The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review
Abstract
1. Introduction
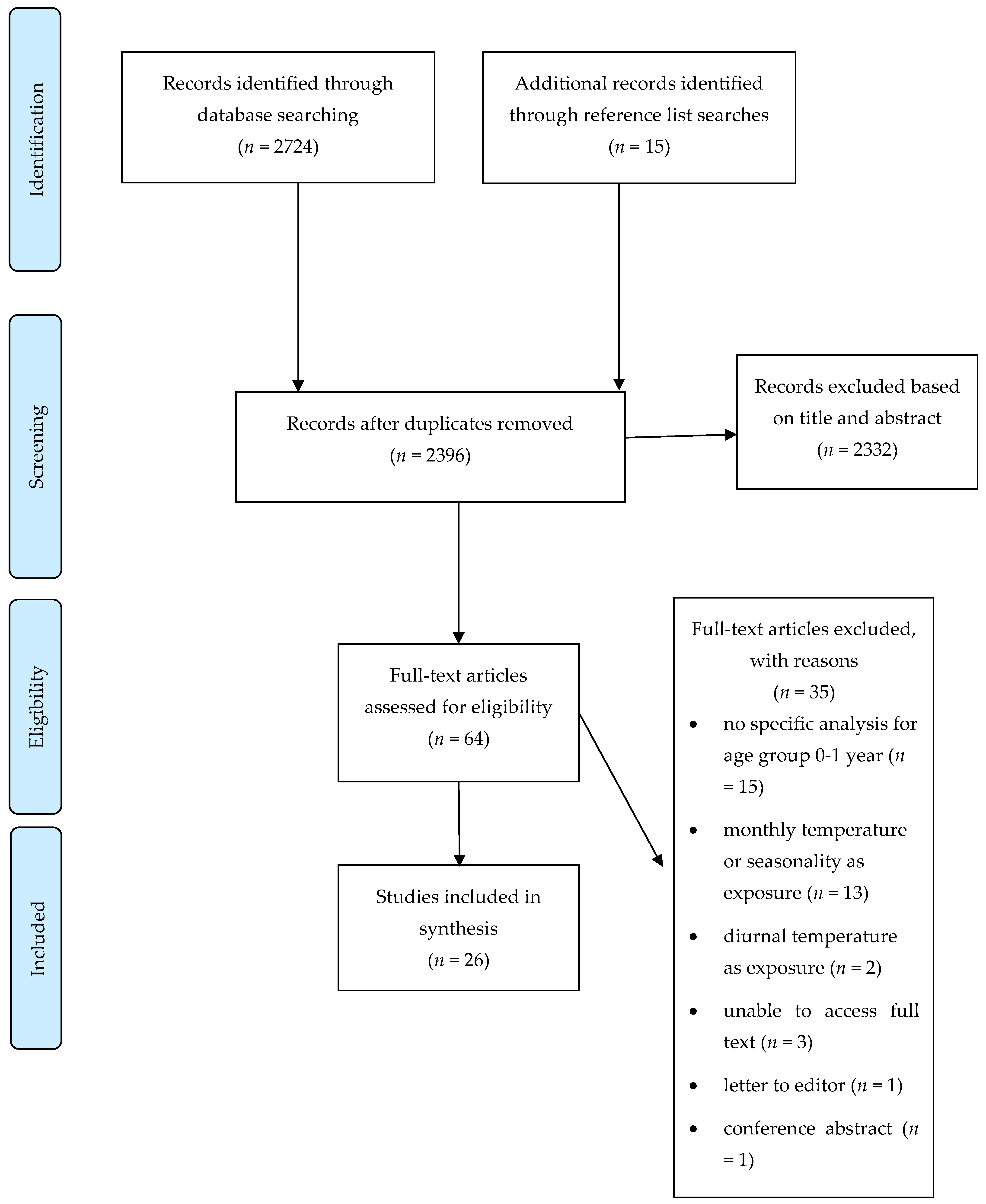
2. Methods
3. Results
3.1. Temperature and Infant Mortality
3.2. Temperature and Infant Morbidity
3.3. Temperature and Other Neonatal Conditions
3.4. Evidence of Effect Modification by Socioeconomic Factors, Race, Rural Versus Urban Areas and Time Periods
3.5. Quality of Studies
3.6. Key Findings
4. Limitations of This Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Climate Change and Health. Geneva. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 20 February 2022).
- IPCC. Climate change 2021: The physical science basis summary for policymakers working group I contribution to the sixth assessment report of the intergovernmental panel on climate change. In Climate Change 2021: The Physical Science Basis; IPCC: Geneva, Switzerland, 2021; 3949p. [Google Scholar]
- Luber, G.; McGeehin, M. Climate change and extreme heat events. Am. J. Prev. Med. 2008, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Basu, R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ. Health 2009, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Remenyi, T.A.; White, C.J.; Johnston, F.H. Heatwave and health impact research: A global review. Health Place 2018, 53, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Hajat, S. Heat stress and public health: A critical review. Annu. Rev. Public Health 2008, 29, 41–55. [Google Scholar] [CrossRef]
- Hajat, S.; Kosatky, T. Heat-related mortality: A review and exploration of heterogeneity. J. Epidemiol. Community Health 2010, 64, 753–760. [Google Scholar] [CrossRef]
- Chersich, M.F.; Pham, M.D.; Areal, A.; Haghighi, M.M.; Manyuchi, A.; Swift, C.P.; Wernecke, B.; Robinson, M.; Hetem, R.; Boeckmann, M.; et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: Systematic review and meta-analysis. BMJ 2020, 371, m3811. [Google Scholar] [CrossRef]
- Gronlund, C.J. Racial and Socioeconomic Disparities in Heat-Related Health Effects and Their Mechanisms: A Review. Curr. Epidemiol. Rep. 2014, 1, 165–173. [Google Scholar] [CrossRef]
- Xu, Z.; Sheffield, P.E.; Su, H.; Wang, X.; Bi, Y.; Tong, S. The impact of heat waves on children’s health: A systematic review. Int. J. Biometeorol. 2014, 58, 239–247. [Google Scholar] [CrossRef]
- Xu, Z.; Etzel, R.A.; Su, H.; Huang, C.; Guo, Y.; Tong, S. Impact of ambient temperature on children’s health: A systematic review. Environ. Res. 2012, 117, 120–131. [Google Scholar] [CrossRef]
- Helldén, D.; Andersson, C.; Nilsson, M.; Ebi, K.L.; Friberg, P.; Alfvén, T. Climate change and child health: A scoping review and an expanded conceptual framework. Lancet Planet Health 2021, 5, e164–e175. [Google Scholar] [CrossRef]
- Sheffield, P.E.; Landrigan, P.J. Global climate change and children’s health: Threats and strategies for prevention. Environ. Health Perspect. 2011, 119, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Scovronick, N.; Sera, F.; Acquaotta, F.; Garzena, D.; Fratianni, S.; Wright, C.Y.; Gasparrini, A. The association between ambient temperature and mortality in South Africa: A time-series analysis. Environ. Res. 2018, 161, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Schinasi, L.H.; Bloch, J.R.; Melly, S.; Zhao, Y.; Moore, K.; de Roos, A.J. High ambient temperature and infant mortality in Philadelphia, Pennsylvania: A case-crossover study. Am. J. Public Health 2020, 110, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J. Pediatric thermoregulation: Considerations in the face of global climate change. Nutrients 2019, 11, 2010. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sung, Y.H.; Wang, S.M.; Lung, H.L.; Chang, J.H.; Hsu, C.H.; Hung, H.F. Short- and Long-Term Outcomes in Very Low Birth Weight Infants with Admission Hypothermia. PLoS ONE 2015, 10, e0131976. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sheffield, P.E.; Hu, W.; Su, H.; Yu, W.; Qi, X.; Tong, S. Climate change and children’s health—A call for research on what works to protect children. Int. J. Environ. Res. Public Health 2012, 9, 3298–3316. [Google Scholar] [CrossRef]
- Kakkad, K.; Barzaga, M.L.; Wallenstein, S.; Azhar, G.S.; Sheffield, P.E. Neonates in Ahmedabad, India, during the 2010 Heat Wave: A climate change adaptation study. J. Environ. Public Health 2014, 2014, 946875. [Google Scholar] [CrossRef]
- Sarofim, M.C.; Saha, S.; Hawkins, M.D.; Mills, D.D.; Hess, J.; Kinney, P.; Schwartz, J.; Juliana St., A. Temperature-Related Death and Illness: The Impacts of Climate Change on Human Health in the United States, A Scientific Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2016; pp. 43–68.
- Basu, R.; Pearson, D.; Sie, L.; Broadwin, R. A Case-Crossover Study of Temperature and Infant Mortality in California. Paediatr. Perinat. Epidemiol. 2015, 29, 407–415. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Hajat, S.; Armstrong, B.; Wilkinson, P. Declining vulnerability to temperature-related mortality in London over the 20th century. Am. J. Epidemiol. 2006, 164, 77–84. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- National Toxicology Program. OHAT Risk of Bias Rating Tool for Human and Animal Studies. 2015. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 18 May 2022).
- Wang, Z.; Taylor, K.; Allman-Farinelli, M.; Armstrong, B.; Askie, L.; Ghersi, D.; Bero, L.A. A systematic review: Tools for assessing methodological quality of human observational studies. NHMRC 2019. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Mannan, I.; Choi, Y.; Coutinho, A.J.; Chowdhury, A.I.; Rahman, S.M.; Seraji, H.R.; Baqui, A.H. Vulnerability of newborns to environmental factors: Findings from community based surveillance data in Bangladesh. Int. J. Environ. Res. Public Health 2011, 8, 3437–3452. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wei, L.; Duan, Y.; Li, H.; Liao, Y.; Lv, Q.; Jiang, H. Short-term effects of meteorological factors and air pollutants on hand, foot and mouth disease among children in Shenzhen, China, 2009–2017. Int. J. Environ. Res. Public Health 2019, 16, 3639. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, T.; Liu, L.; Lv, Q.; Li, X. The Association between Ambient Temperature and Childhood Hand, Foot, and Mouth Disease in Chengdu, China: A Distributed Lag Non-linear Analysis. Sci. Rep. 2016, 6, 27305. [Google Scholar] [CrossRef][Green Version]
- Scrafford, C.G.; Mullany, L.C.; Katz, J.; Khatry, S.K.; Leclerq, S.C.; Darmstadt, G.L.; Tielsch, J.M. Incidence of and risk factors for neonatal jaundice among newborns in southern Nepal. Trop Med. Int. Health 2013, 18, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Lundevaller, E.H.; Schumann, B. Neonatal mortality and temperature in two Northern Swedish rural parishes, 1860–1899—The significance of ethnicity and gender. Int. J. Environ. Res. Public Health 2020, 17, 1216. [Google Scholar] [CrossRef]
- Scalone, F.; Samoggia, A. Neonatal mortality, cold weather, and socioeconomic status in two northern Italian rural parishes, 1820–1900. Demogr. Res. 2018, 39, 525–560. [Google Scholar] [CrossRef]
- Fouillet, A.; Rey, G.; Laurent, F.; Pavillon, G.; Bellec, S.; Guihenneuc-Jouyaux, C.; Hémon, D. Excess mortality related to the August 2003 heat wave in France. Int. Arch. Occup. Environ. Health 2006, 80, 16–24. [Google Scholar] [CrossRef]
- Basu, R.; Ostro, B.D. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am. J. Epidemiol. 2008, 168, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Lee, J.T.; Bell, M.L. Is ambient temperature associated with risk of infant mortality? A multi-city study in Korea. Environ. Res. 2017, 158, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Basagaña, X.; Sartini, C.; Barrera-Gómez, J.; Dadvand, P.; Cunillera, J.; Ostro, B.; Medina-Ramón, M. Heat waves and cause-specific mortality at all ages. Epidemiology 2011, 22, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Linares, C.; García-Herrera, R.; López, C.; Trigo, R. Impact of temperature and air pollution on the mortality of children in Madrid. J. Occup. Environ. Med. 2004, 46, 768–774. [Google Scholar] [CrossRef]
- Jhun, I.; Mata, D.A.; Nordio, F.; Lee, M.; Schwartz, J.; Zanobetti, A. Ambient Temperature and Sudden Infant Death Syndrome in the United States. Epidemiology 2017, 28, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Fraser, W.D.; Smargiassi, A.; Kosatsky, T. Ambient heat and sudden infant death: A case-crossover study spanning 30 years in Montreal, Canada. Environ. Health Perspect. 2015, 123, 712–716. [Google Scholar] [CrossRef]
- Waldhoer, T.; Heinzl, H. Exploring the possible relationship between ambient heat and sudden infant death with data from Vienna, Austria. PLoS ONE 2017, 12, e0184312. [Google Scholar] [CrossRef]
- Scheers-Masters, J.R.; Schootman, M.; Thach, B.T. Heat stress and sudden infant death syndrome incidence: A United States population epidemiologic study. Pediatrics 2004, 113, e586-92. [Google Scholar] [CrossRef]
- Chang, H.P.; Li, C.Y.; Chang, Y.H.; Hwang, S.L.; Su, Y.H.; Chen, C.W. Sociodemographic and meteorological correlates of sudden infant death in Taiwan. Pediatr. Int. 2013, 55, 11–16. [Google Scholar] [CrossRef]
- Xu, Z.; Crooks, J.L.; Black, D.; Hu, W.; Tong, S. Heatwave and infants’ hospital admissions under different heatwave definitions. Environ. Pollut. 2017, 229, 525–530. [Google Scholar] [CrossRef]
- Gosai, A.; Salinger, J.; Dirks, K. Climate and respiratory disease in Auckland, New Zealand. Aust. N. Z. J. Public Health 2009, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Evangelisti, M.; Frassanito, A.; Scagnolari, C.; Pierangeli, A.; Antonelli, G.; Midulla, F. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: An observational study. Environ. Res. 2017, 158, 188–193. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeon, J.S.; Kim, J.K. Weather and its effects on RSV A and B infections in infants and children in Korea. Australas. Med. J. 2017, 10, 997–1002. [Google Scholar]
- Hoeppner, T.; Borland, M.; Babl, F.E.; Neutze, J.; Phillips, N.; Krieser, D.; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Influence of weather on incidence of bronchiolitis in Australia and New Zealand. J. Paediatr. Child Health 2017, 53, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Sekii, K.; Baba, T.; Ueno, D.; Ohishi, A. Seasonal variation in the international normalized ratio of neonates and its relationship with ambient temperature. BMC Pediatr. 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheffield, P.E.; Herrera, M.T.; Kinnee, E.J.; Clougherty, J.E. Not so little differences: Variation in hot weather risk to young children in New York City. Public Health 2018, 161, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A. Hand-Foot-and-Mouth Disease (HFMD): Practice Essentials, Background, Pathophysiology. 2021. Available online: https://emedicine.medscape.com/article/218402-overview (accessed on 18 May 2022).
- Xu, Z.; Hu, W.; Jiao, K.; Ren, C.; Jiang, B.; Ma, W. The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010–2013: A multicity study. BMC Infect. Dis. 2019, 19, 969. [Google Scholar] [CrossRef] [PubMed]
- Kambarami, R.; Chidede, O. Neonatal hypothermia levels and risk factors for mortality in a tropical country. Cent. Afr. J. Med. 2003, 49, 103–106. [Google Scholar] [PubMed]
- Jones, M.E.; Ponsonby, A.-L.; Dwyer, T.; Gilbert, N. The Relation between Climatic Temperature and Sudden Infant Death Syndrome Differs among Communities: Results from an Ecologic Analysis. Epidemiology 1994, 5, 332–336. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Belesova, K.; Boykoff, M.; Montgomery, H. The 2019 report of The Lancet Countdown on health and climate change: Ensuring that the health of a child born today is not defined by a changing climate. Lancet 2019, 394, 1836–1878. [Google Scholar] [CrossRef]
- Colston, J.M.; Zaitchik, B.; Kang, G.; Peñataro Yori, P.; Ahmed, T.; Lima, A.; Kosek, M.N. Use of earth observation-derived hydrometeorological variables to model and predict rotavirus infection (MAL-ED): A multisite cohort study. Lancet Planet Health 2019, 3, e248–e258. [Google Scholar] [CrossRef]
- Carlton, E.J.; Woster, A.P.; DeWitt, P.; Goldstein, R.S.; Levy, K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int. J. Epidemiol. 2016, 45, 117. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Birch, C.E.; Marsham, J.H.; Part, C.; Hajat, S.; Chersich, M.F.; Ebi, K.L.; Luchters, S.; Nakstad, B.; Kovats, S. Past and projected climate change impacts on heat-related child mortality in Africa. Environ. Res. Lett. 2022, 17, 074028. [Google Scholar] [CrossRef]


| PICOS Framework | Inclusion | Exclusion |
|---|---|---|
| People | Results report for infants 0–1 year age group | If infants were included in wider range of age, e.g., 0–2 or 0–5 years old, and not specifically for 0–1 years |
| Exposure and comparator (considered together) | Weather related, high and low temperatures | Non-weather-related temperature exposures, e.g., incubators, baths, or infant body temperature |
| Short term exposure, i.e., temperatures 0–14 days before event | Seasonality, monthly temperature and/or temperature exposure >14 days prior to outcome measures | |
| Indoor and outdoor ambient temperature | Factors such as ozone and pollution that may be secondarily affected by temperature | |
| Exposure after birth | Diurnal temperature range, or mean day-to-day temperature changes | |
| Humidity only as exposure | ||
| Outcomes | Any adverse health outcomes | Birth-related outcomes—pre-term birth, stillbirth, low birth weight, and birth defects |
| Setting | All countries/regions | |
| Type of study design | Quantitative observational study designs | Conference abstracts, case studies, editorials, opinion papers, reviews, protocols, modelling studies, and systematic or narrative reviews |
| Type of paper | English language | Grey literature |
| Peer-reviewed, published literature | ||
| Published from January 2000–July 2020 |
| Main Author (Year) | Research Design | Location, Year of Study | Description of Climate | Main Temperature Exposure Variable(s) | Statistical Analysis | Outcomes Measure (Source of Data) | Effect Estimates |
|---|---|---|---|---|---|---|---|
| All-Cause Mortality | |||||||
| Basu (2008) [35] | Case-crossover | California, USA 1999–2003 | Mean apparent temperature in warm season: 21.4 °C | Mean daily apparent temperature. Lag 0 days | Time-stratified case-crossover | Nonaccidental infant death. Sample size not provided for this age group (routine data) | 4.9% (95%CI = 1.0, 7.1%) increase in mortality for every 4.7 °C increase in temperature (no threshold presented). |
| Basu (2015) [21] | Case-crossover | California, USA, 1999–2011 | Mean temperature in warm season in coastal area, 17.8 °C and in non-coastal area 20.8 °C | Mean daily apparent temperature in warm season. Lag 0–3 days | Time-stratified case-crossover | 12,356 infant deaths (routine data) | Increase in all-cause mortality by 4.4% (95%CI = −0.3, 9.2) per 5.6 °C increase temperature (no threshold presented). For stratified analysis; all-cause mortality in black race/ethnic group: 13.3% (95%CI = 0.6, 27.6). |
| Schinasi (2020) [15] | Case-crossover | Philadelphia, USA, 2000–2015 | Humid, subtropical climate. Mean dry bulb temperature 23.3 °C in warm season | Minimum daily temperature in warm season. Lag 0–3 days | Time-stratified case-crossover | 1522 all-cause infant deaths (routine data) | OR comparing 23.9 °C and 26.1 °C with 4.4 °C were 2.1 (95%CI = 1.2, 3.6) and 2.6 (95%CI = 1.3, 5.0), respectively. Risk of infant mortality increased by 22.4% (95%CI = 5.0, 42.6) for every 1 °C increase in temperature above 23.9 °C. No evidence of effect modification. |
| Son (2017) [36] | Retrospective cohort | 7 cities in South Korea, 2004–2007 | Climate varies across cities. Average 13.0 °C–14.8 °C. | Mean daily temperature averaged 2 weeks before death | Cox proportional hazards model | 557 all-cause infant mortality and SIDS (routine data) | HR for 1 °C increase in temperature during 2 weeks before death; 1.51 (95%CI = 1.45, 1.56) for total mortality and 1.50 (95%CI = 1.35, 1.66) for SIDS. No evidence of effect modification. |
| Basagaña (2011) [37] | Case-crossover | Catalonia, Spain, 1983–2006 | Mediterranean climate. Average maximum temperature 19.9 °C–27.4 °C. 95th percentile of maximum temperature in warm season was 27.3 °C–38.0 °C | Maximum daily temperature during warm season over 95th percentile. Lag 0–6 days | Time-stratified case-crossover | 3144 deaths on hot days (routine data) | RR 1.25 (95%CI = 1.02, 1.53) on hot day as compared to non-hot day. |
| Fouillet (2006) [34] | Heat episode analysis | France, August 2003 | Temperate oceanic climate. During heatwave, temperature exceeded 35° for at least 9 days in most French departments | Daily minimum and maximum temperatures for very hot days | Excess mortality (observed over expected deaths) | Excess mortality in infant population (0.4 million) (routine data) | Excess of 25 deaths in male infants; mortality ratio 1.3 (95%CI = 1.0, 1.6) of observed over expected deaths during same time period. No excess mortality observed in females. |
| Diaz (2004) [38] | Retrospective cohort | Madrid, Spain, 1986–1997 | Mediterranean climate. Mean maximum temperature 19.7 °C. | Maximum, minimum, and temperature in cold wave. Lag 0–7 days | Poisson regression models | Infant mortality (sample size not provided for this age group) (routine data) | 17.4% of deaths are attributable to cold wave. RR 1.21 (95%CI = 1.10, 1.32) per 1 °C, below 6 °C, at lag 4 days. |
| Karlsson (2020) [32] | Retrospective cohort | Northern Sweden 1860 –1899 | Sub-artic climate. Mean monthly temperatures 14.8 °C–15 °C | Mean daily temperature. No lags. | Time-event binomial regression model | 330 neonatal deaths (parish registers) | Temperature at and after birth not associated with increased risk of neonatal mortality for whole study population. Sami infants had a higher mortality risk than winter-born non-Sami infants with lower temperatures on day of birth, HR 1.46 (95%CI = 1.07, 2.01). |
| Scalone (2018) [33] | Retrospective cohort | Northern Italy, 1820–1900 | Prolonged rainy and dry periods with frequent violent weather events. Mean temperatures 1.2 °C–20.7 °C | Minimum daily temperature. No lag. | Multivariate statistical analysis, using event-history techniques | 175 neonatal deaths (parish registers) | Temperature at birth had a significant effect on neonatal mortality; RR 0.911 SE 0.028, p = 0.003; 9% decrease in mortality for each unit increment in temperature at birth. Daily temperature was not associated with increased neonatal mortality RR 1.031 SE 0.928 p = 0.255. Landless rural labourers were at a higher risk for neonatal mortality related to temperature at birth as compared to sharecroppers and farmers during the 1860–1900 period: RR 0.830 SE 0.068 p = 0.023 |
| SIDS | |||||||
| Jhun (2017) [39] | Case-crossover | 210 cities in USA 1972–2006 | 8 climate clusters were created given variability across different cities | Mean temperature. Lag 0–2 days | Time-stratified case-crossover | 60,364 SIDS cases (routine data. ICD 8–795.0, ICD 9–798.0 and ICD 10-R95.0) | 8.6% (95%CI = 3.6, 13.8) increase in SIDS risk for 5.6 °C increase in temperature. 3.1% (95% CI = −5.0, −1.3) decrease in the winter. Summer risks were greater among black infants 18.5% (95%CI = 9.3, 28.5) compared to white infants 3.6% (95%CI = −2.3, 9.9) |
| Auger (2015) [40] | Case-crossover | Montreal, Canada1981–2010 | Continental climate with hot summers and cold winters. Maximum temperatures ranged from −1.5 °C to 33.8 °C on days before SIDS occurred. | Maximum temperature during warm months. Lag 0–2 days | Time-stratified case-crossover | 196 SIDS cases (routine data) | Same-day maximum daily temperature of 24 °C, 27 °C and 30 °C when compared to 20 °C increased the odds of SIDS by 1.41 (95%CI = 1.17, 1.69), 2.12 (95%CI = 1.43, 3.14) and 3.18 (95%CI = 1.76, 5.77), respectively. |
| Waldhoer (2017) [41] | Case-crossover | Vienna, Austria1984–2014 | Continental climate. Mean maximum temperatures on day before and day of SIDS 14.4 °C–28.9 °C | Maximum daily temperature. Lag 0–1 days | Time-stratified case-crossover | 187 SIDS cases (routine data. ICD 10-R95.0, ICD 9–798.0 and mentioned autopsy) | OR for SIDS at same day temperatures of 24 °C, 27 °C and 30 °C compared to 20 °C were 1.05 (95%CI = 0.87, 1.27), 1.13 (95%CI = 0.76, 1.68), and 1.23 (95%CI = 0.67, 2.29), respectively. |
| Scheers-Masters (2004) [42] | Retrospective cohort | Arkansas, Georgia, Kansas and Missouri, USA1980 | Temperate climate | Daily average and maximum temperatures | Chi-squared test for trend. Spearman’s rank correlation coefficient | 111 SIDS cases (ICD 9–79.8) and heat-related mortality (ICD 9–900: Death due to excessive heat due to weather conditions) | No increase in SIDS rate with increasing average (p = 0.713) and maximum temperature (p = 0.362). |
| Chang (2013) [43] | Case-control | Taiwan 1994–2003 | Temperate climate | Daily maximum temperature categorised into percentiles | Log-liner model | 1671 SIDS cases (routine data, ICD 9–789) | Risk of SIDS at the lowest percentile <5th (9.2–14.2 °C) compared to 45th–55th percentile (21.9–23.3 °C) is 2.10 (95%CI = 1.67, 2.64). Daily mean temperature in 85th–95th percentile (26.4–27.3 °C) and >95th percentile (27.3–33.2 °C) associated with reduced risk of SIDS 0.60 (95%CI = 0.46, 0.79) and 0.61 (95%CI = 0.50, 0.75), respectively. |
 Cold exposure.
Cold exposure.| Main Author (Year) | Research Design | Location, Year of Study | Description of Climate | Main Temperature Exposure Variable(s) | Statistical Analysis | Outcomes Measures (Source of Data) | Effect Estimates |
|---|---|---|---|---|---|---|---|
| All-Cause/Heat-Related Hospital Visits and/or Admissions | |||||||
| Sheffield (2018) [13] | Case-crossover | New York City, USA 2005–2011 | Continental climate. | Minimum, maximum and average apparent daily temperature. Lag 0–6 days | Time-stratified case-crossover | 278,114 all-cause emergency department (ED) visits (hospital records, all-cause diagnostic codes) | Positive association with maximum temperature; 0.6% (95%CI = 0.1, 1.1). |
| Xu (2017) [44] | Time-series analysis | Brisbane, Australia2005–2015 | Humid, sub-tropical climate. Mean temperature 9.0 °C–30.9 °C | Mean daily temperature. Nine different heatwave definitions | Poisson generalised additive model and distributed lag non-linear model | 53,792 all-cause hospital admissions (hospital records) | When mean temperature was >97th percentile, the RR of hospital admissions increased by 1.05 (95%CI = 1.01, 1.10), and then further increased to 1.18 (95%CI = 1.05, 1.32) when duration of heatwave increased from 2 days to 4 days. No evidence of effect modification. |
| Kakkad (2014) [19] | Retrospective cohort | Ahmedabad, India 2009–2011 | Warm, dry conditions that often include heat waves. Average monthly maximum of 38.8 °C between March and June. Heat wave in May 2010, maximum temperature 46.8 °C. | Daily maximum temperature. No lags. | Generalised linear models and segmented regression. | Heat-related illness in neonatal admissions—defined as diagnosis of exclusion when body temperature >38 °C with any signs and symptoms such as refusal to feed, signs of dehydration, increased respiratory rate, convulsions and/or lethargy (hospital records) | Above 42 °C, each temperature increase of a degree was associated with a 43% increase in heat-related admissions (95%CI = 9.2, 88). |
| Mannan (2011) [28] | Retrospective cohort | Sylhet district, Bangladesh2004–2006 | Tropical monsoon climate. Monthly average temperatures range 19.3 °C–29.3 °C for Sylhet and 17.7 °C–29.5 °C for Mirzapur | 7 day rolling average temperature and rolling humidity index prior to diagnosis | Multivariable logistic regression. | Very severe disease in 6936 newborns in Sylhet and 5900 newborns in Mirzapur—diagnosed on history using clinical algorithms) (data from 2 cluster randomised controlled trials) | OR for temperature at time of diagnosis of very severe disease were 1.14 (95%CI = 1.08, 1.21) in Sylhet and 1.06 (95%CI = 1.04, 1.07) in Mirzapur. |
| Infectious diseases | |||||||
| Gosai (2009) [45] | Retrospective cohort | Auckland, New Zealand1994–2004 | Temperate oceanic climate. Average monthly temperatures 10.8 °C–19.8 °C | Daily minimum temperature. Lag 0–7 days | Pearson’s correlation | Hospital admissions for respiratory illness (hospital admissions data. No ICD codes given) | Correlation between minimum temperature and respiratory infections and inflammation (r = −0.42 and p < 0.001) and total whooping cough and acute bronchiolitis (r = −0.40 and p < 0.001). |
| Yan (2019) [29] | Time-series analysis | Shenzhen, China 2009–2017 | Subtropical monsoon climate. Mean temperature over period 23.3 °C. | Daily temperature, unclear which index used. Lag with a max of 30 days | Quasi-Poisson regression based on distributed lag nonlinear model | 50,657 HFMD cases (surveillance data—notifiable disease) | Cumulative RR of HFMD over 14 days in 0–1 age group was RR 0.58 (95%CI = 0.4, 0.84) for temperature in 5th percentile and RR 2.03 (95%CI = 1.77, 2.33) in the 95th percentile. 5th percentile was 12.9 °C, 95th was 30 °C and median 24.6 °C. |
| Yin (2015) [30] | Time series analysis | Chengdu, China 2013–2014 | Humid sub-tropical climate. Mean temperature for study period 16.21 °C. | Daily mean temperature. Lag 0–14 days | Poisson generalised linear regression combined with distributed lag non-linear model | 74,247 HFMD cases aged 0–5 years (surveillance data—notifiable disease) | Risk of HFMD significantly increased at temperatures 14, 17.2, 23.2 and 27 compared to 0 °C at lag 0 and lag 14 for infants <1 years old. No increase in HFMD risk for colder temperature exposure of 4.1 °C. For lag 0, RR 1.15 (95%CI = 1.03, 1.29), 1.19 (95%CI = 1.06, 1.34), 1.29 (95%CI = 1.13, 1.44), and 1.31 (95%CI 1.16, 1.48) for 14, 17.2, 23.2, and 27 °C, respectively. For lag 14 RR 1.09 (95%CI = 1.03, 1.16), 1.07 (95%CI = 1.00, 1.14), 1.06 (95%CI = 1.00, 1.13), and 1.03 (95%CI = 1.02, 1.04) for 14, 17.2, 23.2, and 27 °C, respectively. |
| Nenna (2017) [46] | Prospective cohort | Rome, Italy 2004–2014 | Mediterranean climate | Weekly average temperature. No lag | Pearson’s correlation | 723 cases of viral bronchiolitis in hospitalised infants (prospective clinical records) | The number of RSV-positive infants correlated negatively with temperature (r = −0.46, p < 0.001). |
| Kim (2017) [47] | Prospective cohort | Cheonan, South Korea 2006–2014 | Subtropical climate | Mean daily temperature. No lag days | Logistic regression | 2484 infants admitted with RSV A and RSV B (lab confirmed admissions with respiratory symptoms) | RSV A and RSV B infections were negatively correlated with average temperature; −0.056 for RSV A and −0.069 for RSV B infection, with p < 0.001 for both. |
| Hoeppner (2017) [48] | Retrospective cohort | Australia and New Zealand 2009–2011 | Perth—Mediterranean, Melbourne and Auckland-oceanic and Brisbane—humid, subtropical | Minimum temperature aggregated over a week. Lag 0–4 weeks | Linear regression. Poisson and negative binomial regression to verify results | 3876 infants admitted with bronchiolitis (data from prospective, multicentre clinical trial) | Minimum temperature and lag 0): r −0.62 (−0.75, 0.48) p < 0.001. Lag 1: r −0.58 (95%CI = −0.75, −0.43) p < 0.001. Lag 2: r −0.67 (95%CI = −0.75, −0.58) p < 0.001. Lag 3: r −0.34 (95%CI = −0.49, −0.20) p < 0.001 |
| Other neonatal outcomes | |||||||
| Iijima (2016) [49] | Prospective cohort | Hamamatsu, Japan 2012–2013 | Temperate climate. Average temperatures spring 15.3 °C, summer 27.3 °C, autumn, 18.0 °C and winter 7.0 °C. | Mean outdoor and indoor, and wind chill temperature. No lag | Simple and multivariate regression | 498 neonates. International normalised ratio on day 4 after birth (data from healthy neonates) | Significant correlation between INR and outdoor temperature (r = 0.25, p < 0.001). Weakly negative correlation between INR and room temperature (r = −0.13, p = 0.02). |
| Scrafford (2013) [31] | Retrospective cohort | Southern Nepal 2003–2006 | Humid subtropical climate | Minimum daily temperature. No lag days | Bivariate and multivariate analyses | Incidental jaundice in 18,985 neonates, defined as first report of yellow eyes/body based on visual assessment by study staff, not laboratory confirmed (part of nested pair of cluster-randomised, placebo-controlled, community based clinical trial) | OR 1.03 (95%CI = 1.02, 1.05) p < 0.001 for each 1 °C increase in minimum ambient temperature. Adjusted OR 1.04 (95%CI = 1.03, 1.06). |
 Cold exposure.
Cold exposure.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhoo, D.P.; Blake, H.A.; Chersich, M.F.; Nakstad, B.; Kovats, S. The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9109. https://doi.org/10.3390/ijerph19159109
Lakhoo DP, Blake HA, Chersich MF, Nakstad B, Kovats S. The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(15):9109. https://doi.org/10.3390/ijerph19159109
Chicago/Turabian StyleLakhoo, Darshnika Pemi, Helen Abigail Blake, Matthew Francis Chersich, Britt Nakstad, and Sari Kovats. 2022. "The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 15: 9109. https://doi.org/10.3390/ijerph19159109
APA StyleLakhoo, D. P., Blake, H. A., Chersich, M. F., Nakstad, B., & Kovats, S. (2022). The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review. International Journal of Environmental Research and Public Health, 19(15), 9109. https://doi.org/10.3390/ijerph19159109






