Effects of Household Air Pollution (HAP) on Cardiovascular Diseases in Low- and Middle-Income Countries (LMICs): A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Population
2.2.2. Exposure
2.2.3. Comparator
2.3. Outcomes
2.4. Study Designs
2.5. Selection Process
2.6. Data Extraction
2.7. Quality Assessment
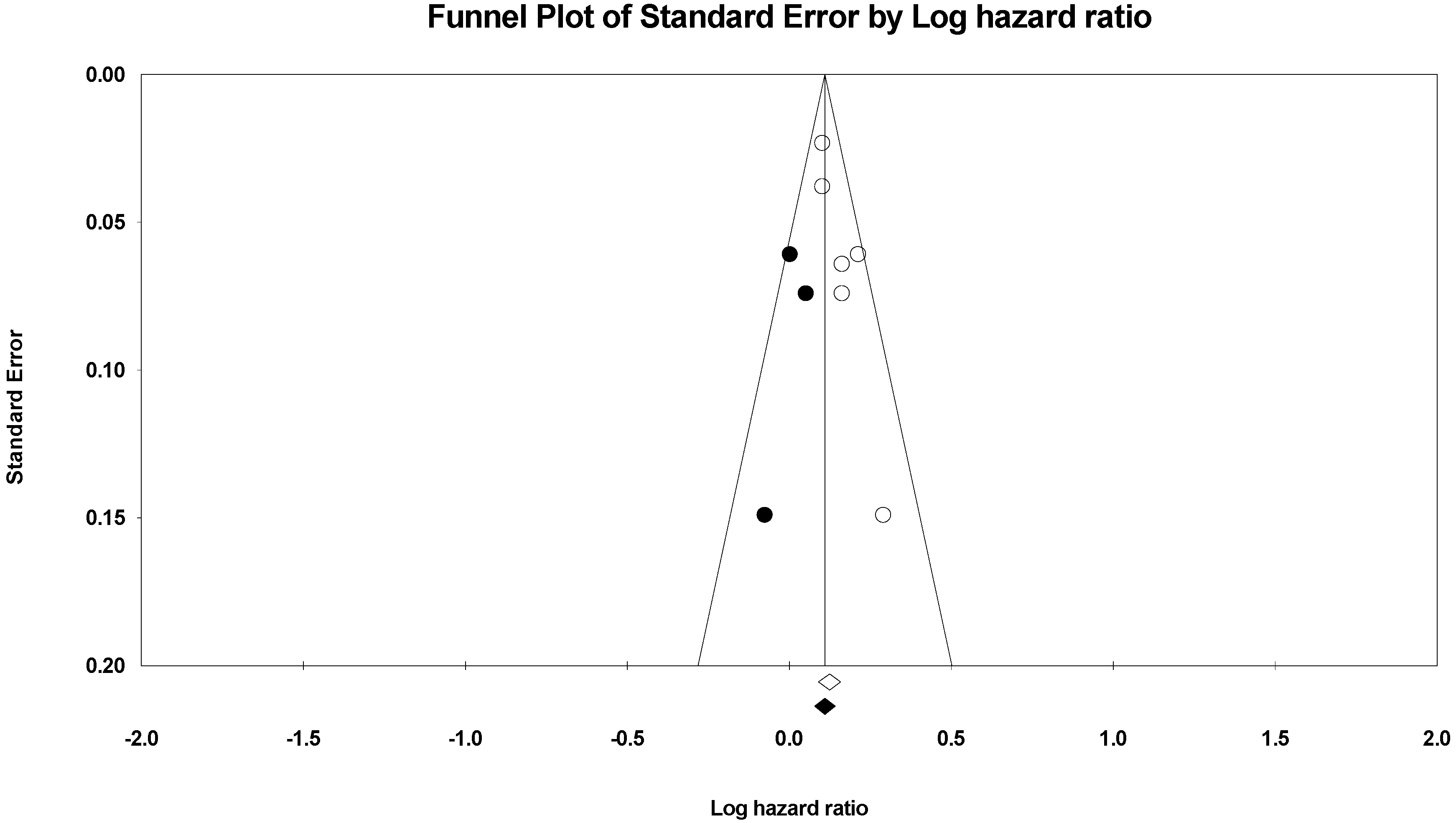
2.8. Statistical Analyses
3. Results
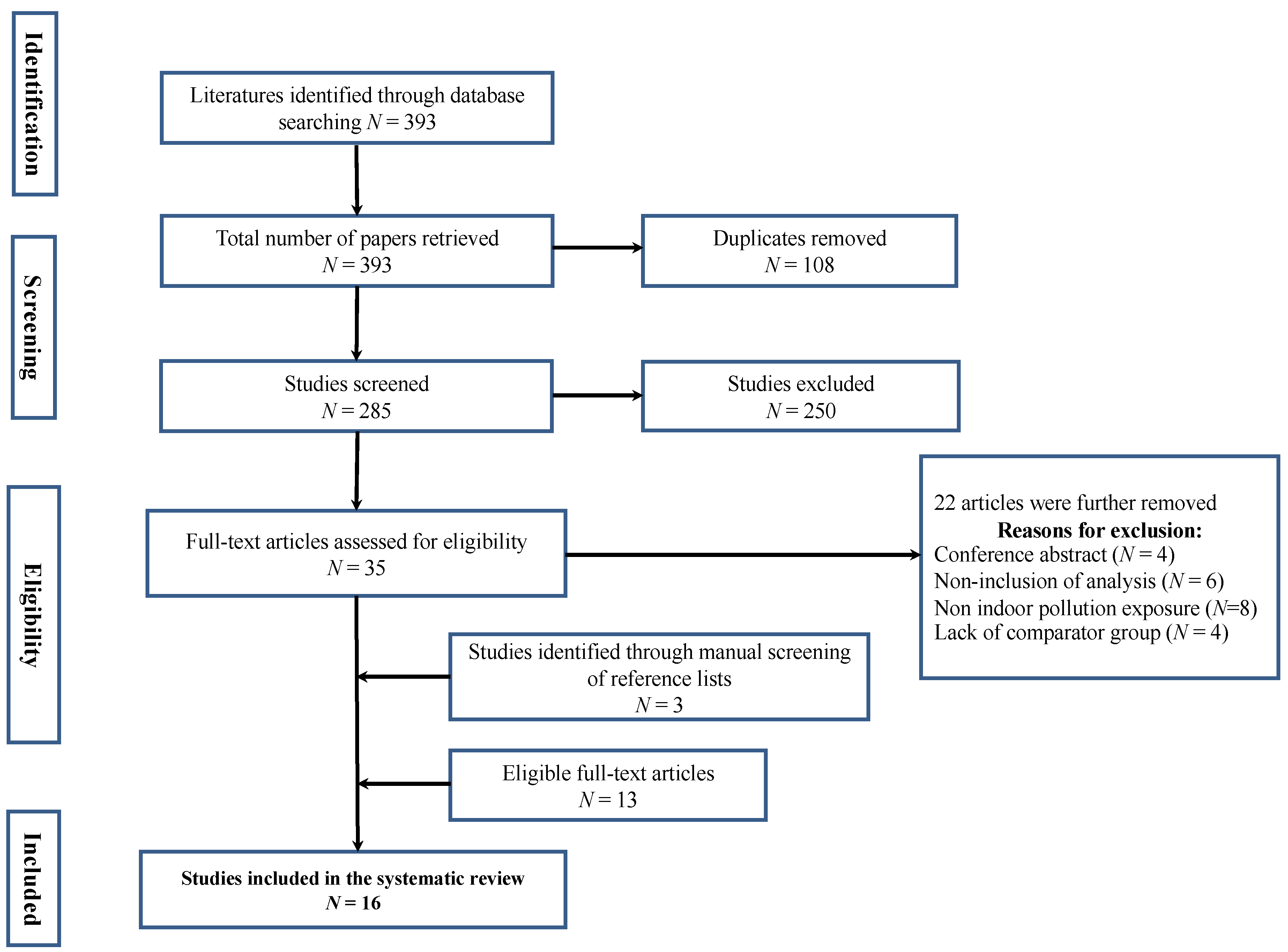
3.1. Search Results
3.2. Characteristics of Selected Studies
3.3. Cardiovascular Risk and Diseases Examined in Selected Studies
3.4. Patients’ Demographic Characteristics
3.5. Association between HAP and Cardiovascular Diseases
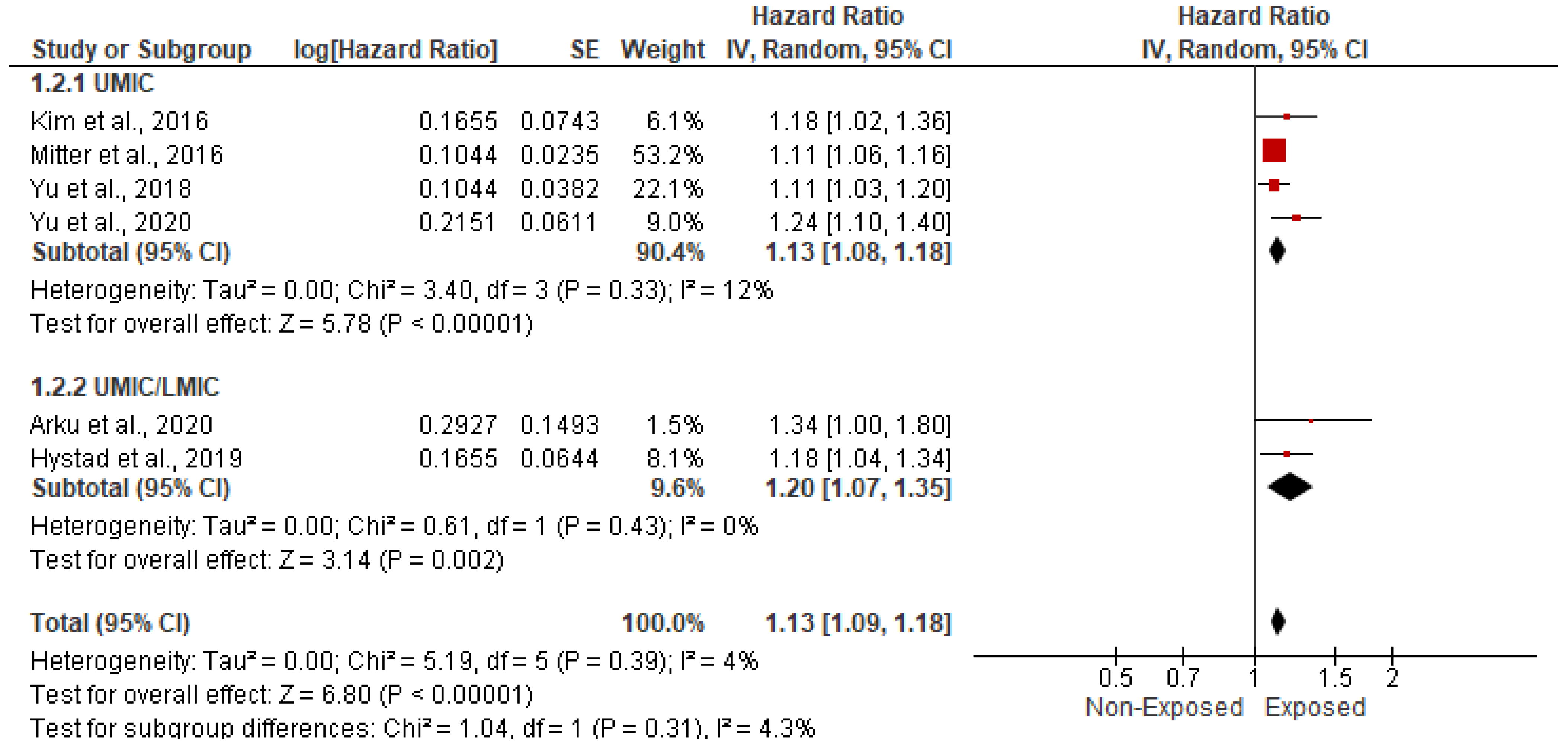
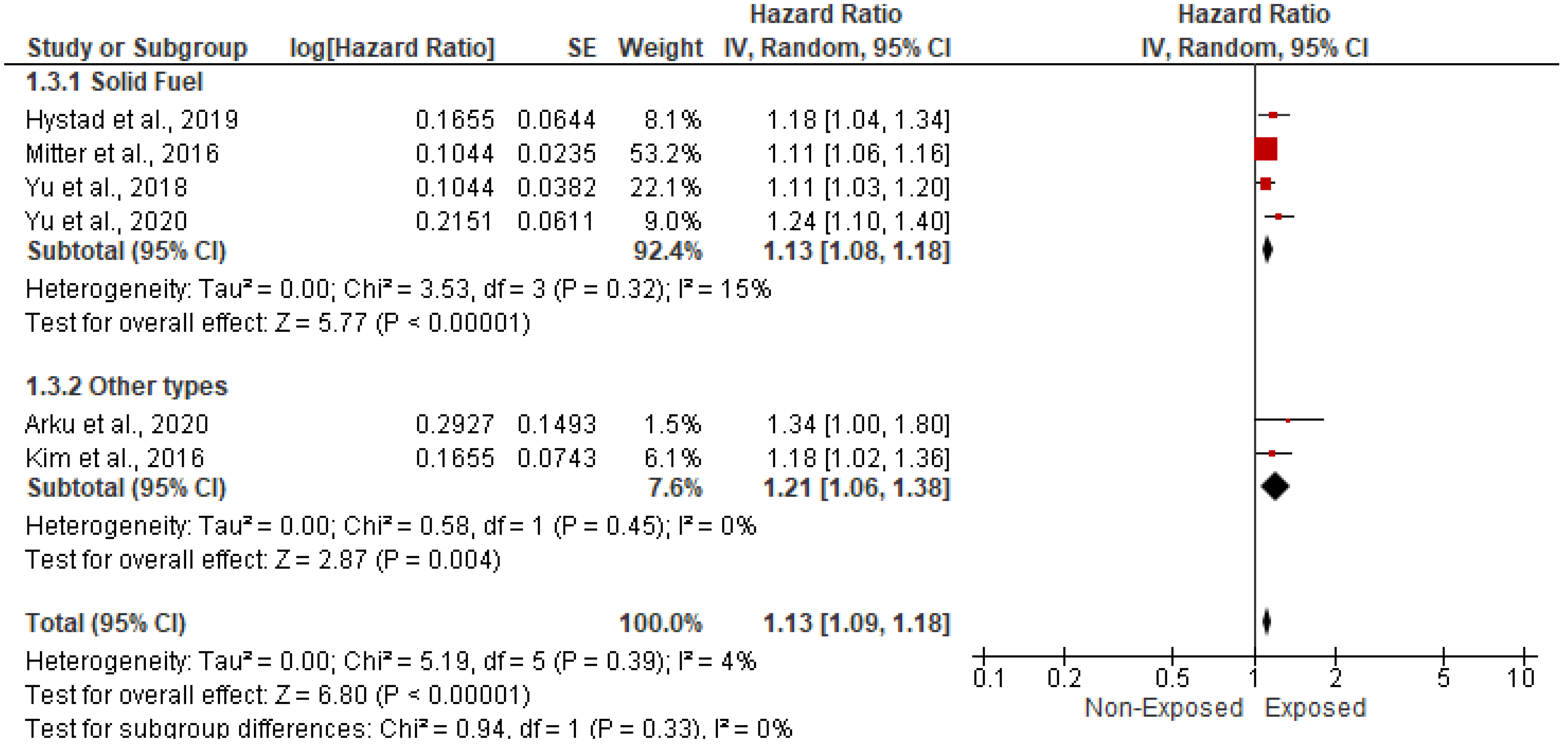
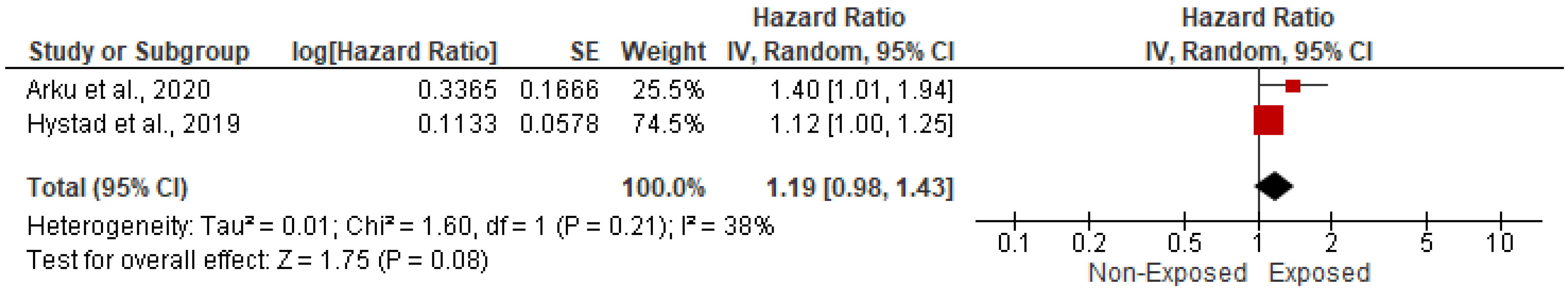
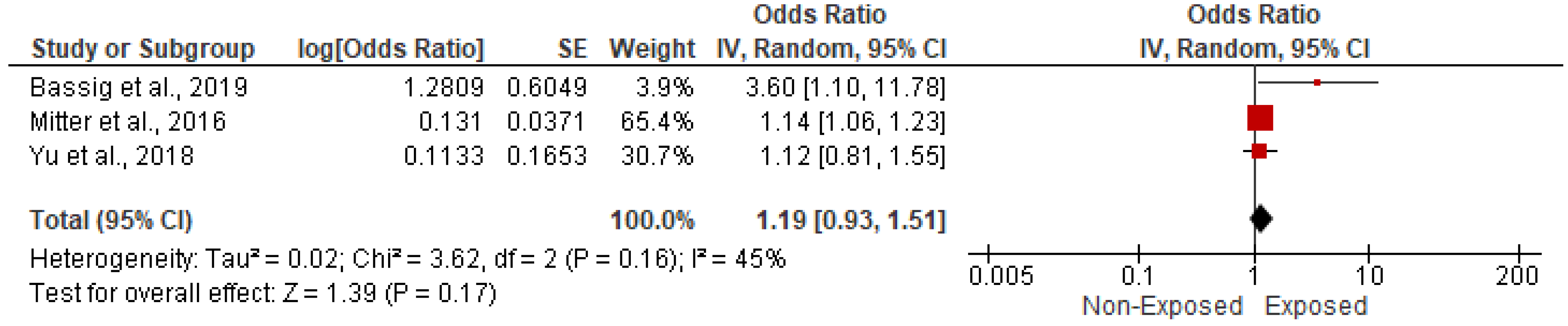
3.5.1. Association with CVD-Related Mortality
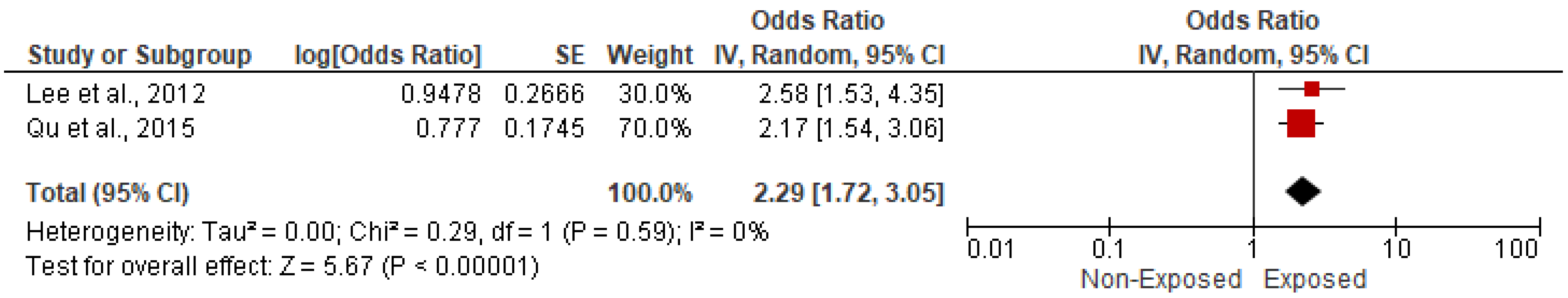
3.5.2. Association with Stroke and Cerebrovascular Accidents
3.5.3. Association with Myocardial Infarction
3.5.4. Association with Ischemic Heart Disease
3.5.5. Association with Heart Failure
3.5.6. Association with Hypertension
3.5.7. Association with Eclampsia/Pre-Eclampsia
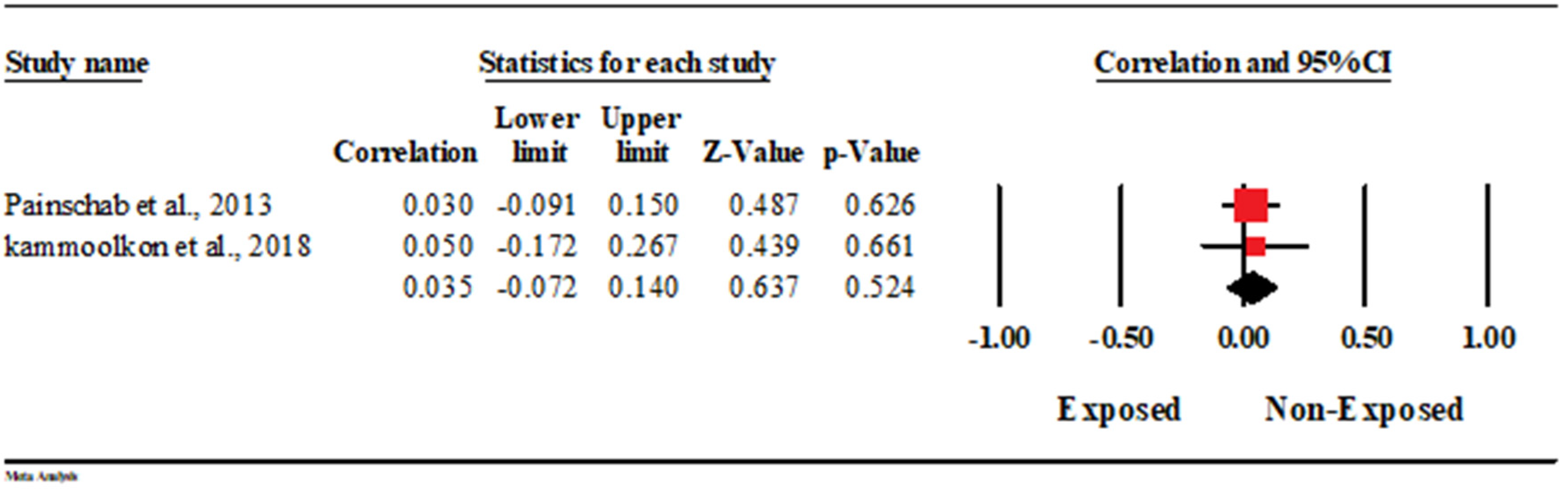
3.5.8. Association with Carotid Intimal Myocardial Thickness (CIMT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Household Air Pollution and Health; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hadley, M.B.; Baumgartner, J.; Vedanthan, R. Developing a clinical approach to air pollution and cardiovascular health. Circulation 2018, 137, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Ochieng, C.; Vardoulakis, S.; Tonne, C. Household air pollution following replacement of traditional open fire with an improved rocket type cookstove. Sci. Total Environ. 2017, 580, 440–447. [Google Scholar] [CrossRef]
- Sapkota, A.; Zaridze, D.; Szeszenia-Dabrowska, N.; Mates, D.; Fabiánová, E.; Rudnai, P.; Janout, V.; Holcatova, I.; Brennan, P.; Boffetta, P.; et al. Indoor air pollution from solid fuels and risk of upper aerodigestive tract cancers in central and eastern Europe. Environ. Res. 2013, 120, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Bing, R.; Kiang, J.; Bashir, S.; Spath, N.; Stelzle, D.; Mortimer, K.; Bularga, A.; Doudesis, D.; Joshi, S.S.; et al. Adverse health effects associated with household air pollution: A systematic review, meta-analysis, and burden estimation study. Lancet Glob. Health 2020, 8, e1427–e1434. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale for Assessing the Quality of Non-Randomized Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, version 3; Biostat: Englewood, NJ, USA, 2007. [Google Scholar]
- Painschab, M.S.; Davila-Roman, V.G.; Gilman, R.H.; Villar, A.D.V.; Pollard, S.L.; Wise, R.; Miranda, J.J.; Checkley, W.; CRONICAS Cohort Study Group. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart 2013, 99, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.J.; Singh, M.P.; Coull, B.A.; Quinn, A.; Yeboah-Antwi, K.; Sabin, L.; Hamer, D.H.; Singh, N.; MacLeod, W.B. Association between wood cooking fuel and maternal hypertension at delivery in central East India. Hypertens Pregnancy 2015, 34, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammoolkon, R.; Taneepanichskul, N.; Pitaknoppakul, N.; Lertmaharit, S.; Lohsoonthorn, V. Incense smoke and increasing carotid intima media thickness: A cross sectional study on the Thai-Vietnamese community. Asia Pac. J. Public Health 2018, 30, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Adu-Bonsaffoh, K.; Vermeulen, R.; Klipstein-Grobusch, K.; Grobbee, D.E.; Browne, J.L.; Downward, G.S. Household fuel use and adverse pregnancy outcomes in a Ghanaian cohort study. Reprod. Health 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Qiu, G.; Chan, K.H.; Lam, K.B.H.; Kurmi, O.P.; Bennett, D.A.; Yu, C.; Pan, A.; Lv, J.; Guo, Y.; et al. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. J. Amer. Med. Assn. 2018, 319, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Seow, W.J.; Shu, X.-O.; Bassig, B.A.; Rothman, N.; Chen, B.E.; Xiang, Y.-B.; Hosgood, H.D.; Ji, B.-T.; Hu, W.; et al. Cooking coal use and all-cause and cause-specific mortality in a prospective cohort study of women in Shanghai, China. Environ. Health Perspect. 2016, 124, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.S.; Vedanthan, R.; Islami, F.; Pourshams, A.; Khademi, H.; Kamangar, F.; Abnet, C.C.; Dawsey, S.M.; Pharoah, P.D.; Brennan, P.; et al. Household fuel use and cardiovascular disease mortality: Golestan Cohort Study. Circulation 2016, 133, 2360–2369. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Qiu, G.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Wang, C.; Pan, A.; Liang, L.; et al. Cooking fuels and risk of all-cuase and cardiopulmonary mortality in urban China: A prospective cohort study. Lancet Glob. Health 2020, 8, e430–e439. [Google Scholar] [CrossRef] [Green Version]
- Hystad, P.; Duong, M.; Brauer, M.; Larkin, A.; Arku, P.; Kurmi, O.P.; Fan, W.Q.; Avezum, A.; Azam, I.; Chifamba, J.; et al. Health effects of household solid fuel use: Findings from 11 countries with the prospective urban and rural epidemiology study. Environ. Health Perspect. 2019, 127, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassig, B.A.; Hosgood, H.D.; Shu, X.; Vermeulen, R.; Chen, B.E.; Katki, H.A.; Seow, W.J.; Hu, W.; Portengen, L.; Ji, B.-T.; et al. Ischeamic heart disease and stroke mortality by specific coal type among non-smoking women with substantial indoor air pollution exposure in China. Int. J. Epidemiol. 2019, 49, 1–13. [Google Scholar]
- Arku, R.E.; Brauer, M.; Duong, M.; Wei, L.; Hu, B.; Ah Tse, L.; Mony, P.K.; Lakshmi, P.V.M.; Pillai, R.K.; Mohan, V.; et al. Adverse health impacts of cooking with kerosine: A multi-country analysis within the prospective urban and rural epidemiology study. Environ. Res. 2020, 188, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Hang, J.-Q.; Zhang, F.-Y.; Dai, H.-L.; Su, L.; Christiani, D.C. In-home solid fuel use and cardiovascular disease: A cross sectional analysis of the Shanghai Putuo study. Environ. Health 2012, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Yamoto, S. Effect of indoor air pollution from biomass and solid fuel combustion on symptoms of preeclampsia/eclampsia in India women. Indoor Air 2014, 25, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, B.S.; Shetty, R.S.; Kamath, A.; Shetty, A. Household cooking fuel use and its health effects among rural women in southern India: A cross sectional study. PLoS ONE 2020, 15, e0231757. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Yan, Z.; Qu, G.; Ikram, M. Household solid fuel use and cardiovascular disease in rural areas in Shanxi, China. Iran J. Public Health 2015, 144, 625–638. [Google Scholar]
- Tiwana, J.; Benziger, C.; Hooper, L.; Pope, K.; Alurkar, V.; Kafle, R.; Sijali, T.R.; Balmes, J.R.; Kaufman, J.D.; Bates, M.N. Biomass fuel use and cardiac function in Nepali women. Glob. Heart 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, F.; Adi, D.; Yang, Y.N.; Xie, X.; Li, X.-M.; Ma, X.; Fu, Z.-Y.; Huang, Y.; Chen, B.-D.; et al. Association between carotid atherosclerosis and different subtypes of hypertension in adult populations: A multiethnic study in Xinjiang, China. PLoS ONE 2017, 12, e0171791. [Google Scholar] [CrossRef] [Green Version]
- Buturak, A.; Genç, A.; Ulus, O.S.; Duygu, E.; Ökmen, A.Ş.; Uyarel, H. Evaluation of the effects of chronic biomass fuel smoke exposure on peripheral endothelial functions: An observational study. Anatol. J. Cardiol. 2011, 11, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Larson, T.; Bolton, S.; Vedal, S. Systolic blood pressure changes in indigenous Bolivian women associated with an improved cookstove intervention. Air Qual. Atmos. Health 2015, 8, 47–53. [Google Scholar] [CrossRef]
- Neupane, M.; Basnyat, B.; Fischer, R.; Froeschl, G.; Wolbers, M.; Rehfuess, E.A. Sustained use of biogas fuel and blood pressure among women in rural Nepal. Environ. Res. 2015, 136, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña, M.B.; Romero, K.M.; Velazquez, E.J.; Davila-Roman, V.G.; Gilman, R.H.; Wise, R.; Miranda, J.J.; Checkley, W. Relationship between daily exposure to biomass fuel smoke and blood pressure in high-altitude Peru. Hypertension 2015, 65, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, J.; Schauer, J.J.; Ezzati, M.; Lu, L.; Cheng, C.; Patz, J.A.; Bautista, L.E. Indoor air pollution and blood pressure in adult women living in rural China. Environ. Health Perspect. 2011, 119, 1390–1395. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, J.; Zhang, Y.; Schauer, J.J.; Huang, W.; Wang, Y.; Ezzati, M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc. Natl. Acad. Sci. USA 2014, 111, 13229–13234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, A.; Mukherjee, B.; Das, D.; Banerjee, A.; Ray, M.R. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air 2011, 21, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.L.; Bazemore, H.; Reynolds, S.J.; Heiderscheidt, J.M.; Conway, S.; Bachand, A.M.; Volckens, J.; Peel, J.L. A baseline evaluation of traditional cook stove smoke exposures and cardiovascular and respiratory health indicatorsamong Nicaraguan women. Int. J. Occup. Environ. Health 2011, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular diseases. Circulation 2010, 121, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Al-shammari, W.A. Indoor air pollution and the risk of cardiovascular diseases. Eur. J. Med. Health Sci. 2020, 2, 459. [Google Scholar] [CrossRef]

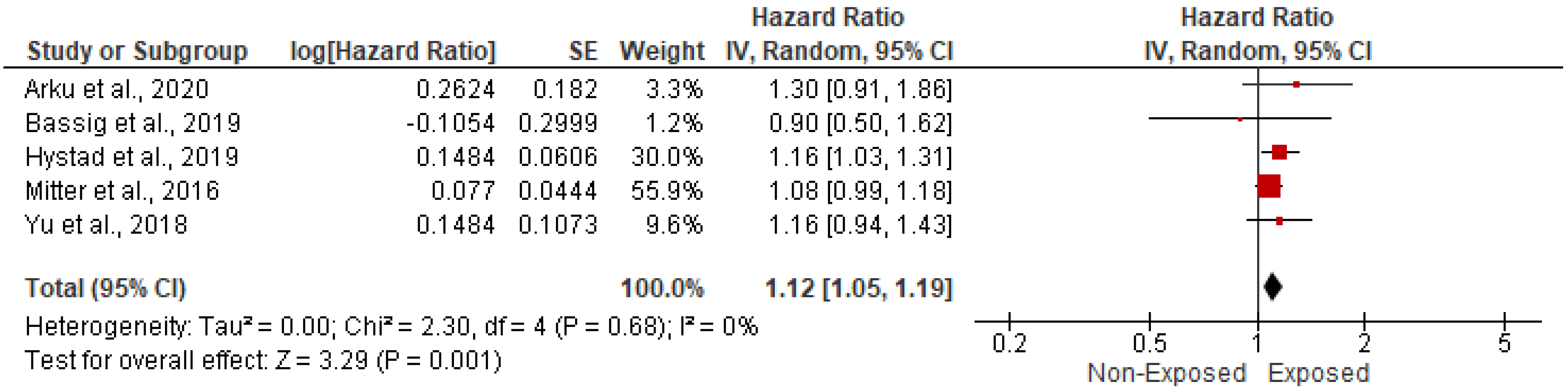
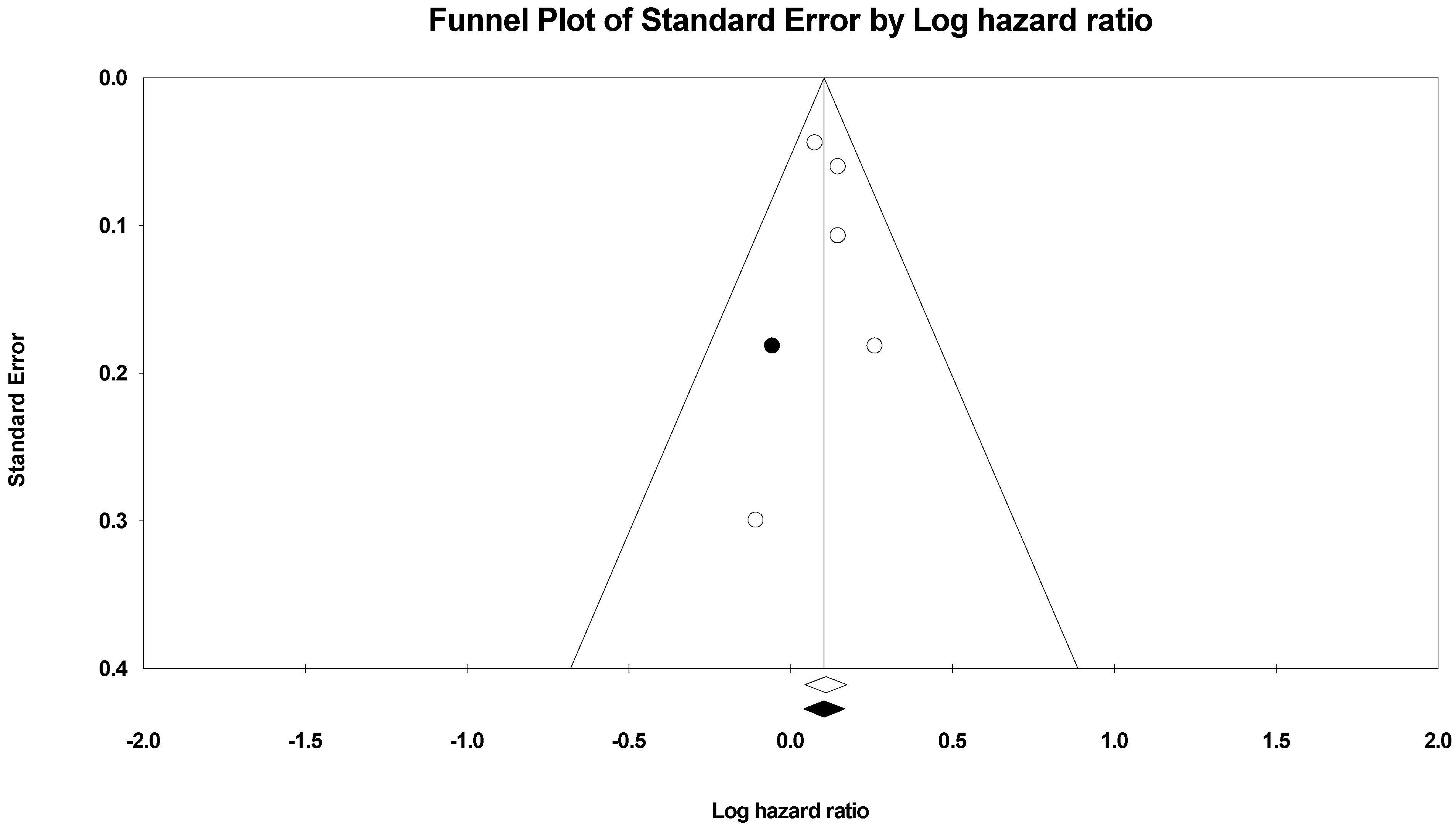
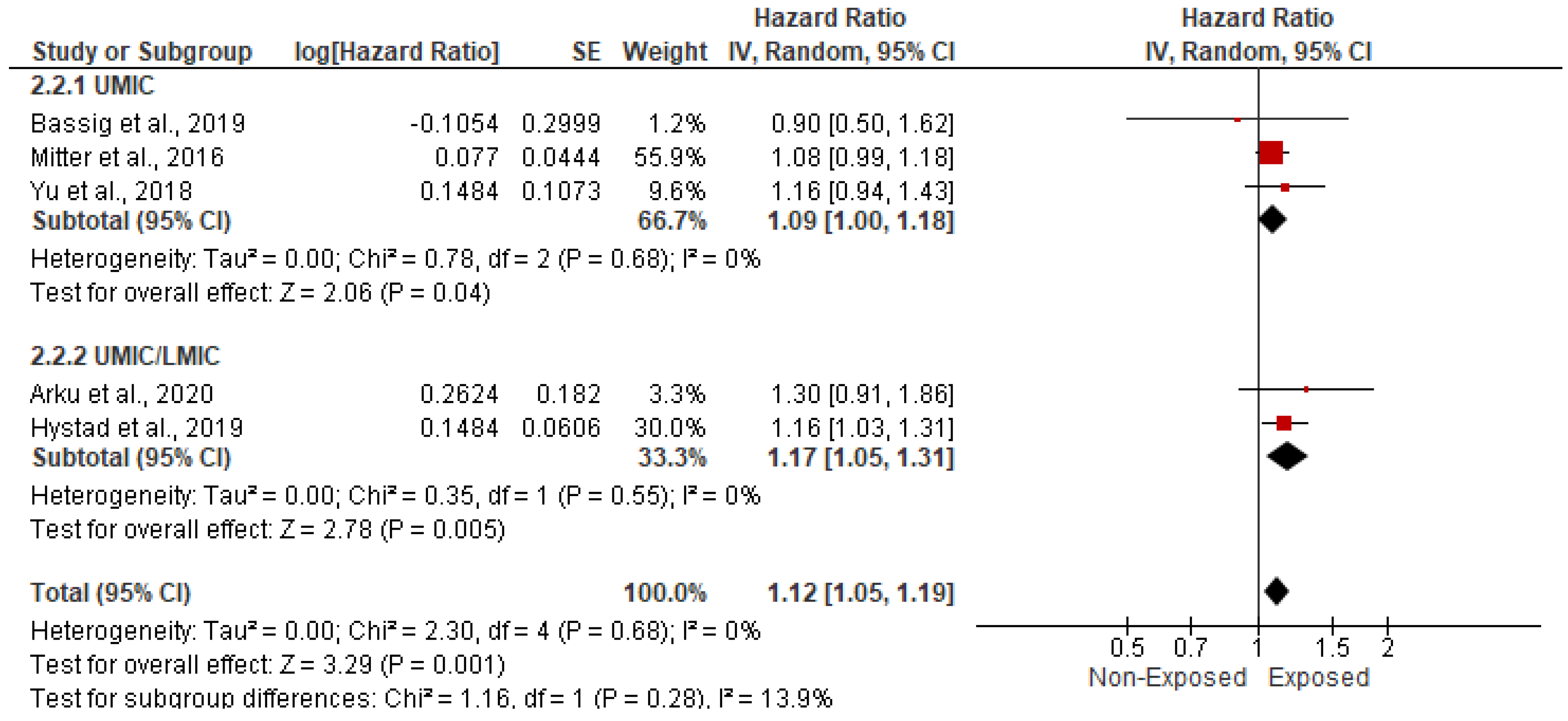
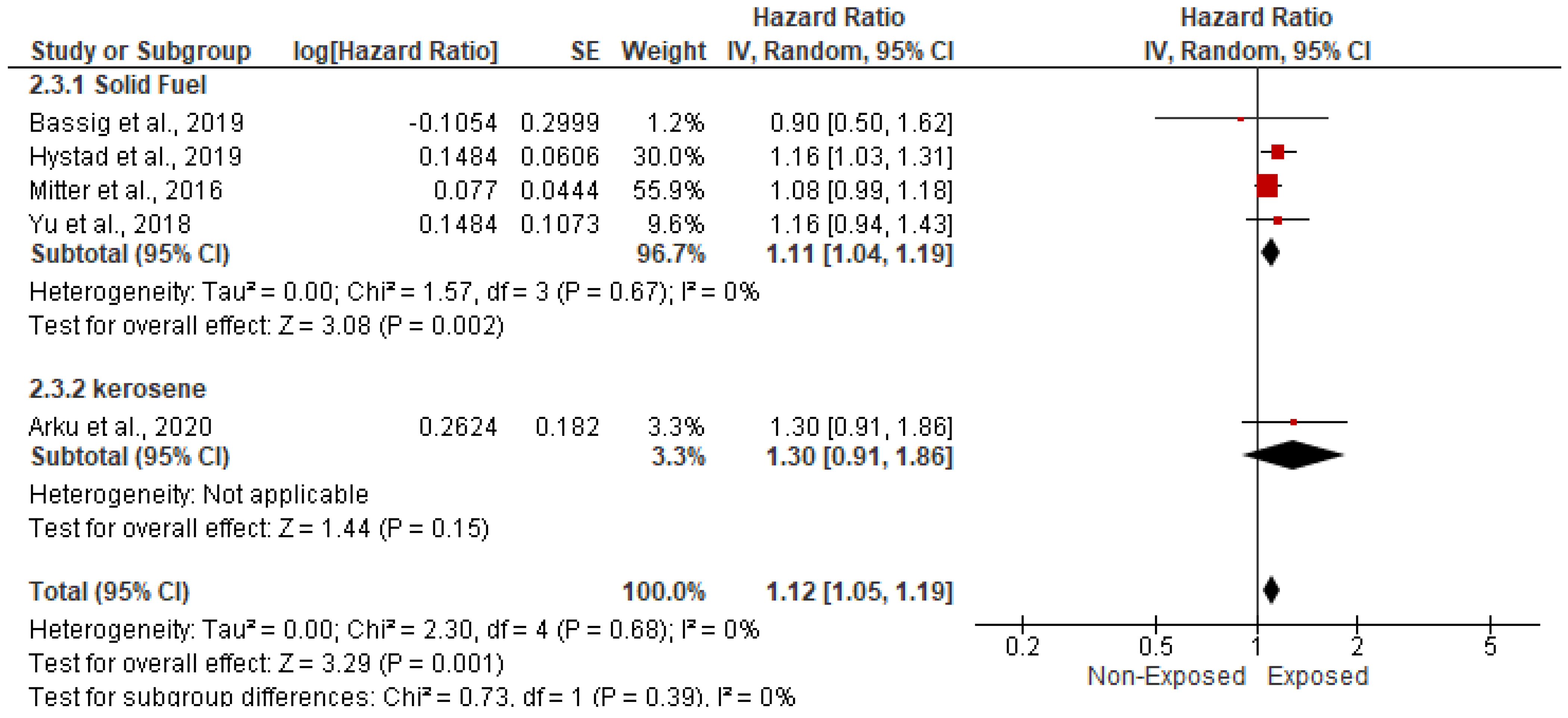


| Study ID | Study Regions | Income Category | Study Design | Study Period | Indoor Pollutants | Sample Size | Age | Gender (Male) | Gender (Female) | Body Mass Index | Completed Primary School | Comorbidities | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Disease | Smoking | Alcohol Drinking | ||||||||||||||||||||||
| Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | Exposed | Non-Exposed | |||||||
| Number | Number | Mean ± SD | Mean ± SD | Number | Number | Number | Number | Mean ± SD | Mean ± SD | Number | Number | Number | Number | Number | Number | Number | Number | |||||||
| 1 | Painschab et al., 2013 [13] | Peru | UMIC | Cross-sectional study | February and October 2011 | PM2.5 | 154 | 112 | 58 ± 12 | 55 ± 12 | 63 | 60 | 91 | 52 | 24 (22–27) * | 27 (23–30) * | 102 | 40 | 2 | 20 | 4 | 12 | NR | NR |
| 2 | Wylie et al., 2015 [14] | India | LMIC | Cross-sectional study | December 2006 To May 2008 | Wood and gas fuel | 1134 | 235 | NR | NR | 0 | 0 | 1134 | 235 | 17 | 8 | NR | NR | NR | NR | 2 | 0 | 23 | 1 |
| 3 | kammoolkon et al., 2018 [15] | Thailand | UMIC | Cross-sectional study | July to August 2016 | Incense exposure | 37 | 43 | 60 ± 10 | 57 ± 11.0 | 8 | 11 | 29 | 33 | 24.13 ± 3.22 | 23.46 ± 3.38 | 22 | 22 | 2 | 0 | 1 | 2 | 4 | 6 |
| 4 | Weber et al., 2020 [16] | Ghana | LMIC | Prospective cohort study | July 2012 to March 2014 | Wood, charcoal, crop residue, and kerosene | 279 | 540 | 27.8 ± 5.6 | 28.5 ± 4.8 | 0 | 0 | 279 | 540 | 25.0 ± 4.6 | 25.8 ± 4.8 | 44 | 206 | NR | NR | NR | NR | NR | NR |
| 5 | Yu et al., 2018 [17] | China | UMIC | Prospective cohort study | June 2004 and January 1, 2014 | Solid fuel exposure | 150,992 | 26,559 | 53.13 ± 10.09 | 48.2 ± 9.6 | 29,231 | 7274 | 121,761 | 19,285 | 23.11 ± 3.36 | 23.7 ± 3.2 | 11,194 | 5498 | NR | NR | 114,228 | 18,857 | 48,496 | 10,199 |
| 6 | Kim et al., 2016 [18] | China | UMIC | Prospective cohort study | 1996 and December 2009 | Household coal | 46,287 | 27,076 | 52.05 ± 9.16 | 51.97 ± 8.88 | 0 | 0 | 46,287 | 27,076 | 24.13 ± 3.46 | 23.81 ± 3.35 | 12,113 | 8377 | 3350 | 2003 | 1429 | 614 | 1104 | 546 |
| 7 | Mitter et al., 2016 [19] | Iran | UMIC | Prospective cohort study | 2004–2008 | Household fuels | 1578 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 8 | Yu et al., 2020 [20] | China | UMIC | Prospective cohort study | From baseline until December 31, 2016 | Solid fuel exposure | 15,381 | 75,785 | 57.1 (10.6) * | 49.2 (10.1) * | 4049 | 28,708 | 11,332 | 47,077 | 22.9 ± 3.4 | 24.4 ± 3.4 | 979 | 38,047 | NR | NR | 3049 | 20,605 | 4257 | 48,213 |
| 9 | Hystad et al., 2019 [21] | Bangladesh, Brazil, Chile, China, Colombia, India, Pakistan, Philippines, South Africa, Tanzania, and Zimbabwe | UMIC/ LMIC | Cross-sectional study | 2002 to 2015 | Solid fuel exposure for cooking | 38,187 | 53,163 | NR | NR | 15,883 | 21,709 | 22,304 | 31,454 | NR | NR | 25,031 | 12,754 | 2984 | 8542 | 9965 | 10,283 | 7269 | 10,630 |
| 10 | Bassig et al., 2019 [22] | China | UMIC | Cross-sectional study | 1 January 1976 to 31 December 2011 | Solid fuel exposure for cooking | 11,188 | 1954 | NR | NR | 0 | 0 | 11,188 | 1954 | NR | NR | 2600 | 286 | NR | NR | NR | NR | NR | NR |
| 11 | Arku et al., 2020 [23] | India, China, South Africa, Tanzania | UMIC/ LMIC | Prospective cohort study | 2001 and 2018 | Kerosene for cooking | 2767 | 28,723 | NR | NR | 2639 | 28,592 | 128 | 131 | NR | NR | NR | NR | 17 | 25 | 66 | 50 | 60 | 44 |
| 12 | Lee et al., 2012 [24] | China | UMIC | Cross-sectional study | August 2007 to July 2009 | Kerosene for cooking | 11,013 | 3055 | NR | NR | 5053 | 1410 | 5960 | 1645 | NR | NR | 4502 | 1562 | NR | NR | 2717 | 498 | NR | NR |
| 13 | Agrawal et al., 2014 [25] | India | LMIC | Cross-sectional study | 2005–2006 | Cooking Smoke | 28,158 | 7969 | NR | NR | 0 | 0 | 28158 | 7969 | NR | NR | 12,959 | NR | NR | 608 | 911 | |||
| 14 | James et al., 2020 [26] | India | LMIC | Cross-sectional study | August 2016 and September 2018 | Wood, crop residues, animal dung, and charcoal | 566 | 582 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 15 | Qu et al., 2015 [27] | China | UMIC | Cross-sectional study | 2010–2012 | Coal, wood fuel, and straw | 11,390 | 2487 | NR | NR | 5615 | 826 | 5775 | 1625 | NR | NR | NR | NR | NR | NR | 5120 | 749 | 6947 | 1720 |
| 16 | Tiwna et al., 2020 [28] | Nepal | LMIC | Case–control study | NR | Wood, biogas, and LPG | 69 | 35 | NR | NR | NR | NR | NR | NR | NR | NR | 4 | 4 | NR | NR | 16 | 6 | 10 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekoya, A.; Tyagi, S.K.; Duru, C.N.; Satia, I.; Paudyal, V.; Kurmi, O.P. Effects of Household Air Pollution (HAP) on Cardiovascular Diseases in Low- and Middle-Income Countries (LMICs): A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9298. https://doi.org/10.3390/ijerph19159298
Adekoya A, Tyagi SK, Duru CN, Satia I, Paudyal V, Kurmi OP. Effects of Household Air Pollution (HAP) on Cardiovascular Diseases in Low- and Middle-Income Countries (LMICs): A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(15):9298. https://doi.org/10.3390/ijerph19159298
Chicago/Turabian StyleAdekoya, Akorede, Sudhir K. Tyagi, Christiana N. Duru, Imran Satia, Vibhu Paudyal, and Om P. Kurmi. 2022. "Effects of Household Air Pollution (HAP) on Cardiovascular Diseases in Low- and Middle-Income Countries (LMICs): A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 15: 9298. https://doi.org/10.3390/ijerph19159298
APA StyleAdekoya, A., Tyagi, S. K., Duru, C. N., Satia, I., Paudyal, V., & Kurmi, O. P. (2022). Effects of Household Air Pollution (HAP) on Cardiovascular Diseases in Low- and Middle-Income Countries (LMICs): A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(15), 9298. https://doi.org/10.3390/ijerph19159298








