Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- Burning sensation, using a visual analogue scale (VAS) from 0–10 (0 = no burning sensation, 10 = extreme burning sensation).
- Dry mouth or xerostomia, using a VAS from 0–10 (0 = no dry mouth sensation, 10 = extreme dry mouth sensation).
- Taste alterations, defined as perceived changes in taste quality (metallic, bitter, sweet, salty and acid).
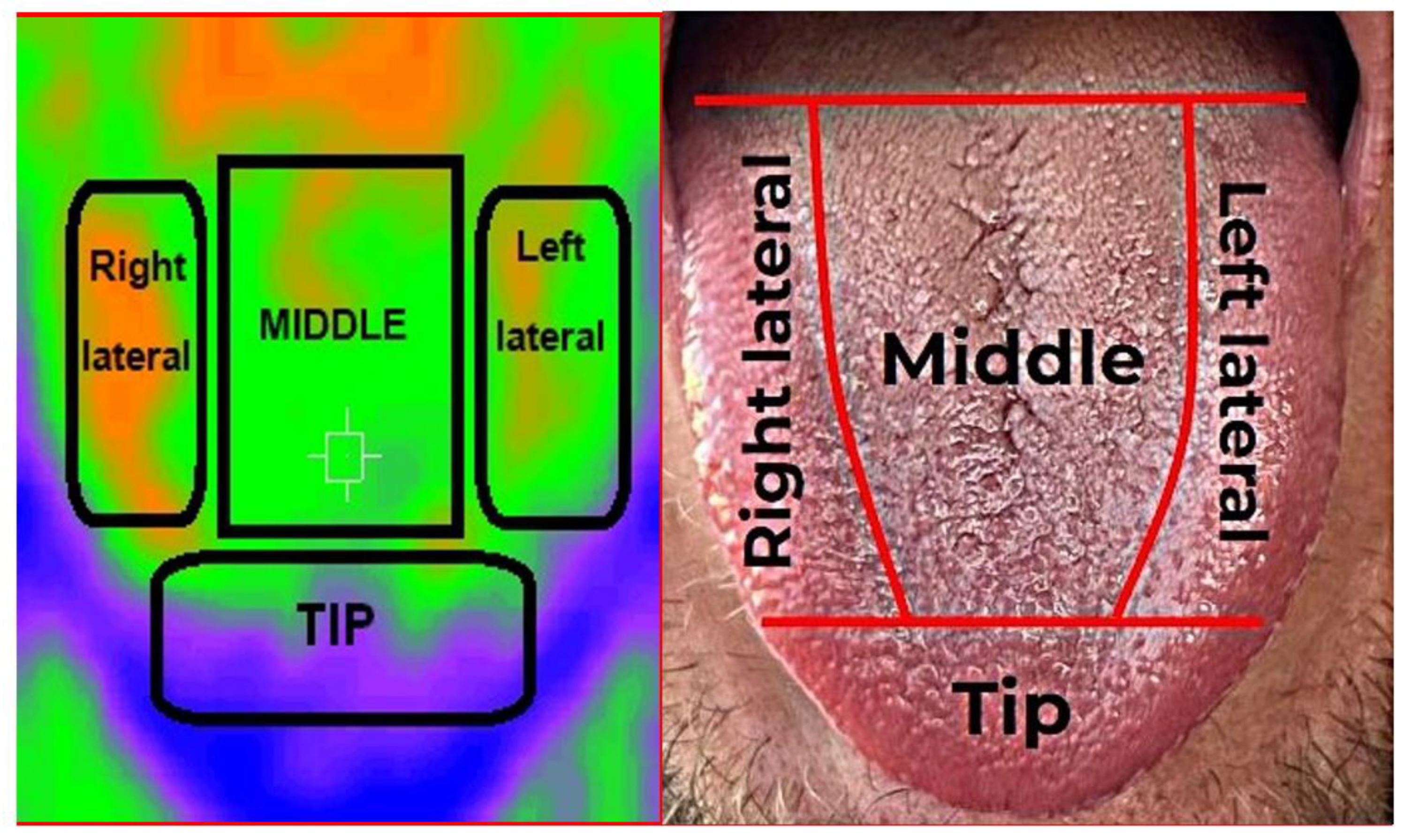
2.2. Recording of Thermographic Images
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, B.; Thoppay, J.R.; De Rossi, S.S.; Ciarrocca, K. Burning Mouth Syndrome. Dermatol. Clin. 2020, 38, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Checchi, L.; Montevecchi, M.; Marini, I.; Giamberardino, M.A. Update on burning mouth syndrome: Overview and patient management. Crit. Rev. Oral Biol. Med. 2003, 14, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Feller, L.; Fourie, J.; Bouckaert, M.; Khammissa, R.A.G.; Ballyram, R.; Lemmer, J. Burning Mouth Syndrome: Aetiopathogenesis and Principles of Management. Pain Res. Manag. 2017, 2017, 1926269. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, S.K. Is burning mouth syndrome a neuropathic pain condition? Pain 2018, 159, 610–613. [Google Scholar] [CrossRef]
- Ritchie, A.; Kramer, J.M. Recent Advances in the Etiology and Treatment of Burning Mouth Syndrome. J. Dent. Res. 2018, 97, 1193–1199. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Andujar-Mateos, P.; Sánchez-Siles, M.; Gómez-García, F. Burning mouth syndrome: Update. Med. Oral Patol. Oral Cir. Bucal 2010, 15, 562–568. [Google Scholar] [CrossRef]
- Jääskeläinen, S.K.; Woda, A. Burning Mouth Syndrome. Cephalgia 2017, 37, 627–647. [Google Scholar] [CrossRef] [Green Version]
- Chimenos-Küstner, E.; de Luca-Monasterios, F.; Schemel-Suárez, M.; Rodríguez de Rivera-Campillo, M.E.; Pérez-Pérez, A.M.; López-López, J. Burning mouth syndrome and associated factors: A case-control retrospective study. Med. Clin. 2017, 148, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Jornet, P.; Molino Pagan, D.; Andujar Mateos, P.; Rodriguez Agudo, C.; Pons-Fuster, A. Circadian rhythms variation of pain in burning mouth syndrome. Geriatr. Gerontol. Int. 2015, 15, 490–495. [Google Scholar] [CrossRef]
- Carreño-Hernández, I.; Cassol-Spanemberg, J.; Rodríguez de Rivera-Campillo, E.; Estrugo-Devesa, A.; López-López, J. Is Burning Mouth Syndrome a Neuropathic Pain Disorder? A Systematic Review. J. Oral Facial Pain Headache 2021, 35, 218–229. [Google Scholar] [CrossRef]
- Madariaga, V.I.; Tanaka, H.; Ernberg, M. Psychophysical characterisation of burning mouth syndrome-A systematic review and meta-analysis. J. Oral Rehabil. 2020, 47, 1590–1605. [Google Scholar] [CrossRef]
- Kolkka-Palomaa, M.; Jääskeläinen, S.K.; Laine, M.A.; Teerijoki-Oksa, T.; Sandell, M.; Forssell, H. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: A review. Oral Dis. 2015, 21, 937–948. [Google Scholar] [CrossRef]
- Freilich, J.E.; Kuten-Shorrer, M.; Treister, N.S.; Woo, S.-B.; Villa, A. Burning mouth syndrome: A diagnostic challenge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 120–124. [Google Scholar] [CrossRef]
- Just, T.; Steiner, S.; Pau, H.W. Oral pain perception and taste in Burning Mouth Syndrome. J. Oral Pathol. Med. 2010, 39, 22–27. [Google Scholar] [CrossRef]
- Boucher, Y.; Simons, C.T.; Faurion, A.; Azérad, J.; Carstens, E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003, 973, 265–274. [Google Scholar] [CrossRef]
- Currie, C.C.; Ohrbach, R.; De Leeuw, R.; Forssell, H.; Imamura, Y.; Jääskeläinen, S.; Koutris, M.; Nasri-Heir, C.; Tan, H.; Renton, T.; et al. Renaming burning mouth syndrome: Implications and use for the Research Diagnostic Criteria for Burning Mouth Syndrome. Pain 2022, 163, e691–e692. [Google Scholar] [CrossRef]
- Ozasa, K.; Noma, N.; Kobayashi, M.; Takizawa, K.; Young, A.; Eliav, E.; Imamura, Y. Association Between Anxiety and Descending Pain Modulation of Thermal Stimuli in Patients with Burning Mouth Syndrome: A Cross-Sectional Study. J. Oral Facial Pain Headache 2022, 36, 67–77. [Google Scholar] [CrossRef]
- Imura, H.; Shimada, M.; Yamazaki, Y.; Sugimoto, K. Characteristic changes of saliva and taste in burning mouth syndrome patients. J. Oral Pathol. Med. 2016, 45, 231–236. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Brucher, S.; Kesztyüs, T. Use of infrared thermography in medical diagnostics: A scoping review protocol. BMJ Open 2022, 12, e059833. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F.G. Infrared thermography for temperature measurement and non-destructive testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damião, C.P.; Montero, J.R.G.; Moran, M.B.H.; de Oliveira Marçal, E.; Silva Carvalho, M.E.; de Farias, C.G.; Brito, I.B.; Saad, M.A.N.; Fontes, C.A.P.; Fainstein, C.; et al. Application of thermography in the diagnostic investigation of thyroid nodules. Endocr. J. 2021, 68, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Jones, B.F. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar] [CrossRef]
- Gabrić, D.; Aumiler, D.; Vuletić, M.; Gjorgievska, E.; Blašković, M.; Mladenov, M.; Pavlić, V. Thermal evaluation by infrared thermography measurement of osteotomies performed with Er:YAG laser, piezosurgery and surgical drill-an animal study. Materials 2021, 14, 3051. [Google Scholar] [CrossRef]
- Jung, C.J.; Jeon, Y.J.; Kim, J.Y.; Kim, K.H. Review on the current trends in tongue diagnosis systems. Integr. Med. Res. 2012, 1, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukkarasu, U.; Umapathy, S.; Krishnan, P.T.; Janardanan, K. Human Tongue Thermography Could Be a Prognostic Tool for Prescreening the Type II Diabetes Mellitus. Evid. Based Complementary Altern. Med. 2020, 2020, 3186208. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y. Relationship between dynamic infrared thermal images and blood perfusion rate of the tongue in anaemia patients. Infrared Phys. Technol. 2018, 89, 27–34. [Google Scholar] [CrossRef]
- Baek, S.W.; Lee, J.M.; Park, Y.B.; Park, Y.J. Relationship between Tongue Temperature Estimated by Infrared Thermography, Tongue Color, and Cold-Heat Pathological Patterns: A Retrospective Chart Review Study. Evid. Based Complementary Altern. Med. 2018, 27, 6841460. [Google Scholar] [CrossRef]
- Lv, C.; Wang, X.; Chen, J.; Yang, N.; Fisk, I. A non-invasive measurement of tongue surface temperature. Food Res. Int. 2022, 116, 499–507. [Google Scholar] [CrossRef]
- Biagioni, P.A.; Longmore, R.B.; McGimpsey, J.G.; Lamey, P.J. Infrared thermography. Its role in dental research with particular reference to craniomandibular disorders. Dentomaxillofac. Radiol. 1996, 25, 119–124. [Google Scholar] [CrossRef]
- Dominguez-Lara, S. Magnitud del efecto para pruebas de normalidad en investigación en salud. Investig. Educ. Médica 2018, 7, 92–93. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Zhang, R.; Luan, J.; Wu, Z. Quantitative assessment of Bell’s palsy-related facial thermal asymmetry using infrared thermography: A preliminary study. J. Therm. Biol. 2021, 100, 103070. [Google Scholar] [CrossRef]
- Gratt, B.; Sickles, E. Electronic facial thermography: An analysis of asymptomatic adult subjects. J. Orofac. Pain 1995, 9, 255–265. [Google Scholar]
- Gratt, B.; Sickle, E.; Ross, J.; Wexler, G. Thermographic assessment of craniomandibular disorders: Diagnostic interpretation versus temperature measurement analysis. J. Orofac. Pain 1994, 8, 278–288. [Google Scholar]
- Machoy, M.; Szyszka-Sommerfeld, L.; Rahnama, M.; Koprowski, R.; Wilczyński, S.; Woźniak, K. Diagnosis of Temporomandibular Disorders Using Thermovision Imaging. Pain Res. Manag. 2020, 2020, 5481365. [Google Scholar] [CrossRef]
- Gzawi, M.; Warawreh, A.; Hijazin, R.; Jafar, H. Clinical evaluation of thermography as a diagnostic tool in oral and maxillo-facial lesions. JRMS 2018, 25, 45–49. [Google Scholar]
- Aboushady, M.A.; Talaat, W.; Hamdoon, Z.; Elshazly, M.T.; Ragy, N.; Bourauel, C.; Talaat, S. Thermography as a non-ionizing quantitative tool for diagnosing periapical inflammatory lesions. BMC Oral Health 2021, 21, 260. [Google Scholar] [CrossRef]
- Iosif, L.; Preoteasa, C.T.; Murariu-Măgureanu, C.; Preoteasa, E. Clinical study on thermography, as modern investigation method for Candida-associated denture stomatitis. Rom. J. Morphol. Embryol. 2016, 57, 191–195. [Google Scholar]
- Zhihao, J.; Kai, Z.; Xiaozuo, L.; Li, X. Analysis of tongue information in coronary artery disease. In Proceedings of the 2008 IEEE International Symposium on IT in Medicine and Education, Xiamen, China, 12–14 December 2008; pp. 287–290. [Google Scholar] [CrossRef]
- Naumova, E.A.; Dierkes, T.; Sprang, J.; Arnold, W.H. The oral mucosal surface and blood vessels. Head Face Med. 2013, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Wolowski, A.; Schwarzbach, N.; Hörning, H. Thermal quantitative sensory testing in burning mouth syndrome. Clin. Oral Investig. 2021, 25, 3059–3066. [Google Scholar] [CrossRef]

| Variables Tongue | Control (n = 35) | Burning Mouth (n = 32) | p Value |
|---|---|---|---|
| Mean ± ST | Mean ± ST | ||
| TªM Dorsum | 33.33 ± 1.0 | 34.12 ± 0.9 | 0.001 * |
| TªM Lateral right | 32.81 ± 1.0 | 33.32 ± 0.8 | 0.029 * |
| TªM Lateral left | 32.73 ± 1.0 | 33.07 ± 0.9 | 0.146 |
| TªM apex | 31.27 ± 1.2 | 31.43 ± 1.2 | 0.600 |
| body temperature | 33.71 ± 0.5 | 33.83 ± 0.4 | 0.288 |
| Variable | Taste Alteration | p Value | ||||
|---|---|---|---|---|---|---|
| No Alterations (n = 8) Mean ± ST | Metallic (n = 11) Mean ± ST | Bitter (n = 5) Mean ± ST | Disgeusia (n = 6) Mean ± ST | Others (n = 2) Mean ± ST | ||
| TªM Dorsum | 34.39 ± 0.8 | 34.16 ± 0.8 | 34.15 ± 0.6 | 33.20 ± 0.8 | 35.10 | 0.038 * |
| TªM Lateral right | 33.56 ± 0.7 | 33.28 ± 0.8 | 33.33 ± 0.9 | 32.73 ± 0.9 | 34.32 ± 0.5 | 0.196 |
| TªM Lateral left | 33.29 ± 0.8 | 33.04 ± 0.9 | 33.19 ± 0.6 | 32.29 ± 0.8 | 34.35 ± 0.2 | 0.072 |
| TªM Apex | 31.45 ± 1.4 | 31.27 ± 0.8 | 32.33 ± 1.1 | 30.59 ± 1.3 | 32.75 ± 1.5 | 0.171 |
| TªM body | 33.90 ± 0.4 | 33.94 ± 0.3 | 33.59 ± 0.4 | 33.69 ± 0.4 | 33.98 | 0.181 |
| TªM environmental | 21.28 ± 2.1 | 21.63 ± 1.1 | 21.35 ± 1.3 | 22.60 ± 1.0 | 22.75 ± 0.8 | 0.203 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas-Rodriguez, E.; Garcia-Martinez, A.; Molino-Pagan, D.; Marin-Martinez, L.; Pons-Fuster, E.; López-Jornet, P. Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 8903. https://doi.org/10.3390/ijerph19158903
Nicolas-Rodriguez E, Garcia-Martinez A, Molino-Pagan D, Marin-Martinez L, Pons-Fuster E, López-Jornet P. Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study. International Journal of Environmental Research and Public Health. 2022; 19(15):8903. https://doi.org/10.3390/ijerph19158903
Chicago/Turabian StyleNicolas-Rodriguez, Elena, Ana Garcia-Martinez, Diana Molino-Pagan, Luis Marin-Martinez, Eduardo Pons-Fuster, and Pia López-Jornet. 2022. "Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study" International Journal of Environmental Research and Public Health 19, no. 15: 8903. https://doi.org/10.3390/ijerph19158903
APA StyleNicolas-Rodriguez, E., Garcia-Martinez, A., Molino-Pagan, D., Marin-Martinez, L., Pons-Fuster, E., & López-Jornet, P. (2022). Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study. International Journal of Environmental Research and Public Health, 19(15), 8903. https://doi.org/10.3390/ijerph19158903







