Ideal P2Y12 Inhibitor in Acute Coronary Syndrome: A Review and Current Status
Abstract
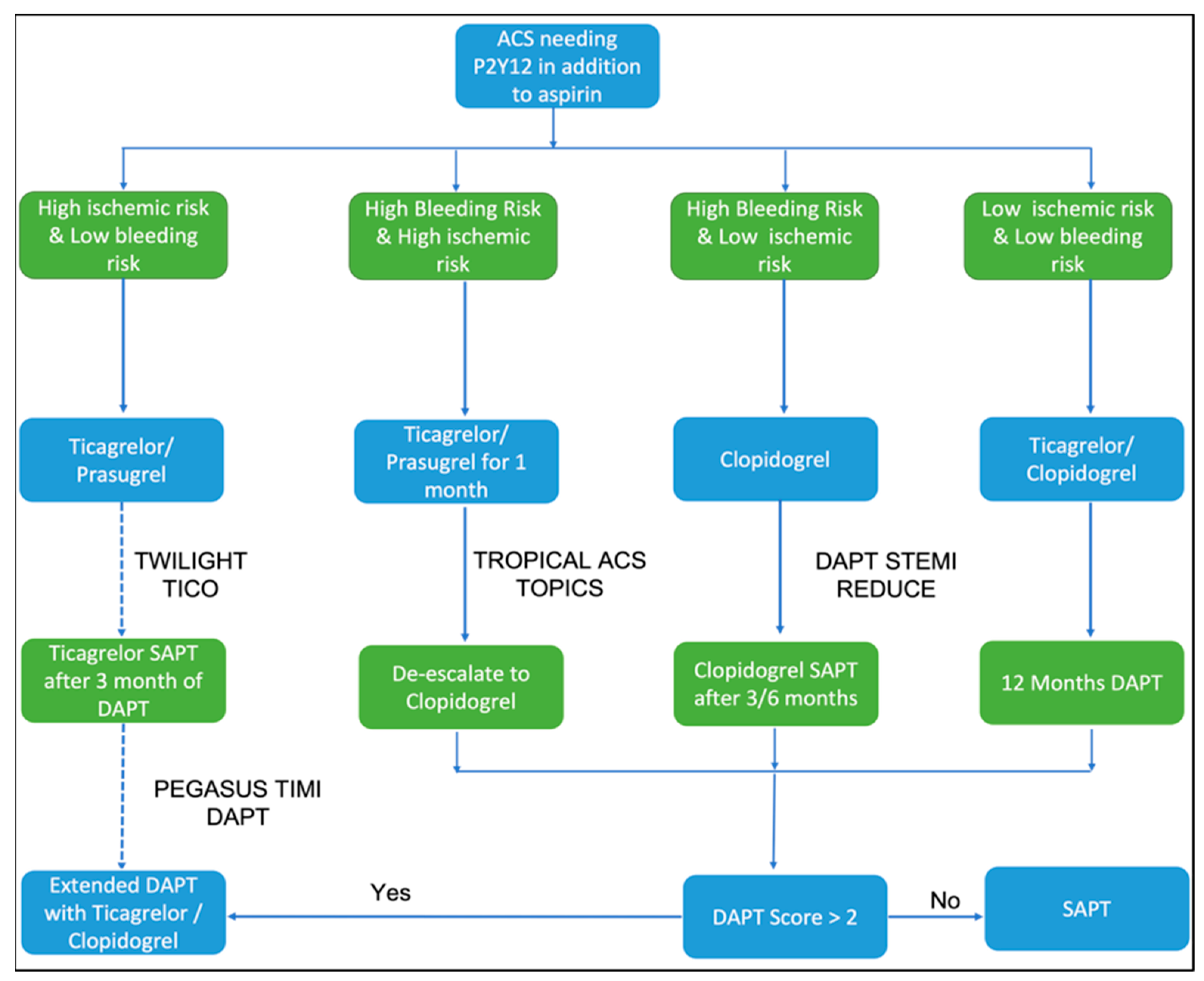
:1. Introduction
2. Evolution of Guidelines over the Years and Journey of Clopidogrel
3. Journey of Newer P2Y12i: Prasugrel, Ticagrelor, and Cangrelor
| Characteristics | Clopidogrel | Prasugrel | Ticagrelor | Ellinogrel | Cangrelor | Selatogrel | References |
|---|---|---|---|---|---|---|---|
| Chemical class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolo-pyrimidine | - | Nonthienopyridine adenosine triphosphate analogue | 2-phenylpyrimidine-4-carboxamide analogue | [1,2,3,34,35] |
| Receptor blockage | Irreversible | Irreversible | Reversible | Reversible | Reversible | Reversible | |
| Prodrug | Yes (prodrug, CYP dependent, 2 steps) | Yes (prodrug, CYP dependent, 1 step) | No | No | No | - | |
| Frequency | Oral, loading dose 300/600 mg, 75 mg once daily | Oral, loading dose 60 mg, then 10 mg/5 mg daily | Oral, loading dose 180 mg, then 90 mg twice daily | IV, single dose | 30 mcg/kg i.v. bolus prior to PCI followed immediately by an infusion of 4 mcg/kg/min continued for at least 2 h or for the duration of the PCI, whichever is longer | 8/16 mg subcutaneous injection | |
| Onset of effect | 2–8 h | 30 min–4 h | 30 min–2 h | Immediate within 2 min | Immediate: 2 min | 15 min, platelet inhibition | |
| Interaction with CYP targeted drugs | CYP2C19 | CYP3A4/CYP2B6 | CYP3A4 inhibitor | - | - | ||
| Effect lasts for | 7–10 days | 7–10 days | 3–5 days | Completely reversed within 24 h | Till infusion | Platelet inhibition maintained for 8 h and reversible within 24 h | |
| Steady state IPA | 40–62% | 70% | 80–90% | - | >90% | - | |
| Dose adjustment in kidney failure | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment | |
| Recommended withdrawal before surgery | 5 days | 7 days | 3–5 days | Normalization of platelet function with in 24 h | Normalization of platelet function within 60 min after discontinuation | Reversible platelet function within 24 h |
4. Individualization of DAPT Agent: Prasugrel vs. Ticagrelor vs. Cangrelor
5. ISAR REACT 5—Game Changer or Watershed
6. Upstream and Downstream P2Y12i Administration
7. P2Y12i in Special Scenarios—CKD and DM
8. Guideline Track and Pending Issues
9. Future Directions
10. Mitigation of Bleeding
11. Conclusions
Funding
Conflicts of Interest
References
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [Green Version]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A report of the ACC/AHA task force on clinical practice guidelines. Circulation 2016, 134, e123–e155, Correction in Circulation 2016, 134, e192–e194. [Google Scholar]
- Tan, J.W.; Chew, D.P.; Kader, M.A.S.A.; Ako, J.; Bahl, V.K.; Chan, M.; Park, K.W.; Chandra, P.; Hsieh, I.-C.; Huan, D.Q.; et al. 2020 Asian Pacific Society of Cardiology Consensus Recommendations on the Use of P2Y12 Receptor Antagonists in the Asia-Pacific Region. Eur. Cardiol. Rev. 2021, 16, e02. [Google Scholar] [CrossRef] [PubMed]
- Schüpke, S.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Kastrati, A.; Menichelli, M.; Neumann, F.-J.; Wöhrle, J.; Bernlochner, I.; Richardt, G.; Witzenbichler, B.; Sibbing, D.; Gewalt, S.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes and Diabetes Mellitus. JACC Cardiovasc. Interv. 2020, 13, 2238–2247. [Google Scholar] [CrossRef]
- Aytekin, A.; Ndrepepa, G.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Wöhrle, J.; Bernlochner, I.; Lahu, S.; Richardt, G.; Witzenbichler, B.; et al. Ticagrelor or Prasugrel in Patients with ST-Segment–Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circulation 2020, 142, 2329–2337. [Google Scholar] [CrossRef]
- Cattaneo, M. P2Y12 receptors: Structure and function. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S10–S16. [Google Scholar] [CrossRef]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus asp1irin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Steinhubl, S.R.; Berger, P.B.; Mann, J.T., III; Fry, E.T.A.; DeLago, A.; Wilmer, C.; Topol, E.J.; CREDO Investigators. Early and sustained dual oral anti platelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002, 288, 2411–2420, Correction in JAMA 2003, 289, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Jiang, L.; Chen, Y.; Xie, J.; Pan, H.; Peto, R.; Collins, R.; Liu, L.; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005, 366, 1607–1621. [Google Scholar] [CrossRef]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, P.H.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with c1opidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Cannon, C.P.; Gibson, C.M.; López-Sendón, J.L.; Montalescot, G.; Theroux, P.; Claeys, M.J.; Cools, F.; Hill, K.A.; Skene, A.M.; et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST segment elevation. N. Eng. J. Med. 2005, 352, 1179–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggish, A.L.; Sabatine, M.S. Clopidogrel use in coronary artery disease. Expert Rev. Cardiovasc. Ther. 2006, 4, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Husted, S. New developments in oral antiplatelet therapy. Eur. Heart J. Suppl. 2007, 9, D20–D27. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.R.; Tanguay, J.-F.; Eikelboom, J.W.; Jolly, S.S.; Joyner, C.D.; Granger, C.B.; Faxon, D.P.; Rupprecht, H.-J.; Budaj, A.; Avezum, A.; et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): A randomised factorial trial. Lancet 2010, 376, 1233–1243. [Google Scholar] [CrossRef]
- Price, M.J.; Berger, P.B.; Teirstein, P.S.; Tanguay, J.-F.; Angiolillo, D.J.; Spriggs, D.; Puri, S.; Robbins, M.; Garratt, K.N.; Bertrand, O.F.; et al. Standard- vs. High-Dose Clopidogrel Based on Platelet Function Testing after Percutaneous Coronary Intervention: The GRAVITAS Randomized Trial. JAMA 2011, 305, 1097–1105. [Google Scholar] [CrossRef]
- Collet, J.-P.; Cuisset, T.; Rangé, G.; Cayla, G.; Elhadad, S.; Pouillot, C.; Henry, P.; Motreff, P.; Carrié, D.; Boueri, Z.; et al. Bedside Monitoring to Adjust Antiplatelet Therapy for Coronary Stenting. N. Engl. J. Med. 2012, 367, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.-H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs. Conventional Clopidogrel Therapy on Ischemic Outcomes after Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roe, M.T.; Armstrong, P.W.; Fox, K.A.; White, H.D.; Prabhakaran, D.; Goodman, S.G.; Cornel, J.H.; Bhatt, D.L.; Clemmensen, P.; Martinez, F.; et al. Prasugrel versus Clopidogrel for Acute Coronary Syndromes without Revascularization. N. Engl. J. Med. 2012, 367, 1297–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalescot, G.; Bolognese, L.; Dudek, D.; Goldstein, P.; Hamm, C.; Tanguay, J.-F.; Berg, J.M.T.; Miller, D.L.; Costigan, T.M.; Goedicke, J.; et al. Pretreatment with Prasugrel in Non–ST-Segment Elevation Acute Coronary Syndromes. N. Engl. J. Med. 2013, 369, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.-A.; Pereira, N. Pharmacogenomic Impact of CYP2C19 Variation on Clopidogrel Therapy in Precision Cardiovascular Medicine. J. Pers. Med. 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Antonino, M.J.; Wei, C.; Teng, R.; Rasmussen, L.; Storey, R.; Nielsen, T.; Eikelboom, J.; et al. Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies: The RESPOND Study. Circulation 2010, 121, 1188–1199. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Shemisa, K.; Vaduganathan, M.; Qamar, A.; Gupta, A.; Garg, S.K.; Kumbhani, D.J.; Mayo, H.; Khalili, H.; Pandey, A.; et al. Premature Ticagrelor Discontinuation in Secondary Prevention of Atherosclerotic CVD: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2454–2464. [Google Scholar] [CrossRef]
- Corpataux, N.; Valgimigli, M. P2Y12 inhibitors during and after acute coronary syndrome, where do we stand in 2020? Hematol. Med. Oncol. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Berwanger, O.; Lopes, R.D.; Moia, D.D.; Fonseca, F.A.; Jiang, L.; Goodman, S.G.; Nicholls, S.J.; Parkhomenko, A.; Averkov, O.; Tajer, C.; et al. Ticagrelor Versus Clopidogrel in Patients with STEMI Treated with Fibrinolysis: TREAT Trial. J. Am. Coll. Cardiol. 2019, 73, 2819–2828. [Google Scholar] [CrossRef]
- Kheiri, B.; Osman, M.; Abdalla, A.; Haykal, T.; Barbarawi, M.; Zayed, Y.; Hicks, M.; Ahmed, S.; Bachuwa, G.; Hassan, M.; et al. Ticagrelor versus clopidogrel after fibrinolytic therapy in patients with ST-elevation myocardial infarction: A systematic review and meta-analysis of randomized clinical trials. J. Thromb. Thrombolysis 2018, 46, 299–303. [Google Scholar] [CrossRef]
- Montalescot, G.; van’t Hof, A.W.V.; Lapostolle, F.; Silvain, J.; Lassen, J.F.; Bolognese, L.; Cantor, W.J.; Cequier, Á.; Chettibi, M.; Goodman, S.G.; et al. Prehospital Ticagrelor in ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2014, 371, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abtan, J.; Steg, P.G.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Abnousi, F.; Prats, J.; Deliargyris, E.N.; et al. Efficacy and Safety of Cangrelor in Preventing Periprocedural Complications in Patients with Stable Angina and Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: The CHAMPION PHOENIX Trial. JACC Cardiovasc. Interv. 2016, 9, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Bhatt, D.L.; Stone, G.W.; White, H.D.; Steg, P.G.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Prats, J.; et al. Consistent reduction in periprocedural myocardial infarction with cangrelor as assessed by multiple definitions: Finding from CHAMPION PHOENIX (Cangrelor versus standard therapy to achieve optimal management of platelet inhibition). Circulation 2016, 134, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steg, P.G.; Bhatt, D.L.; Hamm, C.W.; Stone, G.W.; Gibson, C.M.; Mahaffey, K.W.; Leonardi, S.; Liu, T.; Skerjanec, S.; Day, J.R.; et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 2013, 382, 1981–1992. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ferri, N.; Corsini, A.; Bellosta, S. Pharmacology of the New P2Y12 Receptor Inhibitors: Insights on Pharmacokinetic and Pharmacodynamic Properties. Drugs 2013, 73, 1681–1709. [Google Scholar] [CrossRef]
- Motovska, Z.; Hlinomaz, O.; Miklik, R.; Hromadka, M.; Varvarovsky, I.; Dušek, J.; Knot, J.; Jarkovsky, J.; Kala, P.; Rokyta, R.; et al. Prasugrel Versus Ticagrelor in Patients with Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation 2016, 134, 1603–1612. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Shi, J.-X.; Huang, F. Head to head comparison of Prasugrel versus Ticagrelor in patients with acute coronary syndrome: A systematic review and meta-analysis of randomized trials. BMC Pharmacol. Toxicol. 2017, 18, 80. [Google Scholar] [CrossRef]
- Perrone, M.A.; Pieri, M.; Marchei, M.; Sergi, D.; Bernardini, S.; Romeo, F. Serum free light chains in patients with ST elevation myocardial infarction (STEMI): A possible correlation with left ventricle dysfunction. Int. J. Cardiol. 2019, 292, 32–34. [Google Scholar] [CrossRef]
- Iellamo, F.; Perrone, M.A.; Caminiti, G.; Volterrani, M.; Legramante, J.M. Post-exercise Hypotension in Patients With Coronary Artery Disease. Front Physiol. 2021, 12, 788591. [Google Scholar] [CrossRef]
- Valina, C.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Wöhrle, J.; Bernlochner, I.; Aytekin, A.; Richardt, G.; Witzenbichler, B.; Sibbing, D.; et al. Ticagrelor or Prasugrel in Patients with Non–ST-Segment Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2020, 76, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Khan, S.U.; Kołodziejczak, M.; Kubica, J.; Buccheri, S.; Cannon, C.P.; Gurbel, P.A.; De Servi, S.; Budaj, A.; Bartorelli, A.; et al. Comparative Efficacy and Safety of Oral P2Y12Inhibitors in Acute Coronary Syndrome: Network Meta-Analysis of 52 816 Patients from 12 Randomized Trials. Circulation 2020, 142, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Mojoli, M.; Varbella, F.; Caporale, R.; Rigattieri, S.; Andò, G.; Cirillo, P.; Pierini, S.; Santarelli, A.; Sganzerla, P.; et al. Timing of Oral P2Y12 Inhibitor Administration in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 76, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Angiolillo, D.J. Pre-Treatment with Oral P2Y12 Inhibitors in Acute Coronary Syndromes without ST-Segment Elevation: The Saga Continues. J. Am. Coll. Cardiol. 2019, 73, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Moisi, M.I.; Bungau, S.G.; Vesa, C.M.; Diaconu, C.C.; Behl, T.; Stoicescu, M.; Toma, M.M.; Bustea, C.; Sava, C.; Popescu, M.I. Framing Cause-Effect Relationship of Acute Coronary Syndrome in Patients with Chronic Kidney Disease. Diagnostics 2021, 11, 1518. [Google Scholar] [CrossRef]
- Moisi, M.I.; Rus, M.; Bungau, S.; Zaha, D.C.; Uivarosan, D.; Fratila, O.; Tit, D.M.; Endres, L.; Nistor-Cseppento, D.C.; Popescu, M.I. Acute Coronary Syndromes in Chronic Kidney Disease: Clinical and Therapeutic Characteristics. Medicina 2020, 56, 118. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Caillard, S.; Jesel, L.; El Ghannudi, S.; Ohlmann, P.; Sauleau, E.; Hannedouche, T.; Gachet, C.; Moulin, B.; Morel, O. Association of Estimated GFR with Platelet Inhibition in Patients Treated with Clopidogrel. Am. J. Kidney Dis. 2012, 59, 777–785. [Google Scholar] [CrossRef]
- Konishi, A.; Shinke, T.; Otake, H.; Takaya, T.; Osue, T.; Kinutani, H.; Kuroda, M.; Takahashi, H.; Terashita, D.; Hirata, K.-I.; et al. Impact of residual platelet reactivity under clopidogrel treatment for lesions and the clinical outcome after drug-eluting stent implantation in patients with hemodialysis. J. Cardiol. 2016, 67, 531–537. [Google Scholar] [CrossRef] [Green Version]
- James, S.; Budaj, A.; Aylward, P.; Buck, K.K.; Cannon, C.P.; Cornel, J.H.; Harrington, R.A.; Horrow, J.; Katus, H.; Keltai, M.; et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010, 122, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, O.; D’Ascenzo, F.; Raposeiras-Roubin, S.; Abu-Assi, E.; Peyracchia, M.; Bocchino, P.P.; Kinnaird, T.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; et al. P2Y12 inhibitors in acute coronary syndrome patients with renal dysfunction: An analysis from the RENAMI and BleeMACS projects. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 31–42. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Kang, J.; Kim, M.; Geum, M.J.; Kim, S.; Rhie, S. P2Y12 Antiplatelet Choice for Patients with Chronic Kidney Disease and Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and Mortality following Acute Coronary Syndromes. JAMA J. Am. Med. Assoc. 2007, 298, 765–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angiolillo, D.J.; Jakubowski, J.A.; Ferreiro, J.L.; Tello-Montoliu, A.; Rollini, F.; Franchi, F.; Ueno, M.; Darlington, A.; Desai, B.; Moser, B.A.; et al. Impaired Responsiveness to the Platelet P2Y12 Receptor Antagonist Clopidogrel in Patients with Type 2 Diabetes and Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 1005–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angiolillo, D.J.; Shoemaker, S.B.; Desai, B.; Yuan, H.; Charlton, R.K.; Bernardo, E.; Zenni, M.M.; Guzman, L.A.; Bass, T.A.; Costa, M.A. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: Results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007, 115, 708–716. [Google Scholar] [CrossRef] [Green Version]
- James, S.; Angiolillo, D.J.; Cornel, J.; Erlinge, D.; Husted, S.; Kontny, F.; Maya, J.; Nicolau, J.; Spinar, J.; Storey, R.; et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J. 2010, 31, 3006–3016. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; Angiolillo, D.J.; Meisel, S.; Dalby, A.J.; Verheugt, F.W.; Goodman, S.G.; Corbalan, R.; Purdy, D.A.; Murphy, S.A.; et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation 2008, 118, 1626–1636. [Google Scholar]
- Sweeny, J.M.; Angiolillo, D.J.; Franchi, F.; Rollini, F.; Waksman, R.; Raveendran, G.; Dangas, G.; Khan, N.D.; Carlson, G.F.; Zhao, Y.; et al. Impact of Diabetes Mellitus on the Pharmacodynamic Effects of Ticagrelor Versus Clopidogrel in Troponin-Negative Acute Coronary Syndrome Patients Undergoing Ad Hoc Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2017, 6, e005650. [Google Scholar] [CrossRef]
- Franchi, F.; James, S.K.; Lakic, T.G.; Budaj, A.J.; Cornel, J.H.; Katus, H.A.; Keltai, M.; Kontny, F.; Lewis, B.S.; Storey, R.F.; et al. Impact of Diabetes Mellitus and Chronic Kidney Disease on Cardiovascular Outcomes and Platelet P2Y12 Receptor Antagonist Effects in Patients with Acute Coronary Syndromes: Insights from the PLATO Trial. J. Am. Heart Assoc. 2019, 8, e011139. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.R.; Bainey, K.R.; Cantor, W.J.; Lordkipanidzé, M.; Marquis-Gravel, G.; Robinson, S.D.; Sibbald, M.; So, D.Y.; Wong, G.C.; Abunassar, J.G.; et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology Focused Update of the Guidelines for the Use of Antiplatelet Therapy. Can. J. Cardiol. 2018, 34, 214–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.-P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.A.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Hong, S.J.; Cho, Y.H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.Y.; Cho, S.; Jeon, D.W.; et al. Effect of Ticagrelor Monotherapy vs. Ticagrelor with Aspirin on Major Bleeding and Cardiovascular Events in Patients with Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020, 323, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedhi, E.; Fabris, E.; Van Der Ent, M.; Buszman, P.; Von Birgelen, C.; Roolvink, V.; Zurakowski, A.; Schotborgh, C.E.; Hoorntje, J.C.A.; Eek, C.H.; et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): Randomised, multicentre, non-inferiority trial. BMJ 2018, 363, k3793. [Google Scholar] [CrossRef] [Green Version]
- Verdoia, M.; Suryapranata, H.; Damen, S.; Camaro, C.; Benit, E.; Barbieri, L.; Rasoul, S.; Liew, H.B.; Polad, J.; Ahmad, W.A.W.; et al. Gender differences with short-term vs. 12 months dual antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual therapy stent: 2-years follow-up results of the REDUCE trial. J. Thromb. Thrombolysis 2021, 52, 797–807. [Google Scholar] [CrossRef]
- Storey, R.F.; Gurbel, P.A.; Berg, J.T.; Bernaud, C.; Dangas, G.D.; Frenoux, J.-M.; Gorog, D.A.; Hmissi, A.; Kunadian, V.; James, S.K.; et al. Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur. Heart J. 2019, 41, 3132–3140. [Google Scholar] [CrossRef] [Green Version]
- Sinnaeve, P.; Fahrni, G.; Schelfaut, D.; Spirito, A.; Mueller, C.; Frenoux, J.-M.; Hmissi, A.; Bernaud, C.; Ufer, M.; Moccetti, T.; et al. Subcutaneous Selatogrel Inhibits Platelet Aggregation in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 75, 2588–2597. [Google Scholar] [CrossRef]
- Ungerer, M.; Rosport, K.; Bültmann, A.; Piechatzek, R.; Uhland, K.; Schlieper, P.; Gawaz, M.; Münch, G. Novel Antiplatelet Drug Revacept (Dimeric Glycoprotein VI-Fc) Specifically and Efficiently Inhibited Collagen-Induced Platelet Aggregation without Affecting General Hemostasis in Humans. Circulation 2011, 123, 1891–1899. [Google Scholar] [CrossRef]
- Mojica Muñoz, A.K.; Jamasbi, J.; Uhland, K.; Degen, H.; Münch, G.; Ungerer, M.; Brandl, R.; Megens, R.; Weber, C.; Lorenz, R.; et al. Recombinant GPVI-Fc added to single or dual antiplatelet therapy in vitro prevents plaque-induced platelet thrombus formation. Thromb. Haemost. 2017, 11, 1651–1659. [Google Scholar] [CrossRef]
- Mayer, K.; Hein-Rothweiler, R.; Schüpke, S.; Janisch, M.; Bernlochner, I.; Ndrepepa, G.; Sibbing, D.; Gori, T.; Borst, O.; Holdenrieder, S.; et al. Efficacy and Safety of Revacept, a Novel Lesion-Directed Competitive Antagonist to Platelet Glycoprotein VI, in Patients Undergoing Elective Percutaneous Coronary Intervention for Stable Ischemic Heart Disease: The Randomized, Double-blind, Placebo-Controlled ISAR-PLASTER Phase 2 Trial. JAMA Cardiol. 2021, 6, 753. [Google Scholar] [CrossRef] [PubMed]
- Vootukuri, S.; Li, J.; Nedelman, M.; Thomas, C.; Jiang, J.-K.; Babayeva, M.; Coller, B.S. Preclinical studies of RUC-4, a novel platelet αIIbβ3 antagonist, in non-human primates and with human platelets. J. Clin. Transl. Sci. 2019, 3, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Vootukuri, S.; Shang, Y.; Negri, A.; Jiang, J.K.; Nedelman, M.; Diacovo, T.G.; Filizola, M.; Thomas, C.J.; Coller, B.S. RUC-4: A novel antagonist for prehospital therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2321–2329. [Google Scholar] [CrossRef] [Green Version]
- Kereiakes, D.J.; Henry, T.D.; DeMaria, A.N.; Bentur, O.; Carlson, M.; Seng Yue, C.; Martin, L.H.; Midkiff, J.; Mueller, M.; Meek, T.; et al. First Human Use of RUC-4: A Non activating Second-Generation Small-Molecule Platelet Glycoprotein IIb/IIIa (Integrin αIIbβ3) Inhibitor Designed for Subcutaneous Point-of-Care Treatment of ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e016552. [Google Scholar] [CrossRef] [PubMed]
- Bor, W.L.; Zheng, K.L.; Tavenier, A.H.; Gibson, C.M.; Granger, C.B.; Bentur, O.; Lobatto, R.; Postma, S.; Coller, B.S.; van’t Hof, A.W.J.; et al. Pharmacokinetics, pharmacodynamics, and tolerability of subcutaneous administration of a novel glycoprotein IIb/IIIa inhibitor, RUC-4, in patients with ST-segment elevation myocardial infarction. EuroIntervention 2021, 17, e401–e410. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Sethi, R.; Pradhan, A. Landmark Trials in Cardiology in 2017—Celebrating 40 Years of Angioplasty. Int. J. Angiol. 2018, 27, 167–173. [Google Scholar] [CrossRef]
- Crea, F.; Binder, R.K.; Lüscher, T.F. The year in cardiology 2017: Acute coronary syndromes. Eur. Heart J. 2018, 39, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van’t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients with Acute Coronary Syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef]
- Galli, M.; Benenati, S.; Franchi, F.; Rollini, F.; Capodanno, D.; Biondi-Zoccai, G.; Vescovo, G.M.; Cavallari, L.H.; Bikdeli, B.; Berg, J.T.; et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: A network meta-analysis of 61,898 patients from 15 randomized trials. Eur. Heart J. 2021, 43, 959–967. [Google Scholar] [CrossRef]
- Elzanaty, A.M.; Nazir, S.; Awad, M.T.; Elsheikh, E.; Ahuja, K.R.; Donato, A.; Eltahawy, E.A. Meta-Analysis of the Efficacy and Safety of P2Y12 Inhibitor Monotherapy after Short Course of Dual-Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Intervention. Cardiovasc. Revascularizat. Med. 2020, 21, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Dangas, G.; Angiolillo, D.J.; Cohen, D.J.; Sharma, S.K.; Nicolas, J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dudek, D.; et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur. Heart J. 2020, 41, 3533–3545. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- Favresse, J.; Bayart, J.-L.; Gruson, D.; Bernardini, S.; Clerico, A.; Perrone, M. The underestimated issue of non-reproducible cardiac troponin I and T results: Case series and systematic review of the literature. Clin. Chem. Lab. Med. 2021, 59, 1201–1211. [Google Scholar] [CrossRef]
- Perrone, M.A.; Feola, A.; Pieri, M.; Donatucci, B.; Salimei, C.; Lombardo, M.; Perrone, A.; Parisi, A. The Effects of Reduced Physical Activity on the Lipid Profile in Patients with High Cardiovascular Risk during COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 8858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ali, W.; Mishra, S.; Pradhan, A.; Sethi, R.; Kushwaha, R.; Singh, U.S.; Perrone, M.A. Circulating Soluble Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 (sLOX-1): A Diagnostic Indicator across the Spectrum of Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 5567. [Google Scholar] [CrossRef] [PubMed]




| Study | Atherothrombotic Patient Type | Treatment Regimen | Primary End Points | Result/ Remark |
|---|---|---|---|---|
| CAPRIE (1996) [9] | Recent MI, recent ischemic stroke, or symptomatic PAD | Clopidogrel vs. aspirin | Composite of MI, ischemic stroke, or vascular death | Significant relative-risk reduction of 8.7% in the clopidogrel group (p = 0.043); Clopidogrel more effective than aspirin in reducing ischemic stroke, MI, or vascular death. |
| CURE (2001) [10] | NSTE ACS/unstable angina | Clopidogrel + aspirin vs. placebo + aspirin | Composite of CV death, MI, stroke, or refractory ischemia | Decreased death/MI/stroke by 20% in NSTE ACS/unstable angina patients; Clopidogrel in addition to aspirin has beneficial effects in patients with ACS without ST-segment elevation. |
| CREDO Trial (2002) [11] | Stable CAD or ACS undergoing PCI | Loading with clopidogrel 300 mg or placebo before PCI. Thereafter, all patients received clopidogrel (75 mg) through day 28. Then, day 29 through 12 months, the loading dose group received clopidogrel (75 mg daily), and the control group received aspirin throughout the study | Composite of death, myocardial infarction, or stroke at 1 year | 26.9% relative risk reduction in composite endpoint in the clopidogrel group at 1 year. Clopidogrel pretreatment did not significantly reduce the combined risk of death, MI, or urgent target vessel revascularization at 28 days; Long-term (1 year) clopidogrel therapy significantly reduced the risk of adverse ischemic events. |
| COMMIT Trial (2005) [12] | STEMI, NSTEMI | Clopidogrel + aspirin vs. placebo + aspirin | Composite of death, reinfarction or stroke, death from any cause up to 4 week or till discharge | Significant 9% reduction in death, reinfarction, or stroke. There was also a significant 7% proportional reduction in any death; Adding clopidogrel 75 mg daily to standard treatment safely reduces major vascular events and mortality in hospital. |
| CLARITY-TIMI 28 (2005) [14] | STEMI | Clopidogrel + aspirin vs. placebo + aspirin in addition to standard therapy | Occluded infarct-related artery (TIMI flow grade 0 or 1) on predischarge angiogram or death or recurrent MI before angiography | Decreased death, MI, urgent revascularization by 20%. Decreased occluded artery by 36%; Addition of clopidogrel improves the patency rate of the infarct-related artery and reduces ischemic complications. |
| Current-OASIS 7 (2010) [17] | Acute coronary syndromes with intended early PCI | Double-dose (600 mg on day 1, 150 mg on days 2–7, then 75 mg daily) versus standard dose (300 mg on day 1 then 75 mg daily) clopidogrel, and high-dose (300–325 mg daily) versus low-dose (75–100 mg daily) | Cardiovascular death, myocardial infarction, or stroke at 30 days | Double-dose clopidogrel reduced the rate of the primary outcome (3.9% vs. 4.5%) and definite stent thrombosis (0.7% vs. 1.3%); 7 day double-dose clopidogrel regimen was associated with a reduction in cardiovascular events and stent thrombosis. |
| Study Population | Treatment Strategy | Primary End Point | Result | |
|---|---|---|---|---|
| GRAVITAS trial (2011) [18] | Post PCI patient with drug eluting stent | After platelet function testing patients were given high-dose (150 mg daily) or standard-dose clopidogrel (75 mg daily) | Cardiovascular death, nonfatal MI or stent thrombosis at 6 months | In patients with high on-treatment reactivity after PCI with drug eluting stents, the use of high dose clopidogrel compared with standard dose clopidogrel did not reduce primary outcome |
| ARCTIC-GENE study (2015) [19] | Stable angina/NSTE-ACS undergoing PCI with DES implantation | Platelet function analysis in post PCI patients and clopidogrel dose adjustment | Composite of death, MI, stent thrombosis, stroke, or urgent revascularization at 12 months | No significant difference between two groups |
| TAILOR PCI (2020) [20] | Patients undergoing PCI for ACS or CCS | Genotype guided P2Y12 inhibitor verses conventional (no genotyping, clopidogrel in all) | Composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia at 12 months | No significant difference between two groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, A.; Tiwari, A.; Caminiti, G.; Salimei, C.; Muscoli, S.; Sethi, R.; Perrone, M.A. Ideal P2Y12 Inhibitor in Acute Coronary Syndrome: A Review and Current Status. Int. J. Environ. Res. Public Health 2022, 19, 8977. https://doi.org/10.3390/ijerph19158977
Pradhan A, Tiwari A, Caminiti G, Salimei C, Muscoli S, Sethi R, Perrone MA. Ideal P2Y12 Inhibitor in Acute Coronary Syndrome: A Review and Current Status. International Journal of Environmental Research and Public Health. 2022; 19(15):8977. https://doi.org/10.3390/ijerph19158977
Chicago/Turabian StylePradhan, Akshyaya, Aashish Tiwari, Giuseppe Caminiti, Chiara Salimei, Saverio Muscoli, Rishi Sethi, and Marco Alfonso Perrone. 2022. "Ideal P2Y12 Inhibitor in Acute Coronary Syndrome: A Review and Current Status" International Journal of Environmental Research and Public Health 19, no. 15: 8977. https://doi.org/10.3390/ijerph19158977
APA StylePradhan, A., Tiwari, A., Caminiti, G., Salimei, C., Muscoli, S., Sethi, R., & Perrone, M. A. (2022). Ideal P2Y12 Inhibitor in Acute Coronary Syndrome: A Review and Current Status. International Journal of Environmental Research and Public Health, 19(15), 8977. https://doi.org/10.3390/ijerph19158977









