How Does Hedonic Aroma Impact Long-Term Anxiety, Depression, and Quality of Life in Women with Breast Cancer? A Cross-Lagged Panel Model Analysis
Abstract
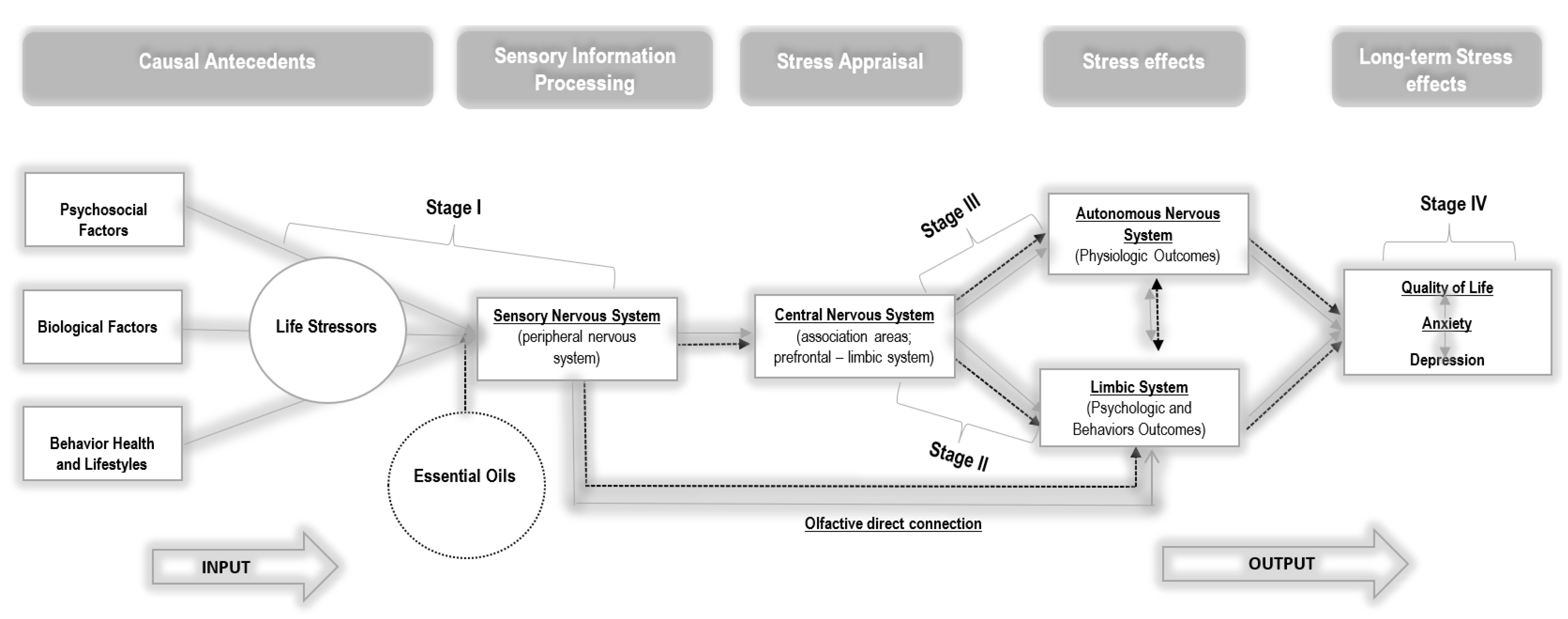
:1. Introduction
2. Methods
2.1. Study Design
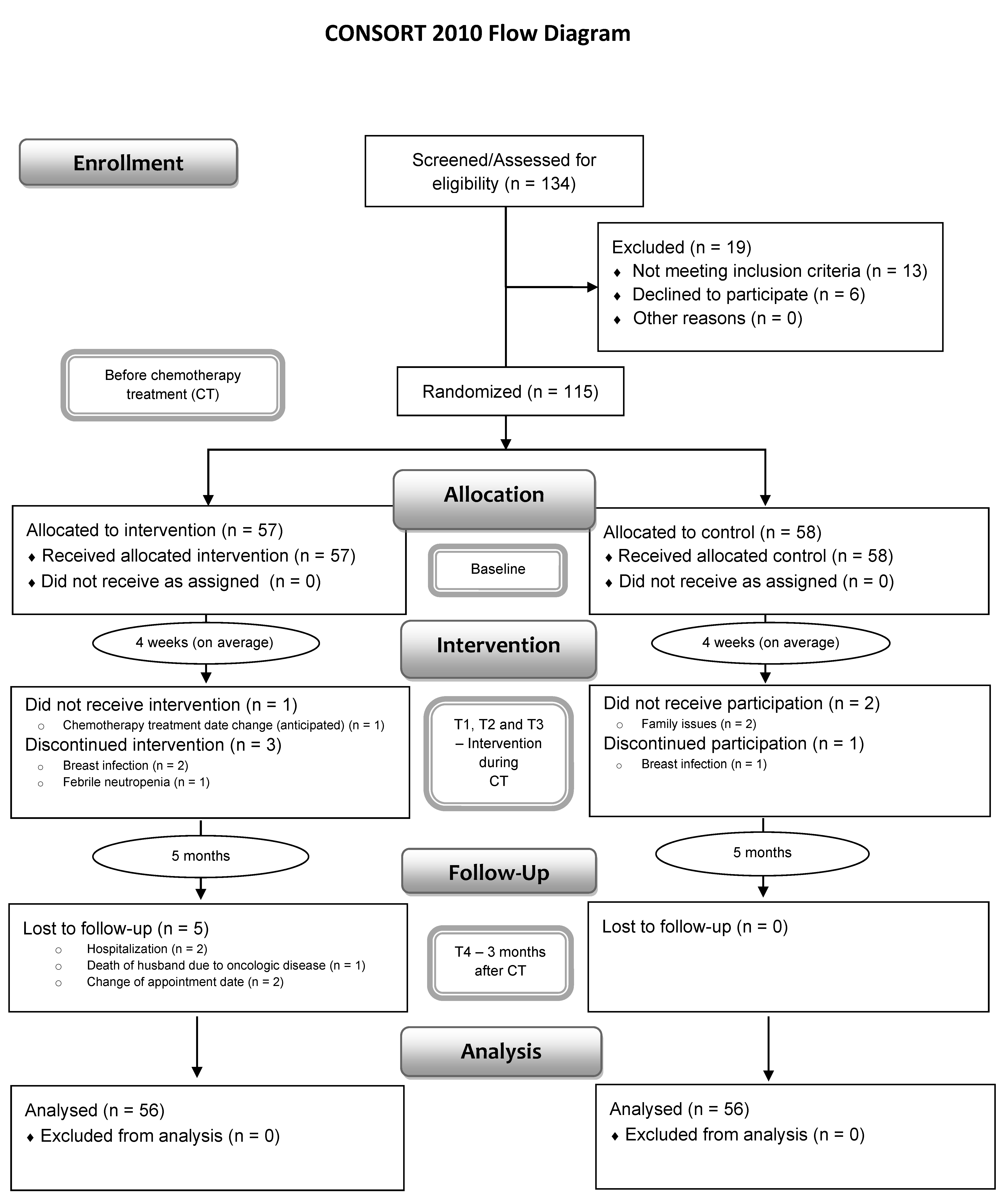
2.2. Sample Size, Participants, and Randomization
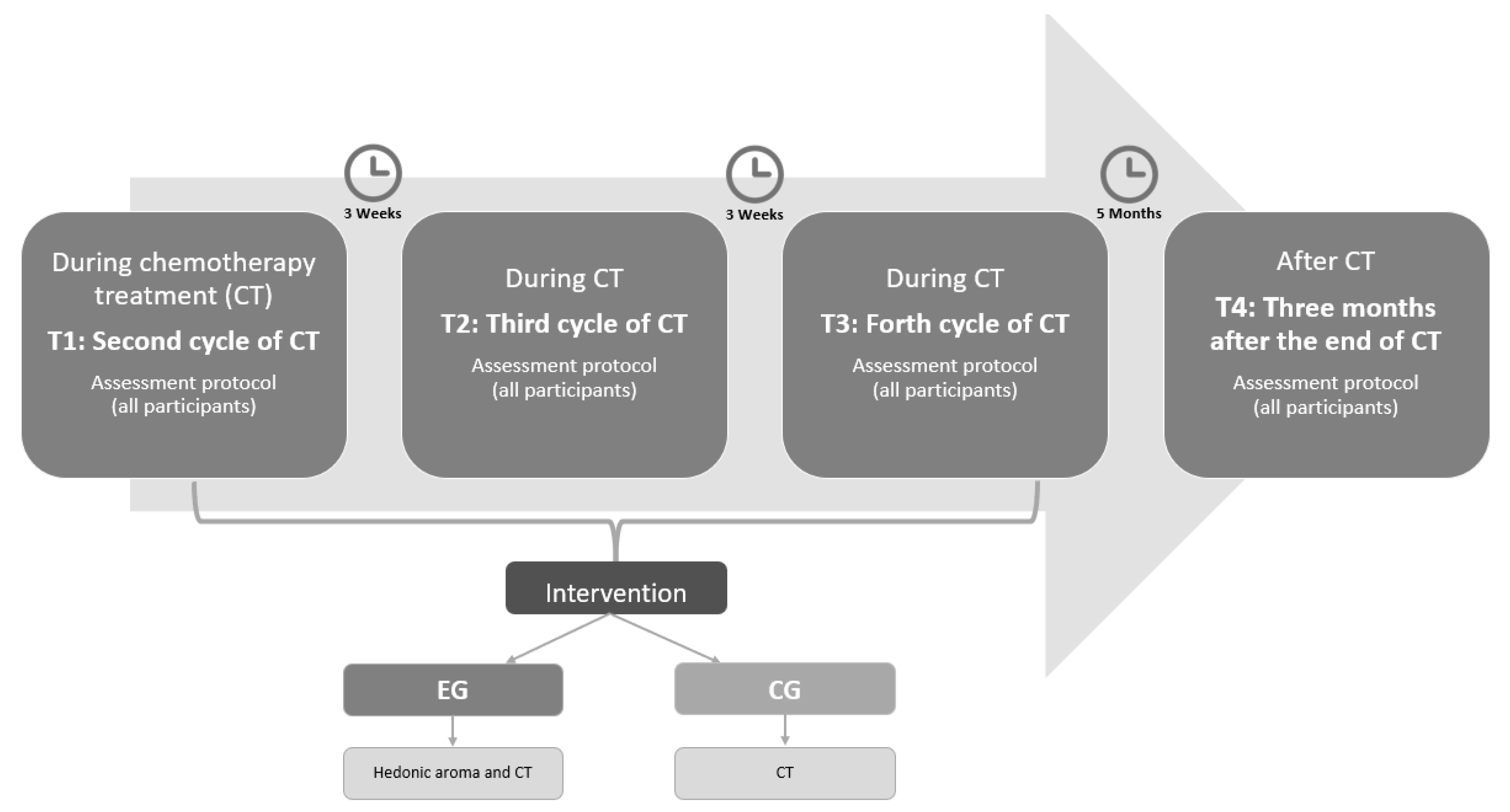
2.3. Intervention
2.4. Measurements
2.5. Other Pre-Specified Outcome Measures
2.6. Data Collection Procedure
2.7. Data Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. Longitudinal Predictions between Anxiety, Depression, and QoL
4. Discussion
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef]
- Piechocki, M.; Koziołek, W.; Sroka, D.; Matrejek, A.; Miziołek, P.; Saiuk, N.; Sledzik, M.; Jaworska, A.; Bereza, K.; Pluta, E.; et al. Trends in incidence and mortality of gynecological and breast cancers in Poland (1980–2018). Clin. Epidemiol. 2022, 14, 95–114. [Google Scholar] [CrossRef]
- Wojtyla, C.; Bertuccio, P.; Wojtyla, A.; La Vecchia, C. European trends in breast cancer mortality, 1980–2017 and predictions to 2025. Eur. J. Cancer 2021, 152, 4–17. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care 2019, 14, 344–353. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef] [Green Version]
- Singer, S.; Blettner, M.; Kreienberg, R.; Janni, W.; Wöckel, A.; Kühn, T.; Felberbaum, R.; Flock, F.; Schwentner, L. Breast cancer patients’ fear of treatment: Results from the multicenter longitudinal study BRENDA II. Breast Care 2015, 10, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, Y.; Feng, Z.; Xu, Y.; Zeng, G. Longitudinal trends in anxiety, depression, and quality of life during different intermittent periods of adjuvant breast cancer chemotherapy. Cancer Nurs. 2018, 41, 62–68. [Google Scholar] [CrossRef]
- Ho, S.S.M.; So, W.K.W.; Leung, D.Y.P.; Lai, E.T.L.; Chan, C.W.H. Anxiety, depression and quality of life in Chinese women with breast cancer during and after treatment: A comparative evaluation. Eur. J. Oncol. Nurs. 2013, 17, 877–882. [Google Scholar] [CrossRef]
- So, W.K.W.; Marsh, G.; Ling, W.M.; Leung, F.Y.; Lo, J.C.K.; Yeung, M.; Li, G.K.H. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur. J. Oncol. Nurs. 2010, 14, 17–22. [Google Scholar] [CrossRef]
- Lazarus, R.; Folkman, S. Stress Appraisal and Coping; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Maass, S.W.M.C.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.M.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Allen, J.D.; Savadatti, S.; Gurmankin Levy, A. The transition from breast cancer ‘patient’ to ‘survivor’. Psycho-Oncology 2009, 18, 71–78. [Google Scholar] [CrossRef]
- Knobf, M.T. Clinical update: Psychosocial responses in breast cancer survivors. Semin. Oncol. Nurs. 2011, 27, e1–e14. [Google Scholar] [CrossRef]
- Clark, L.A.; Watson, D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 1991, 100, 316–336. [Google Scholar] [CrossRef]
- Taira, N.; Shimozuma, K.; Shiroiwa, T.; Ohsumi, S.; Kuroi, K.; Saji, S.; Saito, M.; Iha, S.; Watanabe, T.; Katsumata, N. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res. Treat. 2011, 128, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.; Choudhary, V.; Parganiha, A. Worsening of rest-activity circadian rhythm and quality of life in female breast cancer patients along progression of chemotherapy cycles. Chronobiol. Int. 2017, 34, 609–623. [Google Scholar] [CrossRef]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Supportive Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef]
- Alwhaibi, M.; AlRuthia, Y.; Alhawassi, T.M.; Almalag, H.; Alsalloum, H.; Balkhi, B. Polypharmacy and comorbidities among ambulatory cancer patients: A cross-sectional retrospective study. J. Oncol. Pharm. Pract. 2020, 26, 1052–1059. [Google Scholar] [CrossRef]
- Balducci, L.; Goetz-Parten, D.; Steinman, M.A. Polypharmacy and the management of the older cancer patient. Ann. Oncol. 2013, 24, 36–40. [Google Scholar] [CrossRef]
- Keats, M.R.; Cui, Y.; DeClercq, V.; Grandy, S.A.; Sweeney, E.; Dummer, T.J.B. Burden of multimorbidity and polypharmacy among cancer survivors: A population-based nested case–control study. Supportive Care Cancer 2021, 29, 713–723. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Jafarimanesh, H.; Akbari, M.; Hoseinian, R.; Zarei, M.; Harorani, M. The effect of peppermint (Mentha piperita) extract on the severity of nausea, vomiting and anorexia in patients with breast cancer undergoing chemotherapy: A randomized controlled trial. Integr. Cancer Ther. 2020, 19, 1534735420967084. [Google Scholar] [CrossRef]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complementary Ther. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef]
- Ozkaraman, A.; Dügüm, Ö.; Özen Yılmaz, H.; Usta Yesilbalkan, Ö. Aromatherapy: The effect of lavender on anxiety and sleep quality in patients treated with chemotherapy. Clin. J. Oncol. Nurs. 2018, 22, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Masaoka, Y.; Homma, I. Breathing for olfaction and emotions. In The Biology of Odors: Sources, Olfaction and Response; Logan, E., Weiss, E., Atwood, J.M., Eds.; Nova Biomedical: Waltham, MA, USA, 2011; pp. 295–307. [Google Scholar]
- Lazarus, R.S.; Folkman, S. Transactional theory and research on emotions and coping. Eur. J. Personal. 1987, 1, 141–169. [Google Scholar] [CrossRef]
- Liu, X.; Haagsma, J.; Sijbrands, E.; Buijks, H.; Boogaard, L.; Mackenbach, J.P.; Erasmus, V.; Polinder, S. Anxiety and depression in diabetes care: Longitudinal associations with health-related quality of life. Sci. Rep. 2020, 10, 8307. [Google Scholar] [CrossRef]
- Kim, J.H.; Paik, H.-J.; Jung, Y.J.; Kim, D.; Jo, H.J.; Lee, S.; Kim, H.Y. A prospective longitudinal study about change of sleep, anxiety, depression, and quality of life in each step of breast cancer patients. Oncology 2019, 97, 245–253. [Google Scholar] [CrossRef]
- Pereira, M.; Moreira, C.; Izdebski, P.; Dias, A.C.P.; Nogueira-Silva, C.; Pereira, M.G. The Mid-Term Impact of a Hedonic Aroma on Psychophysiological Indicators in Women with Breast Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. (Manuscript Submitted for Publication); 2022. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Schneider, R. There is something in the air: Testing the efficacy of a new olfactory stress relief method (AromaStick®). Stress Health 2016, 32, 411–426. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Pais-Ribeiro, J.; Pinto, C.; Santos, C. Validation study of the Portuguese version of the QLC-C30-V.3. Psicol. Saúde Doenças 2008, 9, 89–102. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar]
- Simmen, D.; Briner, H.R.; Hess, K. Screeningtest des Geruchssinnes mit Riechdisketten. Laryngo-Rhino-Otologie 1999, 78, 125–130. [Google Scholar] [CrossRef]
- Lee, S.-Y.D.; Stucky, B.D.; Lee, J.Y.; Rozier, R.G.; Bender, D.E. Short Assessment of Health Literacy-Spanish and English: A comparable test of health literacy for Spanish and English speakers. Health Serv. Res. 2010, 45, 1105–1120. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software]; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar]
- Jorgensen, T.D.; Pornprasertmanit, S.; Schoemann, A.M.; Rosseel, Y. semTools: Useful Tools for Structural Equation Modeling. R Package Version 0.5-4. 2021. Available online: https://CRAN.R-project.org/package=semTools (accessed on 12 July 2018).
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Buchanan, T. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In Handbook of Psychophysiology, 4th ed.; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 465–494. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R. Do low levels of stress reactivity signal poor states of health? Biol. Psychol. 2011, 86, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R. Central nervous system integration of the psychological stress response. In Stress and Health: Biological and Psychological Interactions, 3rd ed.; Lovallo, W.R., Ed.; Sage Publishing: New York, NY, USA, 2016; pp. 85–114. [Google Scholar]
- Murison, R. The Neurobiology of Stress. In Neuroscience of Pain, Stress, and Emotion; al’Absi, M., Flaten, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 29–49. [Google Scholar] [CrossRef]
- Fujiwara, R. Psychoneuroimmunological benefits of aromatherapy. Int. J. Aromather. 2002, 12, 77–82. [Google Scholar] [CrossRef]
- Turley, L.W.; Milliman, R.E. Atmospheric effects on shopping behavior: A review of the experimental evidence. J. Bus. Res. 2000, 49, 193–211. [Google Scholar] [CrossRef]
- Amaral, D.G. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol. Psychiatry 2002, 51, 11–17. [Google Scholar] [CrossRef]
- Rolls, E.T. Emotion and Decision-Making Explained; Oxford University Press: Oxford, UK, 2014; pp. 159–189. [Google Scholar]
- Amaral, D.G.; Price, J.L.; Pitkänen, A.; Carmichael, T. Anatomical organization of the primate amygdaloid complex. In The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction; Aggleton, J., Ed.; Wiley-Liss: Hoboken, NJ, USA, 1992; pp. 1–66. [Google Scholar]
- Andreotti, C.; Root, J.C.; Ahles, T.A.; McEwen, B.S.; Compas, B.E. Cancer, coping, and cognition: A model for the role of stress reactivity in cancer-related cognitive decline. Psycho-Oncology 2015, 24, 617–623. [Google Scholar] [CrossRef]
- McCain, N.L.; Gray, D.P.; Walter, J.M.; Robins, J. Implementing a comprehensive approach to the study of health dynamics using the psychoneuroimmunology paradigm. Adv. Nurs. Sci. 2005, 28, 320–332. [Google Scholar] [CrossRef] [Green Version]


| T1 (n = 112) | CG (n = 56) | EG (n = 56) | Differences between Groups d |
|---|---|---|---|
| Freq./M (SD) | Freq./M (SD) | Estimate, p-Value | |
| Sociodemographic variables | |||
| Age | 51.48 (10.34) | 53.50 (10.22) | 2.02, p = 0.295 |
| Local residence: | |||
| Urban | 18 | 19 | −0.08, p = 0.841 |
| Rural | 38 | 37 | |
| Marital status: | |||
| Married/cohabiting | 45 | 43 | 0.213, p = 0.645 |
| Unmarried a | 11 | 13 | |
| Education: | |||
| Primary Studies | 40 | 32 | 0.72, p = 0.071 |
| Secondary studies | 9 | 13 | |
| University degree | 7 | 11 | |
| Professional Situation: | |||
| Active | 1 | 3 | −1.14, p = 0.332 |
| Not active | 55 | 53 | |
| Clinical variables | |||
| Surgery type: | |||
| Lumpectomy | 45 | 44 | 0.11, p = 0.815 |
| Mastectomy b | 11 | 12 | |
| Cancer Stage: | |||
| T1 | 27 | 17 | 0.759, p = 0.055 |
| T2 | 29 | 39 | |
| Axillary lymph node dissection: | |||
| Yes | 29 | 25 | 0.29, p = 0.450 |
| No | 27 | 31 | |
| Number of Chemotherapy Cycles/Cytotoxic Drugs c | |||
| 4 cycles (AC) | 18 | 18 | 0.36, p = 0.297 |
| 6 cycles (FEC-D) | 15 | 9 | |
| 8 cycles (AC-D) | 7 | 4 | |
| 16 cycles (AC-P) | 16 | 25 | |
| Breast Cancer Grade: | |||
| 1 | 7 | 6 | −0.01, p = 0.981 |
| 2 | 35 | 37 | |
| 3 | 14 | 13 | |
| Months since diagnosis: | 2.79 (1.12) | 2.96 (1.32) | 0.06, p = 0.429 |
| Psychological Variables | |||
| QoL | 71.45 (16.08) | 74.4 (14.79) | 0.13, p = 0.356 |
| Anxiety | 6.91 (4.09) | 5.25 (3.53) | −0.28, p = 0.076 |
| Depression | 4.44 (3.63) | 3.93 (2.94) | −0.11, p = 0.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Moreira, C.S.; Izdebski, P.; Dias, A.C.P.; Nogueira-Silva, C.; Pereira, M.G. How Does Hedonic Aroma Impact Long-Term Anxiety, Depression, and Quality of Life in Women with Breast Cancer? A Cross-Lagged Panel Model Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9260. https://doi.org/10.3390/ijerph19159260
Pereira M, Moreira CS, Izdebski P, Dias ACP, Nogueira-Silva C, Pereira MG. How Does Hedonic Aroma Impact Long-Term Anxiety, Depression, and Quality of Life in Women with Breast Cancer? A Cross-Lagged Panel Model Analysis. International Journal of Environmental Research and Public Health. 2022; 19(15):9260. https://doi.org/10.3390/ijerph19159260
Chicago/Turabian StylePereira, Marta, Célia Sofia Moreira, Pawel Izdebski, Alberto C. P. Dias, Cristina Nogueira-Silva, and M. Graça Pereira. 2022. "How Does Hedonic Aroma Impact Long-Term Anxiety, Depression, and Quality of Life in Women with Breast Cancer? A Cross-Lagged Panel Model Analysis" International Journal of Environmental Research and Public Health 19, no. 15: 9260. https://doi.org/10.3390/ijerph19159260
APA StylePereira, M., Moreira, C. S., Izdebski, P., Dias, A. C. P., Nogueira-Silva, C., & Pereira, M. G. (2022). How Does Hedonic Aroma Impact Long-Term Anxiety, Depression, and Quality of Life in Women with Breast Cancer? A Cross-Lagged Panel Model Analysis. International Journal of Environmental Research and Public Health, 19(15), 9260. https://doi.org/10.3390/ijerph19159260







