Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Eligibility Criteria
2.3. Ethical Approval
2.4. Design and Procedures
2.5. Pre-Test Preparation
2.6. Anthropometry
2.7. Incremental Cycling Test
2.8. Ratings of Perceived Exertion (RPE)
2.9. Surface Electromyography (SEMG)
2.10. Statistical Analysis
3. Results
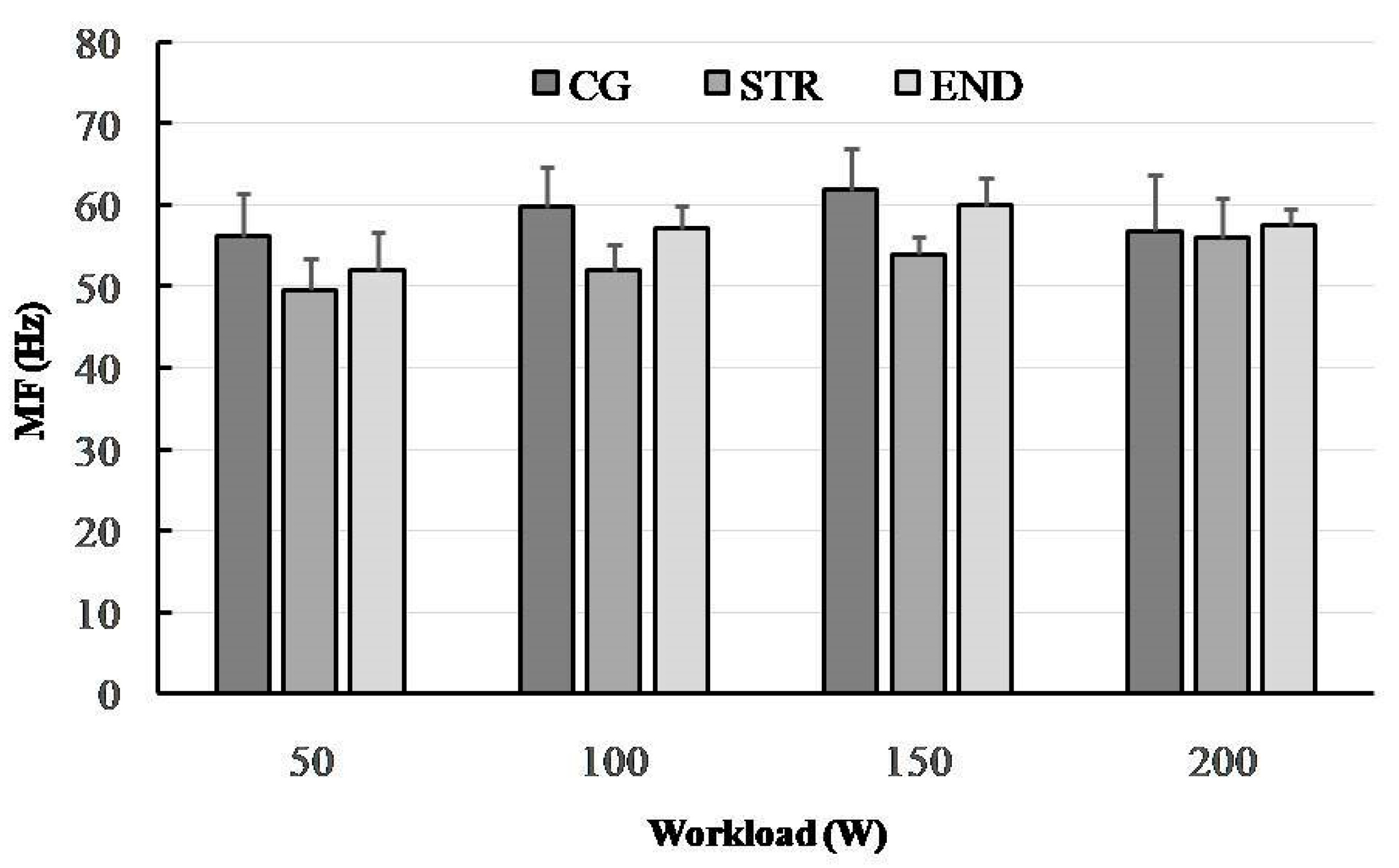
3.1. Median Frequency (MF)
3.2. Mean Power Frequency (MPF)
3.3. Electromyogram Amplitude (AEMG)
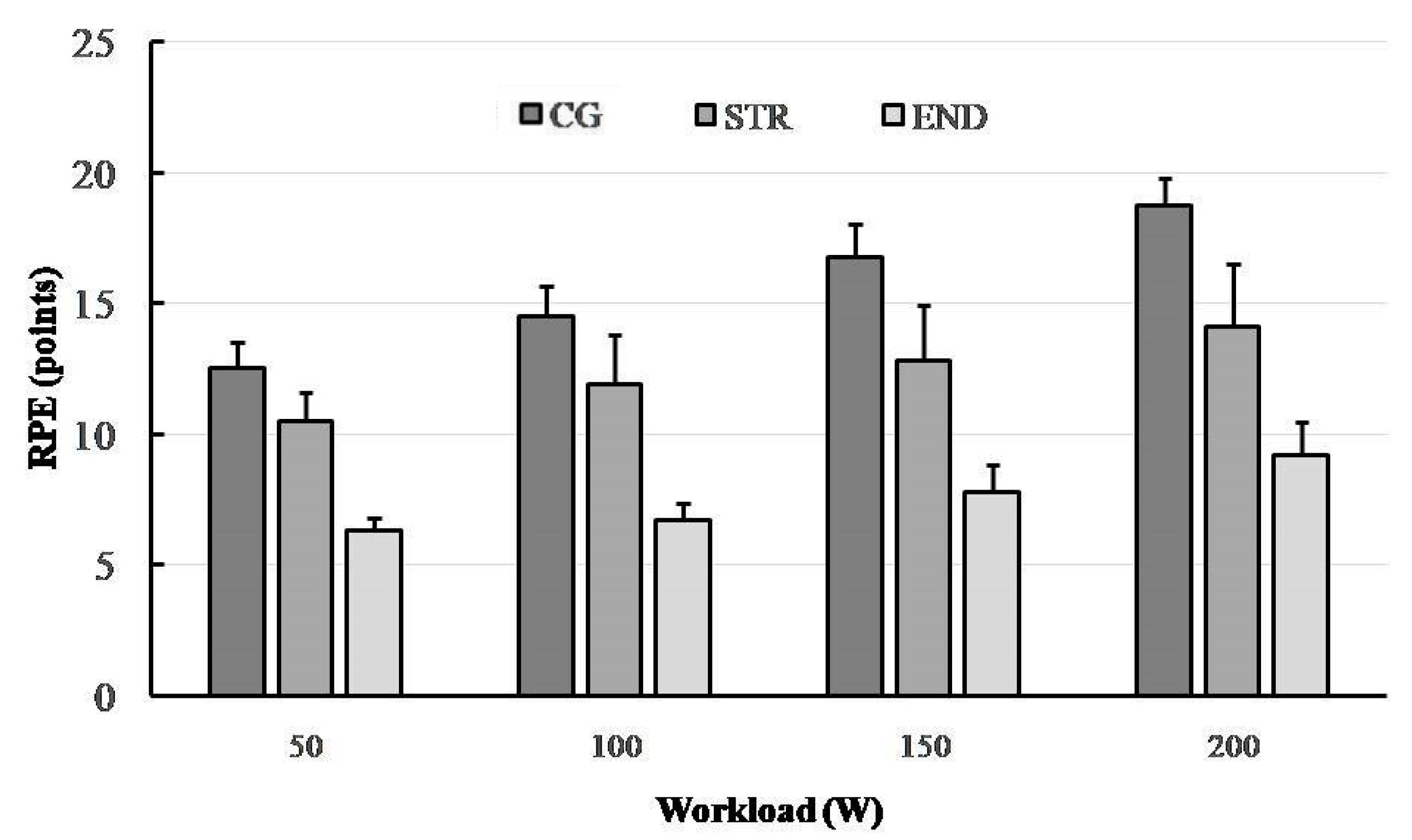
3.4. Ratings of Perceived Exertion (RPE)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamali, A.M.; Saadi, Z.K.; Yahyavi, S.S.; Zarifkar, A.; Aligholi, H.; Nami, M. Transcranial direct current stimulation to enhance athletic performance outcome in experienced bodybuilders. PLoS ONE 2019, 14, e0220363. [Google Scholar] [CrossRef] [Green Version]
- Garrandes, F.; Colson, S.S.; Pensini, M.; Legros, P. Time course of mechanical and neuromuscular characteristics of cyclists and triathletes during a fatiguing exercise. Int. J. Sports Med. 2007, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sleivert, G.G.; Backus, R.D.; Wenger, H.A. Neuromuscular differences between volleyball players, middle distance runners and untrained controls. Int. J. Sports Med. 1995, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Medina, L.; González-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.; Graham, S.R.; Eston, R.G.; Burgess, D.J.; Garrett, L.J.; Jakeman, J.; Norton, K. A novel method of assessment for monitoring neuromuscular fatigue in Australian rules football players. Int. J. Sports Physiol. Perform. 2019, 14, 598–605. [Google Scholar] [CrossRef]
- Astokorki, A.H.; Mauger, A.R. Tolerance of exercise-induced pain at a fixed rating of perceived exertion predicts time trial cycling performance. Scand. J. Med. Sci. Sports 2017, 27, 309–317. [Google Scholar] [CrossRef]
- Glace, B.W.; Kremenic, I.J.; McHugh, M.P. Sex differences in central and peripheral mechanisms of fatigue in cyclists. Eur. J. Appl. Physiol. 2013, 113, 1091–1098. [Google Scholar] [CrossRef]
- Kremenic, I.J.; Glace, B.W.; Ben-Avi, S.S.; Nicholas, S.J.; McHugh, M.P. Central fatigue after cycling evaluated using peripheral magnetic stimulation. Med. Sci. Sports Exerc. 2009, 41, 1461–1466. [Google Scholar] [CrossRef]
- Medved, V.; Medved, S.; Kovač, I. Critical appraisal of surface electromyography (sEMG) as a taught subject and clinical tool in medicine and kinesiology. Front. Neurol. 2020, 11, 560363. [Google Scholar] [CrossRef]
- Graef, J.L.; Smith, A.E.; Kendall, K.L.; Walter, A.A.; Moon, J.R.; Lockwood, C.M.; Beck, T.W.; Cramer, J.T.; Stout, J.R. The relationships among endurance performance measures as estimated from VO2PEAK, ventilatory threshold, and electromyographic fatigue threshold: A relationship design. Dyn. Med. 2008, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Fontes, E.B.; Smirmaul, B.P.; Nakamura, F.Y.; Pereira, G.; Okano, A.H.; Altimari, L.R.; Dantas, J.L.; de Moraes, A.C. The relationship between rating of perceived exertion and muscle activity during exhaustive constant-load cycling. Int. J. Sports Med. 2010, 31, 683–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Self-regulated force and neuromuscular responses during fatiguing isometric leg extensions anchored to a rating of perceived exertion. Appl. Psychophysiol. Biofeedback 2019, 44, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise. Sports Med. 2006, 36, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Balasubramanian, V. Low back pain and muscle fatigue due to road cycling—An sEMG study. J. Bodyw. Mov. Ther. 2007, 11, 260–266. [Google Scholar] [CrossRef]
- Amann, M. Central and peripheral fatigue: Interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 2011, 43, 2039–2045. [Google Scholar] [CrossRef]
- Rossman, M.J.; Garten, R.S.; Venturelli, M.; Amann, M.; Richardson, R.S. The role of active muscle 684 mass in determining the magnitude of peripheral fatigue during dynamic exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R934–R940. [Google Scholar] [CrossRef]
- Thomas, K.; Elmeua, M.; Howatson, G.; Goodall, S. Intensity-dependent contribution of neuromuscular fatigue after constant-load cycling. Med. Sci. Sports Exerc. 2016, 48, 1751–1760. [Google Scholar] [CrossRef]
- Dufour, S.P.; Lampert, E.; Doutreleau, S.T.; Lonsdorfer-Wolf, E.; Billat, V.L.; Piquard, F.R.; Richard, R.U. Eccentric cycle exercise: Training application of specific circulatory adjustments. Med. Sci. Sports Exerc. 2004, 36, 1900–1906. [Google Scholar] [CrossRef]
- Borg, G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef]
- Hebbal, G.V.; Mysorekar, V.R. Evaluation of some tasks used for specifying handedness and footedness. Percept. Mot. Skills 2006, 102, 163–164. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System), version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017; Available online: http://statistica.io (accessed on 7 August 2021).
- IBM. IBM Manuals; IBM ® SPSS ® Statistics Algorithms; IBM Corporation: Armonk, New York, USA, 2020. [Google Scholar]
- Mauchly, J.W. Significance test for sphericity of a normal n-variate distribution. Ann. Math. Statist. 1940, 11, 204–209. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, E.J. Biometry, 3rd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1995. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Academic Press: New York, NY, USA, 1988. [Google Scholar]
- Gergley, J.C. Comparison of two lower-body modes of endurance training on lower-body strength development while concurrently training. J. Strength Cond. Res. 2009, 23, 979–987. [Google Scholar] [CrossRef]
- Nelson, N.L.; Churilla, J.R. A narrative review of exercise-associated muscle cramps: Factors that contribute to neuromuscular fatigue and management implications. Muscle Nerve 2016, 54, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Twomey, R.; Aboodarda, S.J.; Kruger, R.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieën, J.; Hug, F.; Lowery, M.; Merletti, R.; Søgaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Amplitude normalization matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef]
- Laplaud, D.; Hug, F.; Grélot, L. Reproducibility of eight lower limb muscles activity level in the course of an incremental pedaling exercise. J. Electromyogr. Kinesiol. 2006, 16, 158–166. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle fiber type transitions with exercise training: Shifting perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Bergh, U.; Thorstensson, A.; Sjödin, B.E.; Hulten, B.O.; Piehl, K.A.; Karlsson, J. Maximal oxygen uptake and muscle fiber types in trained and untrained humans. Med. Sci. Sport 1978, 10, 151–154. [Google Scholar]
- Fry, A.C.; Schilling, B.K.; Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Thrush, J.T. Muscle fiber characteristics and performance correlates of male Olympic-style weightlifters. J. Strength Cond. Res. 2003, 17, 746–754. [Google Scholar]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Gandhi, V.; Timmons, M.K.; Coelho, A.J. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J. Biomech. 2006, 39, 246–254. [Google Scholar] [CrossRef]
- Bercier, S.; Halin, R.; Ravier, P.; Kahn, J.F.; Jouanin, J.C.; Lecoq, A.M.; Buttelli, O. The vastus lateralis neuromuscular activity during all-out cycling exercise. J. Electromyogr. Kinesiol. 2009, 19, 922–930. [Google Scholar] [CrossRef]
- Hug, F.; Decherchi, P.; Marqueste, T.; Jammes, Y. EMG versus oxygen uptake during cycling exercise in trained and untrained subjects. J. Electromyogr. Kinesiol. 2004, 14, 187–195. [Google Scholar] [CrossRef]
- Singh, V.P.; Kumar, D.K.; Djuwari, D.; Polus, B.; Fraser, S.; Hawley, J.; Giudice, S.L. Strategies to identify muscle fatigue from SEMG during cycling. In Proceedings of the 2004 Intelligent Sensors, Sensor Networks and Information Processing Conference, Melbourne, Australia, 14–17 December 2004; pp. 547–551. [Google Scholar]
- Albertus-Kajee, Y.; Tucker, R.; Derman, W.; Lambert, M. Alternative methods of normalising EMG during cycling. J. Electromyogr. Kinesiol. 2010, 20, 1036–1043. [Google Scholar] [CrossRef]
- Chin, L.M.; Kowalchuk, J.M.; Barstow, T.J.; Kondo, N.; Amano, T.; Shiojiri, T.; Koga, S. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J. Appl. Physiol. 2011, 111, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Baum, B.S.; Li, L. Lower extremity muscle activities during cycling are influenced by load and frequency. J. Electromyogr. Kinesiol. 2003, 13, 181–190. [Google Scholar] [CrossRef]
- Mileva, K.N.; Morgan, J.; Bowtell, J. Differentiation of power and endurance athletes based on their muscle fatigability assessed by new spectral electromyographic indices. J. Sports Sci. 2009, 27, 611–623. [Google Scholar] [CrossRef]
- Sulaiman, M.; Azaman, A.; Salleh, N.A.; Yahya, A. Lower Limb Muscles Activation of Endurance and Power Athletes. In 11th Asian-Pacific Conference on Medical and Biological Engineering. IFMBE Proceedings; Shiraishi, Y., Sakuma, I., Naruse, K., Ueno, A., Eds.; Springer: Cham, Germany, 2021; Volume 82, pp. 111–117. [Google Scholar]
- Chang, K.M.; Liu, S.H.; Wu, X.H. A wireless sEMG recording system and its application to muscle fatigue detection. Sensors 2012, 12, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Nasrabadi, R.; Izanloo, Z.; Sharifnezad, A.; Hamedinia, M.R.; Hedayatpour, N. Muscle fiber conduction velocity of the vastus medilais and lateralis muscle after eccentric exercise induced-muscle damage. J. Electromyogr. Kinesiol. 2018, 43, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Calder, K.M.; Stashuk, D.W.; McLean, L. Physiological characteristics of motor units in the brachioradialis muscle across fatiguing low-level isometric contractions. J. Electromyogr. Kinesiol. 2008, 18, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Ogata, H.; Yunoki, T.; Arimitsu, T.; Yano, T. Effect of blood lactate concentration and the level of oxygen uptake immediately before a cycling sprint on neuromuscular activation during repeated cycling sprints. J. Physiol. Anthropol. 2006, 25, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Z. Influence mechanism of running sportswear fatigue based on BP neural network. Eurasip. J. Adv. Signal Process. 2021, 61, 1–5. [Google Scholar] [CrossRef]
- Luomala, M.J.; Oksa, J.; Salmi, J.A.; Linnamo, V.; Holmér, I.; Smolander, J.; Dugué, B. Adding a cooling vest during cycling improves performance in warm and humid conditions. J. Therm. Biol. 2012, 37, 47–55. [Google Scholar] [CrossRef]
- García-García, O.; Cuba-Dorado, A.; Riveiro-Bozada, A.; Carballo-López, J.; Álvarez-Yates, T.; López-Chicharro, J. A maximal incremental test in cyclists causes greater peripheral fatigue in biceps femoris. Res. Quar. Exerc. Sport 2020, 91, 460–468. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, A.; Ma, G.; Ye, Y.; Li, R.; Lu, T. A comparative study of EMG indices in muscle fatigue evaluation based on grey relational analysis during all-out cycling exercise. Biomed. Res. Int. 2018, 2018, 9341215. [Google Scholar] [CrossRef] [Green Version]
- Gerdle, B.; Karlsson, S.; Crenshaw, A.G.; Elert, J.; Fridén, J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur. J. Appl. Physiol. 2000, 81, 2–10. [Google Scholar] [CrossRef]
- Balagué, N.; Hristovski, R.; García, S.; Aguirre, C.; Vázquez, P.; Razon, S.; Tenenbaum, G. Dynamics of perceived exertion in constant-power cycling: Time-and workload-dependent thresholds. Res. Quar. Exerc. Sport 2015, 86, 371–378. [Google Scholar] [CrossRef]
- Green, J.M.; McIntosh, J.R.; Hornsby, J.; Timme, L.; Gover, L.; Mayes, J.L. Effect of exercise duration on session RPE at an individualized constant workload. Eur. J. Appl. Physiol. 2009, 107, 501–507. [Google Scholar] [CrossRef]
- Macdonald, J.H.; Farina, D.; Marcora, S.M. Response of electromyographic variables during incremental and fatiguing cycling. Med. Sci. Sports Exerc. 2008, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Moritani, T.O.; Takaishi, T.E.; Matsumoto, T.A. Determination of maximal power output at neuromuscular fatigue threshold. J. Appl. Physiol. 1993, 74, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Cochrane-Snyman, K.C.; Housh, T.J.; Smith, C.M.; Hill, E.C.; Jenkins, N.D.; Schmidt, R.J.; Johnson, G.O. Inter-individual variability in the patterns of responses for electromyography and mechanomyography during cycle ergometry using an RPE-clamp model. Eur. J. Appl. Physiol. 2016, 116, 1639–1649. [Google Scholar] [CrossRef]
- Lander, P.J.; Butterly, R.J.; Edwards, A.M. Self-paced exercise is less physically challenging than enforced constant pace exercise of the same intensity: Influence of complex central metabolic control. Br. J. Sports Med. 2009, 43, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurasz, M.; Boraczyński, M.; Wójcik, Z.; Gronek, P. Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise. Int. J. Environ. Res. Public Health 2022, 19, 8839. https://doi.org/10.3390/ijerph19148839
Jurasz M, Boraczyński M, Wójcik Z, Gronek P. Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise. International Journal of Environmental Research and Public Health. 2022; 19(14):8839. https://doi.org/10.3390/ijerph19148839
Chicago/Turabian StyleJurasz, Maciej, Michał Boraczyński, Zbigniew Wójcik, and Piotr Gronek. 2022. "Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise" International Journal of Environmental Research and Public Health 19, no. 14: 8839. https://doi.org/10.3390/ijerph19148839






