Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus
Abstract
:1. Introduction
- To assess the clinical outcomes of patients treated using CURATE.AI technology.
- To evaluate the staff experiences of implementing and using the CURATE.AI system in clinical practice and identify the facilitators and barriers to its use.
- To elicit the opinions and experiences of patients receiving CURATE.AI guided care.
- To optimise the CURATE.AI algorithm through exploratory analysis, including artificial intelligence algorithms.
2. Materials and Methods
2.1. The CURATE.AI System
2.2. Retrospective Study
2.3. Prospective Study
2.3.1. Participants
Inclusion Criteria
Exclusion Criteria
2.3.2. Outcome Measures
- Proportion achieving blood pressure and glycaemic control at four months.
- Average number of days until blood pressure or glycaemic control is first achieved.
- Proportion relapsing after disease control is achieved (i.e., high blood pressure).
- Proportion compliant with clinic follow-up.
- Proportion compliant with home monitoring of blood pressure or glucose.
- Number of dropouts.
- Proportion of dosing decisions recommended by CURATE.AI but not implemented at the physician’s discretion and the magnitude of difference.
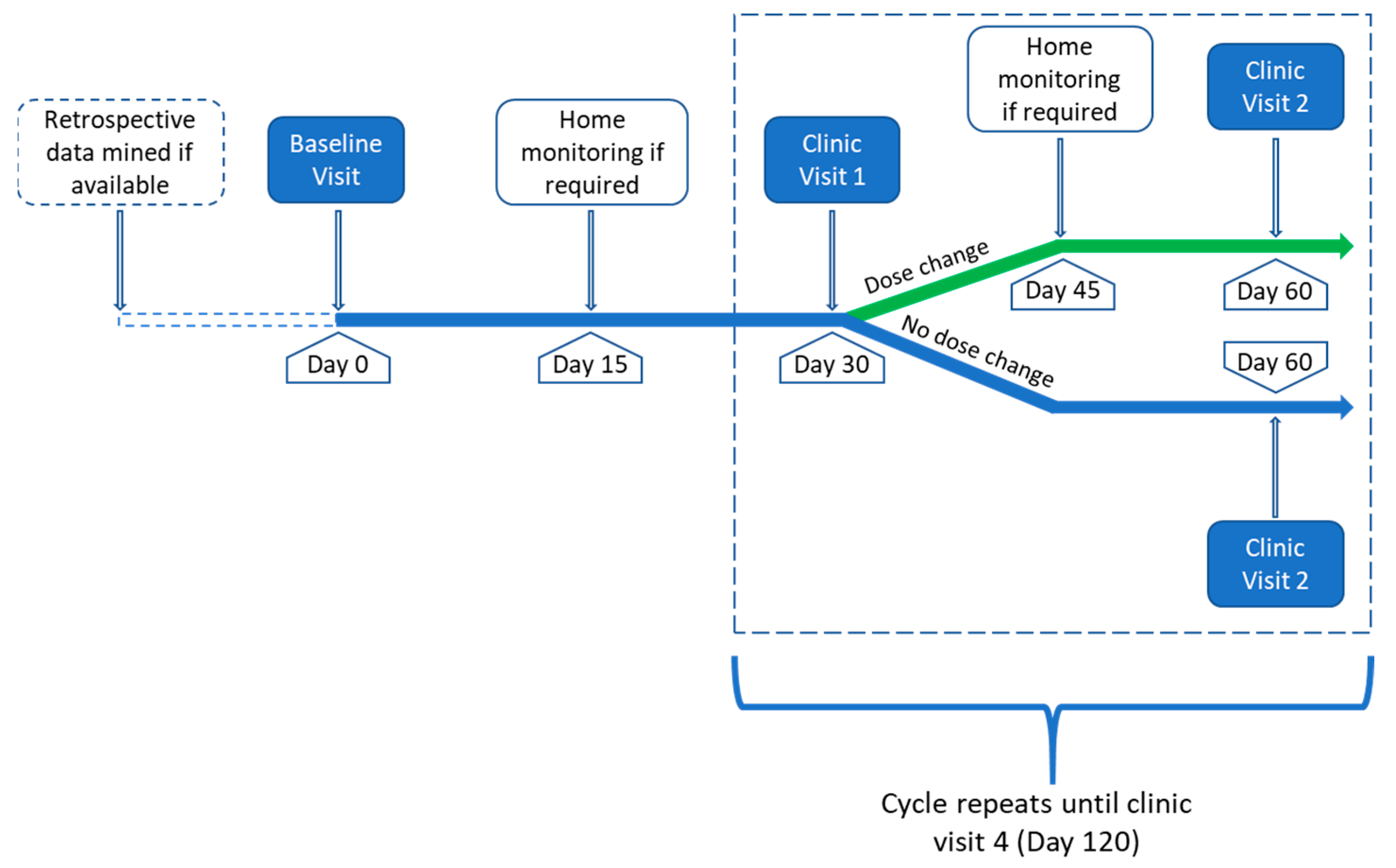
2.3.3. Study Procedures
2.3.4. Implementation Evaluation
Patient Survey
Staff Interviews
- Has CURATE.AI impacted your workload?
- What challenges have you faced when using CURATE.AI?
- What have been the expected and unexpected outcomes, both positive and negative, of using CURATE.AI?
2.3.5. Data Analysis
3. Discussion
Potential Strengths and Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases Geneva: WHO. 2021. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 25 May 2021).
- Whelton, P.K. The Elusiveness of Population-Wide High Blood Pressure Control. Annu. Rev. Public Health 2015, 36, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Walraven, I.; Mast, M.R.; Hoekstra, T.; Jansen, A.P.; Rauh, S.P.; Rutters, F.R.; van der Heijden, A.A.; Elders, P.J.; Moll, A.C.; Polak, B.C.; et al. Real-world evidence of suboptimal blood pressure control in patients with type 2 diabetes. J. Hypertens. 2015, 33, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Cook, J.; Wang, Y.; Peck, R.; Weiner, D. Drug Dosing Recommendations for All Patients: A Roadmap for Change. Clin. Pharmacol. Ther. 2021, 109, 65–72. [Google Scholar] [CrossRef]
- Scheen, A.J. Precision medicine: The future in diabetes care? Diabetes Res. Clin. Pract. 2016, 117, 12–21. [Google Scholar] [CrossRef]
- Smith, R.J.; Nathan, D.M.; Arslanian, S.A.; Groop, L.; Rizza, R.A.; Rotter, J.I. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: What we know and what we need to know. J. Clin. Endocrinol. Metab. 2010, 95, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Gallo, M.; Candido, R.; De Micheli, A.; Esposito, K.; Gentile, S.; Medea, G. Personalized therapy algorithms for type 2 diabetes: A phenotype-based approach. Pharmgenomics Pers. Med. 2014, 7, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Green, E.D.; Gunter, C.; Biesecker, L.G.; Di Francesco, V.; Easter, C.L.; Feingold, E.A.; Felsenfeld, A.L.; Kaufman, D.J.; Ostrander, E.A.; Pavan, W.J.; et al. Strategic vision for improving human health at The Forefront of Genomics. Nature 2020, 586, 683–692. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Genomics and Precision Health US: CDC. 2020. Available online: https://www.cdc.gov/genomics/about/precision_med.htm (accessed on 6 May 2021).
- Cheung, K.L.; Durusu, D.; Sui, X.; de Vries, H. How recommender systems could support and enhance computer-tailored digital health programs: A scoping review. Digit. Health 2019, 5, 2055207618824727. [Google Scholar] [CrossRef] [Green Version]
- Bin Sawad, A.; Narayan, B.; Alnefaie, A.; Maqbool, A.; Mckie, I.; Smith, J.; Yuksel, B.; Puthal, D.; Prasad, M.; Kocaballi, A.B. A Systematic Review on Healthcare Artificial Intelligent Conversational Agents for Chronic Conditions. Sensors 2022, 22, 2625. [Google Scholar] [CrossRef]
- Bossen, D.; Broekema, A.; Visser, B.; Brons, A.; Timmerman, A.; van Etten-Jamaludin, F.; Braam, K.; Engelbert, R. Effectiveness of Serious Games to Increase Physical Activity in Children With a Chronic Disease: Systematic Review With Meta-Analysis. J. Med. Internet Res. 2020, 22, e14549. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, S.; Takeuchi, W.; Chalkidis, G.; Rodriguez-Loya, S.; Kuwata, J.; Flynn, M.; Turner, K.M.; Sakaguchi, F.H.; Weir, C.; Kramer, H.; et al. Leveraging Artificial Intelligence to Improve Chronic Disease Care: Methods and Application to Pharmacotherapy Decision Support for Type-2 Diabetes Mellitus. Methods Inf. Med. 2021, 60, e32–e43. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, L.; Gao, F.; He, S.J.; Zhao, H.J.; Fang, Y.; Yang, J.M.; An, Y.; Ye, Z.W.; Dong, Z. Integration of Artificial Intelligence, Blockchain, and Wearable Technology for Chronic Disease Management: A New Paradigm in Smart Healthcare. Curr. Med. Sci. 2021, 41, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, K.; Herrero, P.; Georgiou, P. Deep Learning for Diabetes: A Systematic Review. IEEE J. Biomed. Health Inform. 2021, 25, 2744–2757. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Lee, D.K.; Silva, A.; Datta, N.; Kee, T.; Eriksen, C.; Weigle, K.; Agopian, V.; Kaldas, F.; Farmer, D.; et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci. Transl. Med. 2016, 8, 333ra49. [Google Scholar] [CrossRef] [Green Version]
- Pantuck, A.J.; Lee, D.K.; Kee, T.; Wang, P.; Lakhotia, S.; Silverman, M.H.; Mathis, C.; Drakaki, A.; Belldegrun, A.S.; Ho, C.M.; et al. Modulating BET Bromodomain Inhibitor ZEN-3694 and Enzalutamide Combination Dosing in a Metastatic Prostate Cancer Patient Using CURATE.AI, an Artificial Intelligence Platform. Adv. Ther. 2018, 1, 1800104. [Google Scholar] [CrossRef]
- Lee, D.K.; Chang, V.Y.; Kee, T.; Ho, C.M.; Ho, D. Optimizing Combination Therapy for Acute Lymphoblastic Leukemia Using a Phenotypic Personalized Medicine Digital Health Platform: Retrospective Optimization Individualizes Patient Regimens to Maximize Efficacy and Safety. SLAS Technol. 2017, 22, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, A.; Khong, J.; Kee, T. CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence. SLAS Technol. 2020, 25, 95–105. [Google Scholar] [CrossRef]
- N1 Labs. N1 Labs Singapore: National University of Singapore. 2022. Available online: https://n1labs.org/ (accessed on 1 March 2022).
- Mokwe, E.; Ohmit, S.E.; Nasser, S.A.; Shafi, T.; Saunders, E.; Crook, E.; Dudley, A.; Flack, J.M. Determinants of Blood Pressure Response to Quinapril in Black and White Hypertensive Patients. Hypertension 2004, 43, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, T.P.; Suonsyrjä, T.; Hannila-Handelberg, T.; Paavonen, K.J.; Miettinen, H.E.; Strandberg, T.; Tikkanen, I.; Tilvis, R.; Pentikäinen, P.J.; Virolainen, J.; et al. Predictors of Antihypertensive Drug Responses: Initial Data from a Placebo-Controlled, Randomized, Cross-Over Study With Four Antihypertensive Drugs (The GENRES Study)*. Am. J. Hypertens. 2007, 20, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Wee, L.E.; Koh, G.C.-H. Individual and neighborhood social factors of hypertension management in a low-socioeconomic status population: A community-based case–control study in Singapore. Hypertens. Res. 2012, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.M. Precision Medicine in Type 2 Diabetes: Using Individualized Prediction Models to Optimize Selection of Treatment. Diabetes 2020, 69, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.B.; Groop, L. Precision medicine in type 2 diabetes. J. Intern. Med. 2019, 285, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Sendon, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease: The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar] [PubMed]
- Davidson, M.B.; Peters, A.L. An overview of metformin in the treatment of type 2 diabetes mellitus. Am. J. Med. 1997, 102, 99–110. [Google Scholar] [CrossRef]
- Health Promotion Board. National Population Health Survey Singapore: HPB. 2021. Available online: https://www.hpb.gov.sg/community/national-population-health-survey (accessed on 25 May 2021).
- Chu, A.H.; Ng, S.H.; Koh, D.; Müller-Riemenschneider, F. Reliability and Validity of the Self- and Interviewer-Administered Versions of the Global Physical Activity Questionnaire (GPAQ). PLoS ONE 2015, 10, e0136944. [Google Scholar] [CrossRef]
- Keating, X.D.; Zhou, K.; Liu, X.; Hodges, M.; Liu, J.; Guan, J.; Phelps, A.; Castro-Piñero, J. Reliability and Concurrent Validity of Global Physical Activity Questionnaire (GPAQ): A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4128. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.T.; Hill, M.N.; Bone, L.R.; Levine, D.M. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog. Cardiovasc. Nurs. 2000, 15, 90–96. [Google Scholar] [CrossRef]
- Durso, S.C. Hypertension: What You Need to Know as You Age US: John Hopkins University. 2022. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/high-blood-pressure-hypertension/hypertension-what-you-need-to-know-as-you-age (accessed on 14 June 2022).
- Hirst, J.A.; Stevens, R.J.; Farmer, A.J. Changes in HbA1c level over a 12-week follow-up in patients with type 2 diabetes following a medication change. PLoS ONE 2014, 9, e92458. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.E.; Sezate, G.S.; Lin, W.; Weber, C.A.; Dawson, J.D.; Carter, B.L.; Bergus, G.R. Indication-specific 6-h systolic blood pressure thresholds can approximate 24-h determination of blood pressure control. J. Hum. Hypertens. 2011, 25, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, D.; Madai, V.I. From Bit to Bedside: A Practical Framework for Artificial Intelligence Product Development in Healthcare. Adv. Intell. Syst. 2020, 2, 2000052. [Google Scholar] [CrossRef]
- Ministry of Health. Patient Experience Survey (PES) Singapore: MOH. 2018. Available online: https://www.nuh.com.sg/epes.html (accessed on 18 February 2020).
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management NICE Guideline [NG136]; NICE: London, UK, 2019. [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2018, 41, S1–S159. [Google Scholar]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593. [Google Scholar] [CrossRef] [Green Version]
| Data Variables | Baseline a | Visit 1 a | Visit 2 a | Visit 3 a | Visit 4 |
|---|---|---|---|---|---|
| CURATE.AI variables: | |||||
| Retrospective dose-response data mined | X | ||||
| Hba1C a or blood glucose | X | X | X | X | X |
| Blood pressure | X | X | X | X | X |
| Other data captured: | |||||
| Demographics | X | ||||
| Weight, BMI, hip-waist ratio | X | X | X | X | X |
| Medical history (morbidities, medication) | X | ||||
| Renal function | X | X | |||
| Liver function | X | X | |||
| Details of physical activity (16 items) | X | X | |||
| Details of diet (26 items) | X | X | |||
| Medication adherence (9 items) | X | X | |||
| Change in medications | X | X | X | X | X |
| Disagreements between CURATE.AI and physician | X | X | X | X | X |
| Patient survey | X | ||||
| NASSS Domain | Data Sources |
|---|---|
| 1A/1B: What is the nature of the condition?/What are the relevant sociocultural factors and comorbidities? |
|
| 2A: What are the key features of the technology? |
|
| 2B: What kind of knowledge does the technology bring into play? |
|
| 2C: What knowledge and support are required to use the technology? |
|
| 3B: What is the technology’s desirability, efficacy, safety, and cost effectiveness? |
|
| 4A: What changes in staff roles, practices, and identities are implied? |
|
| AI Framework Domain | Data Sources |
| Data form: Data access, the structure of data, appropriateness of data, data plan |
|
| Model development form: Determine the best algorithm type, scalability of the algorithm |
|
| Model development build: Pilot test performance of the algorithm |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhopadhyay, A.; Sumner, J.; Ling, L.H.; Quek, R.H.C.; Tan, A.T.H.; Teng, G.G.; Seetharaman, S.K.; Gollamudi, S.P.K.; Ho, D.; Motani, M. Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 8979. https://doi.org/10.3390/ijerph19158979
Mukhopadhyay A, Sumner J, Ling LH, Quek RHC, Tan ATH, Teng GG, Seetharaman SK, Gollamudi SPK, Ho D, Motani M. Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2022; 19(15):8979. https://doi.org/10.3390/ijerph19158979
Chicago/Turabian StyleMukhopadhyay, Amartya, Jennifer Sumner, Lieng Hsi Ling, Raphael Hao Chong Quek, Andre Teck Huat Tan, Gim Gee Teng, Santhosh Kumar Seetharaman, Satya Pavan Kumar Gollamudi, Dean Ho, and Mehul Motani. 2022. "Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus" International Journal of Environmental Research and Public Health 19, no. 15: 8979. https://doi.org/10.3390/ijerph19158979






