Change in Bone Mineral Density in Stroke Patients with Osteoporosis or Osteopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.2.1. BMD Test
2.2.2. Biochemical Markers and Hematology Tests
2.2.3. Functional Evaluation
2.2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Changes in BMD
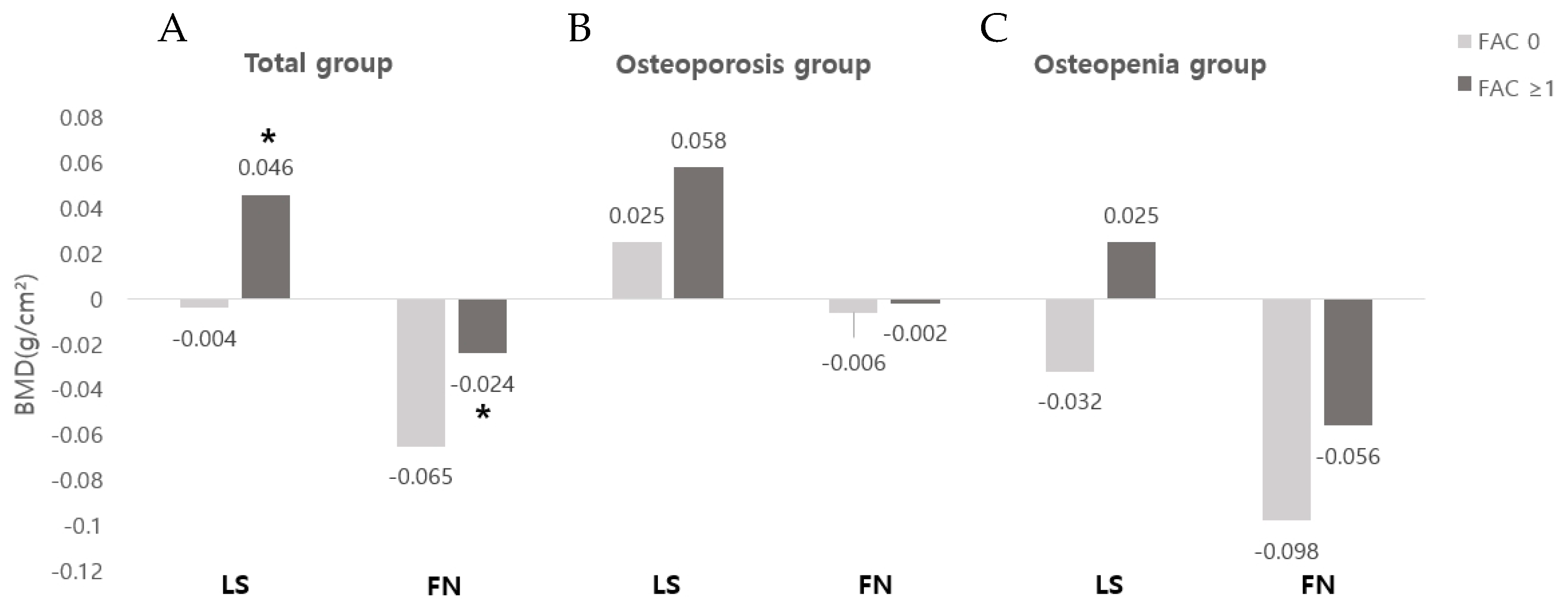
3.3. Correlation between Functional Evaluation and Changes in BMD
3.4. Correlation between BMD and Clinical Variables in Stroke Patients
3.5. Changes in Biochemical Markers and Hematology Tests
3.6. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: Highlights of the conference. South Med. J. 2001, 94, 569–573.
- Carda, S.; Cisari, C.; Invernizzi, M.; Bevilacqua, M. Osteoporosis after stroke: A review of the causes and potential treatments. Cerebrovasc. Dis. 2009, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.C.; Moore, S.W.; Cancellaro, V.A.; Harvill, L.M. Long-term effects of strokes on bone mass. Am. J. Phys. Med. Rehabil. 1995, 74, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lee, J.G.; Kim, B.J.; Kim, J.Y.; Lee, K.J.; Han, M.K.; Park, J.M.; Kang, K.; Cho, Y.J.; Park, H.K.; et al. The Epidemiology of Fracture in Patients with Acute Ischemic Stroke in Korea. J. Korean Med. Sci. 2019, 34, e164. [Google Scholar] [CrossRef]
- Dennis, M.S.; Lo, K.M.; McDowall, M.; West, T. Fractures after stroke: Frequency, types, and associations. Stroke 2002, 33, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Kang, E.; Im, S.; Ko, Y.J.; Im, S.A.; Lee, J.I. Prevalence of pre-stroke low bone mineral density and vertebral fracture in first stroke patients. Bone 2008, 43, 183–186. [Google Scholar] [CrossRef]
- Kanis, J.; Oden, A.; Johnell, O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke 2001, 32, 702–706. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Risedronate therapy for prevention of hip fracture after stroke in elderly women. Neurology 2005, 64, 811–816. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J., 3rd. Clinical practice. Osteopenia. N. Engl. J. Med. 2007, 356, 2293–2300. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals 2020, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Tanvetyanon, T.; Stiff, P.J. Management of the adverse effects associated with intravenous bisphosphonates. Ann. Oncol. 2006, 17, 897–907. [Google Scholar] [CrossRef]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones 2009, 8, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meissner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Burnfield, J.M.; Josephson, K.R.; Powers, C.M.; Rubenstein, L.Z. The influence of lower extremity joint torque on gait characteristics in elderly men. Arch. Phys. Med. Rehabil. 2000, 81, 1153–1157. [Google Scholar] [CrossRef]
- Kulmala, J.P.; Ayramo, S.; Avela, J. Knee extensor and flexor dominant gait patterns increase the knee frontal plane moment during walking. J. Orthop. Res. 2013, 31, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Honda, Y.; Kuno, H.; Oizumi, K. Menatetrenone ameliorates osteopenia in disuse-affected limbs of vitamin D- and K-deficient stroke patients. Bone 1998, 23, 291–296. [Google Scholar] [CrossRef]
- Whitaker, M.; Guo, J.; Kehoe, T.; Benson, G. Bisphosphonates for osteoporosis—Where do we go from here? N. Engl. J. Med. 2012, 366, 2048–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuzer, G.; Ataman, S.; Suldur, N.; Atay, M. Bone mineral density in patients with stroke. Int. J. Rehabil. Res. 2002, 25, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R. How drugs decrease fracture risk: Lessons from trials. J. Musculoskelet. Neuronal Interact. 2002, 2, 198–200. [Google Scholar]
- Chen, Y.C.; Wu, J.C.; Liu, L.; Huang, W.C.; Cheng, H.; Chen, T.J.; Thien, P.F.; Lo, S.S. Hospitalized osteoporotic vertebral fracture increases the risk of stroke: A population-based cohort study. J. Bone Miner. Res. 2013, 28, 516–523. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Chen, Z.; Xie, X.; Gu, F.; Bi, S.; Yu, T. Effects of Bisphosphonates Treatments in Osteopenic Older Women: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 892091. [Google Scholar] [CrossRef]
- Ramnemark, A.; Nyberg, L.; Borssen, B.; Olsson, T.; Gustafson, Y. Fractures after stroke. Osteoporos. Int. 1998, 8, 92–95. [Google Scholar] [CrossRef]
- Sahin, L.; Ozoran, K.; Gunduz, O.H.; Ucan, H.; Yucel, M. Bone mineral density in patients with stroke. Am. J. Phys. Med. Rehabil. 2001, 80, 592–596. [Google Scholar] [CrossRef]
- Wolff, I.; van Croonenborg, J.J.; Kemper, H.C.; Kostense, P.J.; Twisk, J.W. The effect of exercise training programs on bone mass: A meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos. Int. 1999, 9, 1–12. [Google Scholar] [CrossRef]
- Jorgensen, L.; Jacobsen, B.K.; Wilsgaard, T.; Magnus, J.H. Walking after stroke: Does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos. Int. 2000, 11, 381–387. [Google Scholar] [CrossRef]
- Borschmann, K.; Iuliano, S.; Ghasem-Zadeh, A.; Churilov, L.; Pang, M.Y.C.; Bernhardt, J. Upright activity and higher motor function may preserve bone mineral density within 6 months of stroke: A longitudinal study. Arch. Osteoporos. 2018, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilibeck, P.D.; Sale, D.G.; Webber, C.E. Exercise and bone mineral density. Sports Med. 1995, 19, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; Reeve, J.; Warburton, E.A. Falls, fractures, and osteoporosis after stroke: Time to think about protection? Stroke 2002, 33, 1432–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga de Castro Machado, A.; Hannon, R.; Eastell, R. Monitoring alendronate therapy for osteoporosis. J. Bone Miner. Res. 1999, 14, 602–608. [Google Scholar] [CrossRef]
- Paker, N.; Bugdayci, D.; Tekdos, D.; Dere, C.; Kaya, B. Relationship between bone turnover and bone density at the proximal femur in stroke patients. J. Stroke Cerebrovasc. Dis. 2009, 18, 139–143. [Google Scholar] [CrossRef]
- Trento, L.K.; Pietropolli, A.; Ticconi, C.; Gravotta, E.; De Martino, M.U.; Fabbri, A.; Piccione, E. Role of type I collagen C telopeptide, bone-specific alkaline phosphatase and osteocalcin in the assessment of bone status in postmenopausal women. J. Obstet. Gynaecol. Res. 2009, 35, 152–159. [Google Scholar] [CrossRef]
- Hannon, R.; Eastell, R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos. Int. 2000, 11 (Suppl. S6), S30–S44. [Google Scholar] [CrossRef]
- Shinchuk, L.M.; Morse, L.; Huancahuari, N.; Arum, S.; Chen, T.C.; Holick, M.F. Vitamin D deficiency and osteoporosis in rehabilitation inpatients. Arch. Phys. Med. Rehabil. 2006, 87, 904–908. [Google Scholar] [CrossRef]
- Uluduz, D.; Adil, M.M.; Rahim, B.; Gilani, W.I.; Rahman, H.A.; Gilani, S.I.; Qureshi, A.I. Vitamin D deficiency and osteoporosis in stroke survivors: An analysis of National Health and Nutritional Examination Survey (NHANES). J. Vasc. Interv. Neurol. 2014, 7, 23–28. [Google Scholar]
- Poole, K.E.; Loveridge, N.; Barker, P.J.; Halsall, D.J.; Rose, C.; Reeve, J.; Warburton, E.A. Reduced vitamin D in acute stroke. Stroke 2006, 37, 243–245. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Osteoporosis (n = 63) | Osteopenia (n = 34) | |
|---|---|---|---|
| Mean age (year) | 73.6 ± 9.6 | 72.5 ± 9.4 | |
| Duration (month) | 4.3 ± 3.5 | 4.7 ± 3.7 | |
| Sex | Male | 5 | 4 |
| Female | 58 | 30 | |
| Type of stroke | Infarction | 44 | 29 |
| Hemorrhage | 19 | 5 | |
| MMT of hip and knee | Below F grade | 17 | 8 |
| F grade and over | 46 | 26 | |
| MBI (total) | ≤32 | 20 | 9 |
| >32 | 43 | 25 | |
| FAC | 0 | 15 | 6 |
| ≥1 | 48 | 28 | |
| Vitamin D | Normal (≥30 ng/mL) | 18 | 8 |
| Insufficiency (11–29 ng/mL) | 39 | 23 | |
| Deficiency (≤10 ng/mL) | 6 | 3 | |
| BMD Measures (g/cm²) | Osteoporosis | p-Value | |
|---|---|---|---|
| T0 | T1 | ||
| LS | 0.667 ± 0.141 | 0.722 ± 0.141 | 0.01 * |
| FN | 0.524 ± 0.073 | 0.526 ± 0.081 | 0.74 |
| BMD Measures (g/cm²) | Osteopenia | p-Value | |
| T0 | T1 | ||
| LS | 0.855 ± 0.053 | 0.865 ± 0.061 | 0.09 |
| FN | 0.674 ± 0.091 | 0.615 ± 0.057 | 0.01 * |
| LS (g/cm²) | FN (g/cm²) | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| MMT of hip and knee | 0.05 | 0.72 | 0.11 | 0.49 |
| FAC | 0.09 | 0.44 | 0.05 | 0.62 |
| MBI | 0.1 | 0.38 | 0.06 | 0.53 |
| OC | −0.16 | 0.32 | −0.19 | 0.07 |
| CTX | −0.08 | 0.42 | −0.23 | 0.08 |
| Vit D deficiency | 0.18 | 0.66 | 0.59 | 0.12 |
| Vit D insufficiency | 0.19 | 0.12 | 0.1 | 0.43 |
| Vit D normal | 0.07 | 0.71 | 0.19 | 0.33 |
| Blood Test Level | Osteoporosis | p-Value | |
|---|---|---|---|
| T0 | T1 | ||
| OC (ng/mL) | 13.2 ± 5.5 | 11.36 ± 5.1 | 0.02 * |
| CTX (ng/mL) | 0.3 ± 0.2 | 0.21 ± 0.1 | 0.01 * |
| Vitamin D (ng/mL) | 24.4 ± 10.5 | 23.1 ± 10.0 | 0.1 |
| Blood Test Level | Osteopenia | p-Value | |
| T0 | T1 | ||
| OC (ng/mL) | 12.71 ± 6.4 | 11.18 ± 2.5 | 0.4 |
| CTX (ng/mL) | 0.31 ± 0.2 | 0.28 ± 0.1 | 0.7 |
| Vitamin D (ng/mL) | 23.6 ± 10.3 | 22.5 ± 9.9 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Joo, M.-C. Change in Bone Mineral Density in Stroke Patients with Osteoporosis or Osteopenia. Int. J. Environ. Res. Public Health 2022, 19, 8954. https://doi.org/10.3390/ijerph19158954
Lee D-H, Joo M-C. Change in Bone Mineral Density in Stroke Patients with Osteoporosis or Osteopenia. International Journal of Environmental Research and Public Health. 2022; 19(15):8954. https://doi.org/10.3390/ijerph19158954
Chicago/Turabian StyleLee, Do-Hee, and Min-Cheol Joo. 2022. "Change in Bone Mineral Density in Stroke Patients with Osteoporosis or Osteopenia" International Journal of Environmental Research and Public Health 19, no. 15: 8954. https://doi.org/10.3390/ijerph19158954
APA StyleLee, D.-H., & Joo, M.-C. (2022). Change in Bone Mineral Density in Stroke Patients with Osteoporosis or Osteopenia. International Journal of Environmental Research and Public Health, 19(15), 8954. https://doi.org/10.3390/ijerph19158954






