.44 ^ | 25.08 ± 3.01 ^ | 0.189 |
| BMI: women/men (kg/m2) | 25.27 ± 4.17 ^/27.01 ± 2.87 ^ | 24.55 ± 3.12 ^/25.81 ± 2.87 ^ | 0.530/0.531 |
| BMI > 30 kg/m2 | 11 (19%) | 0 (0%) | 0.039 |
| Hypertension | 28 (49%) | 2 (9%) | 0.014 |
| Diabetes (stable) | 7 (12%) | 0 (0%) | 0.098 |
| Cigarette smoking | 23 (40%) | 5 (23%) | 0.258 |
| Total cholesterol > 5.0 mmol/L | 19 (33%) | 16 (70%) | 0.180 |
| Triglycerides > 2.3 mmol/L | 5 (9%) | 1 (4%) | 0.870 |
| HDL cholesterol < 1.0 mmol/L | 26 (46%) | 1 (4%) | 0.007 |
| LDL cholesterol > 3.0 mmol/L | 30 (53%) | 20 (87%) | 0.185 |
| C (n = 23) Me/IQR | CAD (n = 57) Me/IQR | p * | AMI (n = 27) Me/IQR | p ** | SA (n = 30) Me/IQR | p *** | p **** | |
|---|---|---|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | 5.87/1.24 | 4.70/1.63 | 0.008 | 5.12/2.53 | 0.355 | 4.24/1.39 | 0.001 | 0.018 |
| LDL cholesterol (mmol/L) | 3.67/1.12 | 3.08/1.39 | 0.119 | 3.52/1.88 | 0.763 | 2.61/0.92 | 0.003 | 0.003 |
| HDL cholesterol (mmol/L) | 1.45/0.70 | 1.06/0.34 | <0.001 | 1.06/0.34 | 0.001 | 1.01/0.22 | <0.001 | 0.203 |
| Triglycerides (mmol/L) | 1.16/0.62 | 1.20/0.62 | 0.953 | 1.07/0.59 | 0.224 | 1.33/0.60 | 0.038 | 0.011 |
| LDL-C/HDL-C | 2.46/1.70 | 2.90/1.22 | 0.020 | 3.39/1.17 | 0.003 | 2.80/0.76 | 0.002 | 0.003 |
| vWF (%) | 89.0/40.0 | 147.0/65.5 | <0.001 | 182.0/55.0 | <0.001 | 124.0/42.0 | <0.001 | <0.001 |
| sE-selectin (ng/mL) | 30.2/10.0 | 26.0/17.5 | 0.111 | 29.0/15.0 | 0.953 | 22.5/32.3 | 0.007 | 0.006 |
| sICAM-1 (ng/mL) | 250.0/64.0 | 311.0/118.5 | 0.001 | 311.0/140.0 | <0.001 | 306.7/121.5 | 0.002 | 0.366 |
| sCD40L (pg/mL) | 139.0/337.0 | 410.0/322.0 | 0.030 | 394.0/665.0 | 0.326 | 443.5/200.0 | 0.047 | 0.367 |
| C (n = 23) | CAD (n = 57) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCH | HDL-C | LDL-C | LDL-C/ HDL-C | TG | TCH | HDL-C | LDL-C | LDL-C/ HDL-C | TG | |
| vWF | r = −0.005 p = 0.980 | r = −0.080 p = 0.717 | r = 0.144 p = 0.512 | r = 0.127 p = 0.565 | r = −0.046 p = 0.833 | r = −0.030 p = 0.823 | r = −0.263 p = 0.048 | r = 0.070 p = 0.604 | r = 0.372 p = 0.004 | r = −0.142 p = 0.293 |
| sE-selectin | r = −0.172 p = 0.434 | r = −0.171 p = 0.436 | r = −0.048 p = 0.830 | r = 0.074 p = 0.737 | r = 0.124 p = 0.573 | r = −0.288 p = 0.053 | r = −0.359 p = 0.014 | r = −0.219 p = 0.145 | r = 0.104 p = 0.491 | r = −0.175 p = 0.245 |
| sICAM-1 | r = 0.118 p = 0.591 | r = −0.172 p = 0.431 | r = 0.161 p = 0.462 | r = 0.217 p = 0.320 | r = 0.396 p = 0.061 | r = 0.167 p = 0.214 | r = 0.004 p = 0.977 | r = 0.192 p = 0.153 | r = 0.314 p = 0.017 | r = 0.166 p = 0.219 |
| sCD40L | r = −0.270 p = 0.212 | r = 0.252 p = 0.245 | r = −0.205 p = 0.349 | r = −0.311 p = 0.149 | r = −0.528 p = 0.010 | r = −0.013 p = 0.923 | r = −0.326 p = 0.013 | r = 0.060 p = 0.659 | r = 0.271 p = 0.041 | r = 0.054 p = 0.659 |
| AMI (n = 27) | SA (n = 30) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCH | HDL-C | LDL-C | LDL-C/ HDL-C | TG | TCH | HDL-C | LDL-C | LDL-C/ HDL-C | TG | |
| vWF | r = −0.410 p = 0.033 | r = −0.516 p = 0.006 | r = −0.329 p = 0.094 | r = 0.268 p = 0.177 | r = 0.181 p = 0.366 | r = −0.155 p = 0.413 | r= −0.394 p = 0.031 | r = −0.114 p = 0.547 | r = −0.097 p = 0.694 | r = 0.035 p = 0.854 |
| sE-selectin | r = −0.238 p = 0.233 | r = −0.306 p = 0.120 | r = −0.214 p = 0.284 | r = 0.157 p = 0.435 | r =−0.006 p = 0.976 | r = −0.436 p = 0.062 | r = −0.511 p = 0.025 | r = −0.314 p = 0.190 | r = 0.104 p = 0.491 | r = −0.358 p = 0.133 |
| sICAM-1 | r = 0.212 p = 0.288 | r = −0.227 p = 0.256 | r = 0.234 p = 0.241 | r = 0.592 p = 0.001 | r = 0.517 p = 0.006 | r = 0.079 p = 0.680 | r = 0.171 p = 0.365 | r = 0.140 p = 0.461 | r = 0.040 p = 0.833 | r = −0.045 p = 0.814 |
| sCD40L | r = −0.026 p = 0.897 | r =−0.357 p = 0.067 | r = 0.033 p = 0.870 | r = −0.311 p = 0.149 | r = 0.129 p = 0.520 | r = 0.069 p = 0.716 | r = −0.207 p = 0.273 | r = 0.207 p = 0.272 | r = 0.259 p = 0.167 | r = −0.187 p = 0.322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampka, M.; Olszewska-Słonina, D.; Hołyńska-Iwan, I.; Grąbczewska, Z.; Obońska, K.; Cwynar, A.; Stępowska, J.; Szewczyk-Golec, K. Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 8637. https://doi.org/10.3390/ijerph19148637
Lampka M, Olszewska-Słonina D, Hołyńska-Iwan I, Grąbczewska Z, Obońska K, Cwynar A, Stępowska J, Szewczyk-Golec K. Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(14):8637. https://doi.org/10.3390/ijerph19148637
Chicago/Turabian StyleLampka, Magdalena, Dorota Olszewska-Słonina, Iga Hołyńska-Iwan, Zofia Grąbczewska, Karolina Obońska, Anna Cwynar, Justyna Stępowska, and Karolina Szewczyk-Golec. 2022. "Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 14: 8637. https://doi.org/10.3390/ijerph19148637
APA StyleLampka, M., Olszewska-Słonina, D., Hołyńska-Iwan, I., Grąbczewska, Z., Obońska, K., Cwynar, A., Stępowska, J., & Szewczyk-Golec, K. (2022). Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study. International Journal of Environmental Research and Public Health, 19(14), 8637. https://doi.org/10.3390/ijerph19148637







