Perception of COVID-19 Booster Dose Vaccine among Healthcare Workers in India and Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Measures
2.4. Socio-Demographic and Past Vaccination History Information
2.5. COVID-19 Booster Vaccine Perception
2.6. Statistical Analysis
3. Results
3.1. Participants (Demographics)
3.2. Willingness to Take CBD
3.3. Reasons for Not Willing to Take CBD
3.4. Perception about CBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.W.; Gao, H.; Wong, J.Y.; Xiao, J.; Shiu, E.Y.C.; Ryu, S.; Cowling, B.J. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg. Infect. Dis. 2020, 26, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Schaffer DeRoo, S.; Pudalov, N.J.; Fu, L.Y. Planning for a COVID-19 vaccination program. JAMA 2020, 323, 2458–2459. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, Z.; Huang, J.; Yan, N.; Chen, Q.; Huang, F.; Zhang, Y.; Akinwunmi, O.M.; Akinwunmi, B.O.; Zhang, C.J.P.; et al. A comparison of vaccine hesitancy of COVID-19 vaccination in china and the united states. Vaccines 2021, 9, 649. [Google Scholar] [CrossRef]
- Remmel, A. Covid vaccines and safety: What the research says. Nature 2021, 590, 538–540. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ 2021, 372, n149. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Larson, H.J.; Clarke, R.M.; Jarrett, C.; Eckersberger, E.; Levine, Z.; Schulz, W.S.; Paterson, P. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1599–1609. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Elbarazi, I.; Al-Hamad, S.; Aldhaheri, R.; Sheek-Hussein, M.; Narchi, H. Vaccine hesitancy and its determinants among arab parents: A cross-sectional survey in the united arab emirates. Hum. Vaccines Immunother. 2020, 16, 3163–3169. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the uk. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in england. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against COVID-19 by bnt162b2 booster across age groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef]
- Patalon, T.; Gazit, S.; Pitzer, V.E.; Prunas, O.; Warren, J.L.; Weinberger, D.M. Short term reduction in the odds of testing positive for SARS-CoV-2; a comparison between two doses and three doses of the bnt162b2 vaccine. medRxiv 2021. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mrna-1273 vaccine recipients. Clin. Infect. Dis 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Pyhala, R.; Alanko, S.; Forsten, T.; Haapa, K.; Kinnunen, L.; Jaaskivi, M.; Visakorpi, R.; Valle, M. Early kinetics of antibody response to inactivated influenza vaccine. Clin. Diagn. Virol. 1994, 1, 271–278. [Google Scholar] [CrossRef]
- Gallagher, T.; Lipsitch, M. Postexposure effects of vaccines on infectious diseases. Epidemiol. Rev. 2019, 41, 13–27. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Wood, R.; Gribben, C.; Caldwell, D.; Bishop, J.; Weir, A.; Kennedy, S.; Reid, M.; Smith-Palmer, A.; Goldberg, D.; et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: Nationwide linkage cohort study. BMJ 2020, 371, m3582. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef]
- Wagner, A.L.; Masters, N.B.; Domek, G.J.; Mathew, J.L.; Sun, X.; Asturias, E.J.; Ren, J.; Huang, Z.; Contreras-Roldan, I.L.; Gebremeskel, B.; et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines 2019, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Verger, P.; Fressard, L.; Collange, F.; Gautier, A.; Jestin, C.; Launay, O.; Raude, J.; Pulcini, C.; Peretti-Watel, P. Vaccine hesitancy among general practitioners and its determinants during controversies: A national cross-sectional survey in france. EBioMedicine 2015, 2, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Toth-Manikowski, S.M.; Swirsky, E.S.; Gandhi, R.; Piscitello, G. COVID-19 vaccination hesitancy among health care workers, communication, and policy-making. Am. J. Infect. Control 2022, 50, 20–25. [Google Scholar] [CrossRef]
- Pal, S.; Shekhar, R.; Kottewar, S.; Upadhyay, S.; Singh, M.; Pathak, D.; Kapuria, D.; Barrett, E.; Sheikh, A.B. COVID-19 vaccine hesitancy and attitude toward booster doses among us healthcare workers. Vaccines 2021, 9, 1358. [Google Scholar] [CrossRef]
- Yoshida, M.; Kobashi, Y.; Kawamura, T.; Shimazu, Y.; Nishikawa, Y.; Omata, F.; Zhao, T.; Yamamoto, C.; Kaneko, Y.; Nakayama, A.; et al. Factors associated with COVID-19 vaccine booster hesitancy: A retrospective cohort study, fukushima vaccination community survey. Vaccines 2022, 10, 515. [Google Scholar] [CrossRef]
- WHO. Health Workforce. Available online: https://www.who.int/data/gho/data/themes/health-workforce (accessed on 10 July 2022).
- Hawlader, M.D.H.; Rahman, M.L.; Nazir, A.; Ara, T.; Haque, M.M.A.; Saha, S.; Barsha, S.Y.; Hossian, M.; Matin, K.F.; Siddiquea, S.R.; et al. COVID-19 vaccine acceptance in south asia: A multi-country study. Int. J. Infect. Dis. 2022, 114, 1–10. [Google Scholar] [CrossRef]
- Reiter, P.L.; Pennell, M.L.; Katz, M.L. Acceptability of a COVID-19 vaccine among adults in the united states: How many people would get vaccinated? Vaccine 2020, 38, 6500–6507. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19): Vaccines. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(COVID-19)-vaccines (accessed on 13 July 2022).
- CDC. Covid Vaccines: Frequently Asked Questions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/faq.html (accessed on 13 July 2022).
- Karan, A.; Negandhi, H.; Hussain, S.; Zapata, T.; Mairembam, D.; De Graeve, H.; Buchan, J.; Zodpey, S. Size, composition and distribution of health workforce in india: Why, and where to invest? Hum. Resour. Health 2021, 19, 39. [Google Scholar] [CrossRef]
- MOHFA. Health Statistics. Available online: https://main.mohfw.gov.in/documents/Statistics (accessed on 10 July 2022).
- MOH. Statistical Yearbook. Available online: https://www.moh.gov.sa/en/Ministry/Statistics/book/Pages/default.aspx (accessed on 10 July 2022).
- Biswas, N.; Mustapha, T.; Khubchandani, J.; Price, J.H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J. Community Health 2021, 46, 1244–1251. [Google Scholar] [CrossRef]
- Kukreti, S.; Lu, M.Y.; Lin, Y.H.; Strong, C.; Lin, C.Y.; Ko, N.Y.; Chen, P.L.; Ko, W.C. Willingness of taiwan’s healthcare workers and outpatients to vaccinate against COVID-19 during a period without community outbreaks. Vaccines 2021, 9, 246. [Google Scholar] [CrossRef]
- Fares, S.; Elmnyer, M.M.; Mohamed, S.S.; Elsayed, R. COVID-19 vaccination perception and attitude among healthcare workers in egypt. J. Prim. Care Community Health 2021, 12, 21501327211013303. [Google Scholar] [CrossRef]
- Elharake, J.A.; Galal, B.; Alqahtani, S.A.; Kattan, R.F.; Barry, M.A.; Temsah, M.H.; Malik, A.A.; McFadden, S.M.; Yildirim, I.; Khoshnood, K.; et al. COVID-19 vaccine acceptance among health care workers in the kingdom of saudi arabia. Int. J. Infect. Dis. 2021, 109, 286–293. [Google Scholar] [CrossRef]
- Sung, M.; Huang, Y.; Duan, Y.; Liu, F.; Jin, Y.; Zheng, Z. Pharmaceutical industry’s engagement in the global equitable distribution of COVID-19 vaccines: Corporate social responsibility of eul vaccine developers. Vaccines 2021, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, G.; Kashyap, A.; Singh, G.; Singh Sandhu, H.; Khilji, I.; Singh Gambhir, R. Attitude and acceptance of COVID-19 vaccine amongst medical and dental fraternity—A questionnaire survey. Rocz. Państwowego Zakładu Hig. 2021, 72, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Rana, R.K.; Kumari, M.; Jha, R.R.; Bhushan, R.; Verma, R.K. A cross sectional study exploring determinants for vaccine awareness, belief and hesitancy among health care professionals regarding COVID-19 vaccine, findings from a teaching hospital based in coal capital of india. J. Fam. Med. Prim. Care 2021, 10, 4578–4585. [Google Scholar]
- CDC. Possible Side Effects after Getting a COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (accessed on 5 April 2022).
- Jain, J.; Saurabh, S.; Kumar, P.; Verma, M.K.; Goel, A.D.; Gupta, M.K.; Bhardwaj, P.; Raghav, P.R. COVID-19 vaccine hesitancy among medical students in india. Epidemiol. Infect. 2021, 149, e132. [Google Scholar] [CrossRef]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of influenza vaccination intention and behavior–A systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mohanan, L.; Pokorna, A. COVID-19 vaccine booster hesitancy (vbh) of healthcare workers in czechia: National cross-sectional study. Vaccines 2021, 9, 1437. [Google Scholar] [CrossRef]
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A narrative review of COVID-19 vaccines. Vaccines 2021, 10, 62. [Google Scholar] [CrossRef]
- Alhasan, K.; Aljamaan, F.; Temsah, M.H.; Alshahrani, F.; Bassrawi, R.; Alhaboob, A.; Assiri, R.; Alenezi, S.; Alaraj, A.; Alhomoudi, R.I.; et al. COVID-19 delta variant: Perceptions, worries, and vaccine-booster acceptability among healthcare workers. Healthcare 2021, 9, 1566. [Google Scholar] [CrossRef]
- Khan, W.H.; Hashmi, Z.; Goel, A.; Ahmad, R.; Gupta, K.; Khan, N.; Alam, I.; Ahmed, F.; Ansari, M.A. COVID-19 pandemic and vaccines update on challenges and resolutions. Front. Cell Infect. Microbiol. 2021, 11, 690621. [Google Scholar] [CrossRef]
- Koh, S.W.C.; Tan, H.M.; Lee, W.H.; Mathews, J.; Young, D. COVID-19 vaccine booster hesitancy among healthcare workers: A retrospective observational study in singapore. Vaccines 2022, 10, 464. [Google Scholar] [CrossRef]

| Demographic Items | India | Saudi Arabia | p-Value | ||
|---|---|---|---|---|---|
| Number (%) | Number (%) | ||||
| Sample size, n | 530 (64) | 303 (36) | |||
| Gender | |||||
| Male | 154 (29) | 219 (72) | <0.001 * | ||
| Female | 376 (71) | 84 (28) | |||
| Age interval (in years) | |||||
| 21–30 | 392 (74) | 214 (71) | 0.333 | ||
| 31–40 | 66 (12) | 46 (15) | |||
| >40 | 72 (14) | 43 (14) | |||
| Highest educational level | |||||
| Undergraduate | 413 (78) | 137 (45) | <0.001 * | ||
| Post-graduate | 100 (19) | 123 (41) | |||
| PhD | 17 (3) | 43 (14) | |||
| Residence | |||||
| Rural | 197 (37) | 143 (47) | <0.001 * | ||
| Urban | 333 (63) | 160 (53) | |||
| Have you received all the necessary vaccines in your lifetime? | Yes | No | Yes | No | 0.794 |
| 410 (77%) | 120 (23%) | 232 (77%) | 71 (23%) | ||
| Do you know about the COVID-19 vaccine? | 521 (98) | 9 (2) | 256(84) | 47(16) | <0.001 * |
| Have you received two doses of COVID-19 vaccines? | 514 (97) | 16 (3) | 241 (79) | 62 (21) | <0.001 * |
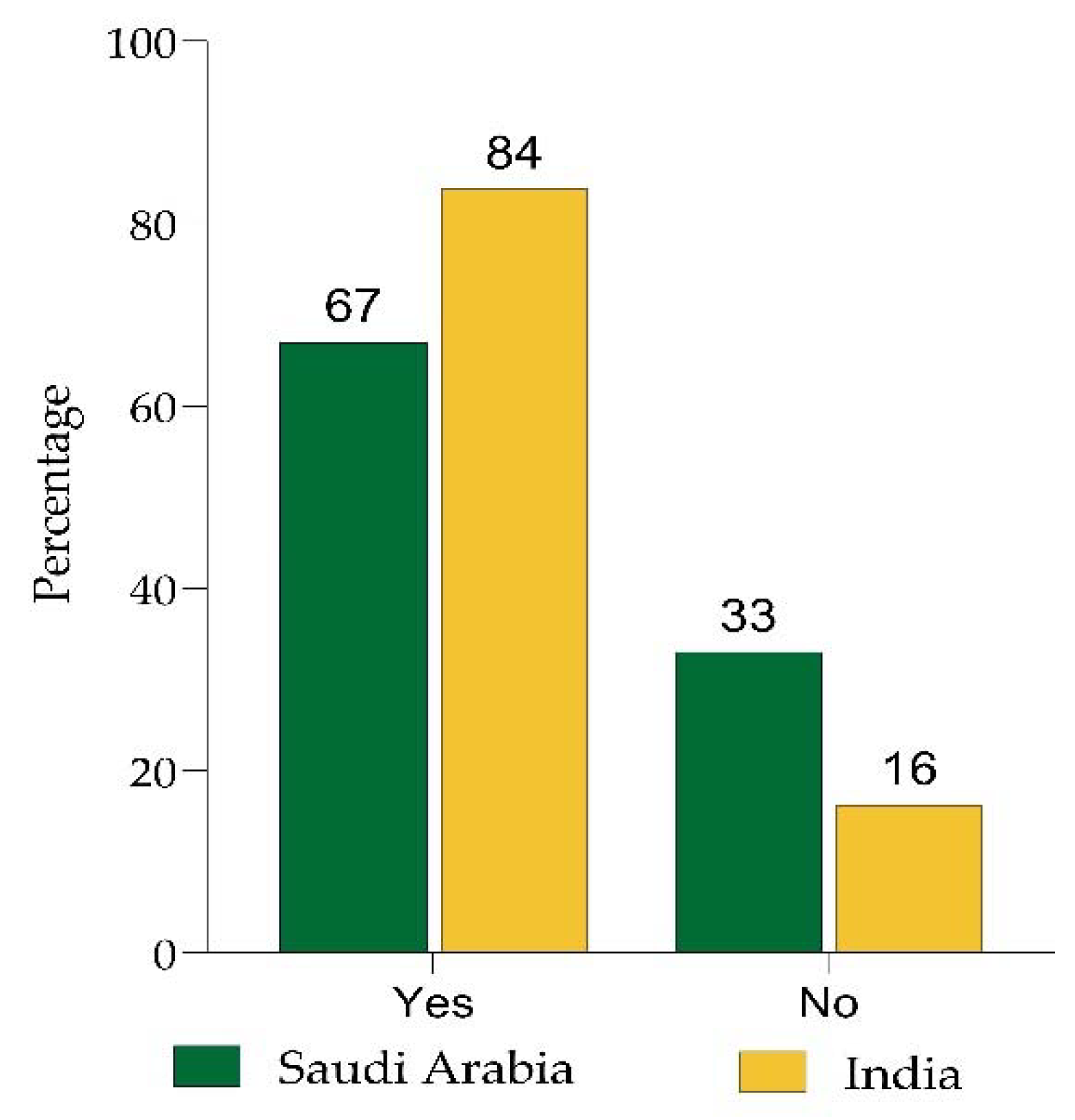
| Are you willing to take the COVID-19 booster vaccine or take it without any hesitation? | 444 (84) | 86 (16) | 203 (67) | 100 (33) | <0.001 * |
| Questions | India | Saudi Arabia | p Value | ||
|---|---|---|---|---|---|
| Number (%) | Number (%) | ||||
| Yes | No | Yes | No | ||
| Do you believe that the COVID-19 vaccine is safe? | 442 (83) | 88 (17) | 206 (68%) | 97 (32%) | <0.001 * |
| Do you think that COVID-19 booster vaccination has adverse reactions? | 76 (14) | 454 (86) | 135 (44) | 168 (56) | <0.001 * |
| Do you encourage your family/friends/relatives to get the booster COVID-19 vaccine? | 420 (79) | 110 (201) | 191 (73) | 112 (27) | <0.001 * |
| Do you believe the COVID-19 booster vaccine can reduce the spread of COVID-19? | 335 (63) | 195 (367) | 183 (60) | 120 (40) | 0.421 |
| Do you believe the COVID-19 booster vaccine can reduce the complications associated with COVID-19? | 387 (73) | 143 (27) | 207 (68) | 96 (32) | 0.149 |
| Do you think that if everyone in society maintains the preventive measures, the COVID-19 pandemic can be eradicated without vaccination? | 200 (38) | 330 (62) | 147 (48) | 156 (52) | 0.002* |
| Do you think Pharmaceutical companies have developed safe and effective COVID-19 vaccines? | 307 (58) | 223 (42) | 175 (58) | 128 (42) | 0.926 |
| Have you received COVID- 19 booster dose because it is mandatory? | 150 (28) | 380 (72) | 169 (56) | 134 (44) | <0.001 * |
| Do you think Mix-Matching the booster dose is safe and effective? | 87 (16) | 443 (84) | 148 (49) | 155 (51) | <0.001 * |
| Do you believe that only high-risk individuals such as health care workers and elderly persons with other diseases only need a booster dose? | 94 (18) | 436 (82) | 146 (48) | 157 (52) | <0.001 * |
| India | Saudi Arabia | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval for OR | 95% Confidence Interval for OR | |||||||
| Sig. | OR | Lower | Upper | Sig. | OR | Lower | Upper | |
| Age | 0.003 | 1.10 | 0.61 | 1.99 | 0.749 | 1.08 | 0.66 | 1.77 |
| Sex (Male) | 0.001 | 1.21 | 0.54 | 2.72 | 0.338 | 1.47 | 0.67 | 3.21 |
| Education | 0.147 | 0.51 | 0.20 | 1.27 | 0.481 | 1.19 | 0.73 | 1.95 |
| Residence (Urban) | 0.001 | 0.65 | 1.31 | 1.37 | 0.363 | 1.38 | 0.69 | 2.82 |
| Do you think that COVID-19 booster vaccination has adverse reactions? (Yes) | 0.285 | 1.59 | 0.68 | 3.73 | 0.604 | 0.83 | 0.42 | 1.66 |
| Do you encourage your family/friends/relatives to get the booster COVID-19 vaccine? (Yes) | 0 | 0.03 | 0.01 | 0.06 | 0 | 0.13 | 0.06 | 0.28 |
| Do you believe that the COVID-19 booster vaccine can reduce the spread of COVID-19? (Yes) | 0.653 | 0.82 | 0.35 | 1.93 | 0.687 | 0.86 | 0.41 | 1.80 |
| Do you believe the COVID-19 booster vaccine can reduce the complications associated with COVID-19? (Yes) | 0.244 | 0.61 | 0.26 | 1.41 | 0 | 0.21 | 0.10 | 0.45 |
| Do you think that if everyone in society maintains the preventive measures, the COVID-19 pandemic can be eradicated without vaccination? (Yes) | 0.394 | 0.73 | 0.35 | 1.51 | 0.623 | 1.19 | 0.59 | 2.41 |
| Do you think Pharmaceutical companies have developed safe and effective COVID-19 vaccines? (Yes) | 0.073 | 0.50 | 0.24 | 1.07 | 0.009 | 0.38 | 0.18 | 0.79 |
| Have you received COVID-19 booster dose because it is mandatory? (Yes) | 0.013 | 0.32 | 0.13 | 0.79 | 0.164 | 0.60 | 0.29 | 1.24 |
| Do you think Mix-Matching the booster dose is safe and effective? (Yes) | 0.001 | 1.16 | 0.35 | 3.81 | 0.19 | 1.59 | 0.27 | 1.29 |
| Do you believe that only high-risk individuals such as health care workers and elderly persons with other diseases only need a booster dose? (Yes) | 0.055 | 2.28 | 0.98 | 5.27 | 0.147 | 1.77 | 0.82 | 3.82 |
| Constant | 0 | 57.61 | 0 | 11.51 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellappally, S.; Naik, S.; Alsadon, O.; Al-Kheraif, A.A.; Alayadi, H.; Alsiwat, A.J.; Kumar, A.; Hashem, M.; Varghese, N.; Thomas, N.G.; et al. Perception of COVID-19 Booster Dose Vaccine among Healthcare Workers in India and Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 8942. https://doi.org/10.3390/ijerph19158942
Vellappally S, Naik S, Alsadon O, Al-Kheraif AA, Alayadi H, Alsiwat AJ, Kumar A, Hashem M, Varghese N, Thomas NG, et al. Perception of COVID-19 Booster Dose Vaccine among Healthcare Workers in India and Saudi Arabia. International Journal of Environmental Research and Public Health. 2022; 19(15):8942. https://doi.org/10.3390/ijerph19158942
Chicago/Turabian StyleVellappally, Sajith, Sachin Naik, Omar Alsadon, Abdulaziz Abdullah Al-Kheraif, Haya Alayadi, Areej Jaber Alsiwat, Aswini Kumar, Mohamed Hashem, Nibu Varghese, Nebu George Thomas, and et al. 2022. "Perception of COVID-19 Booster Dose Vaccine among Healthcare Workers in India and Saudi Arabia" International Journal of Environmental Research and Public Health 19, no. 15: 8942. https://doi.org/10.3390/ijerph19158942







