Transfer of Metals to the Aerosol Generated by an Electronic Cigarette: Influence of Number of Puffs and Power
Abstract
:1. Introduction
2. Materials and Methods
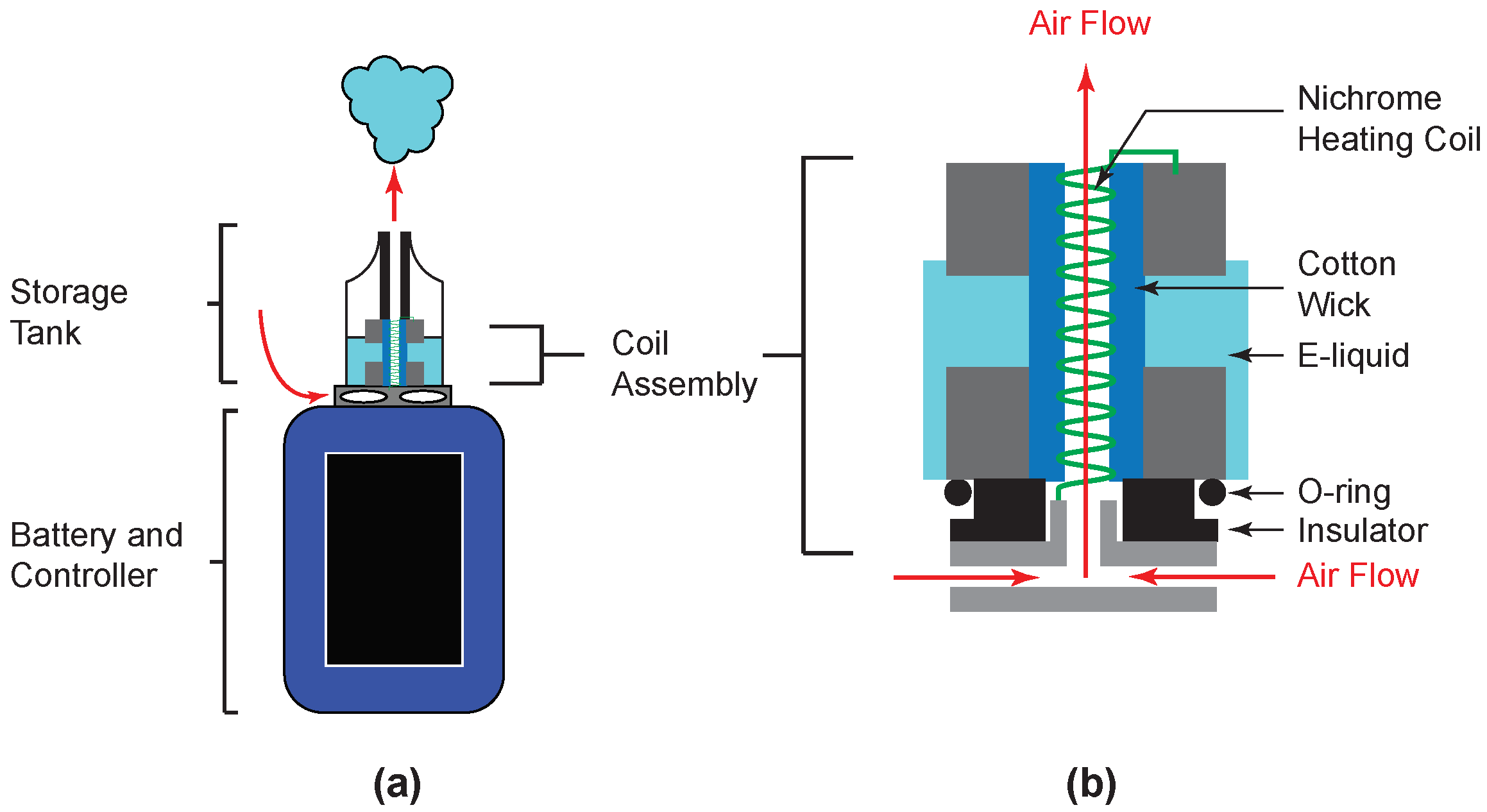
2.1. Electronic Cigarette
2.2. Apparatus
3. Results
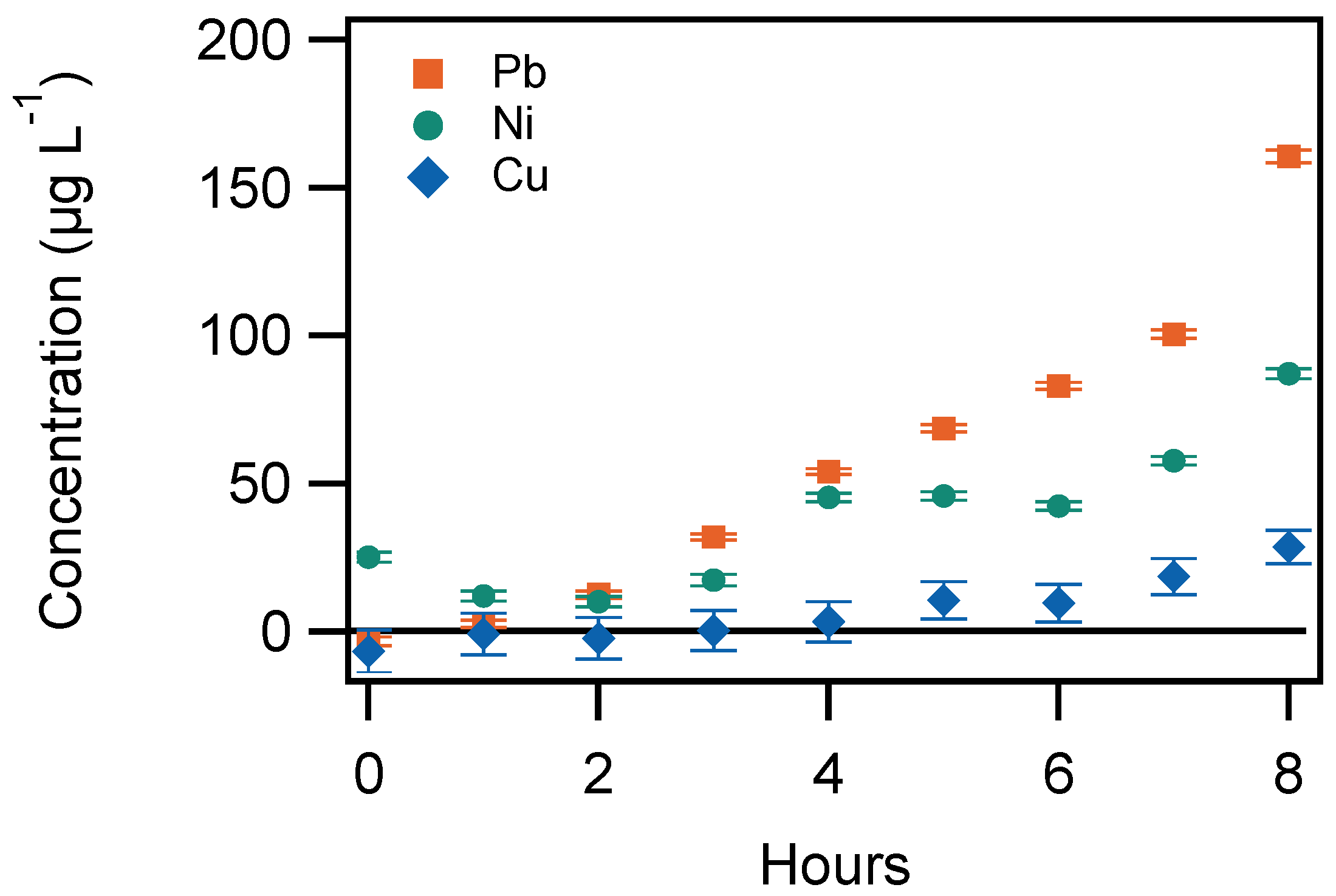
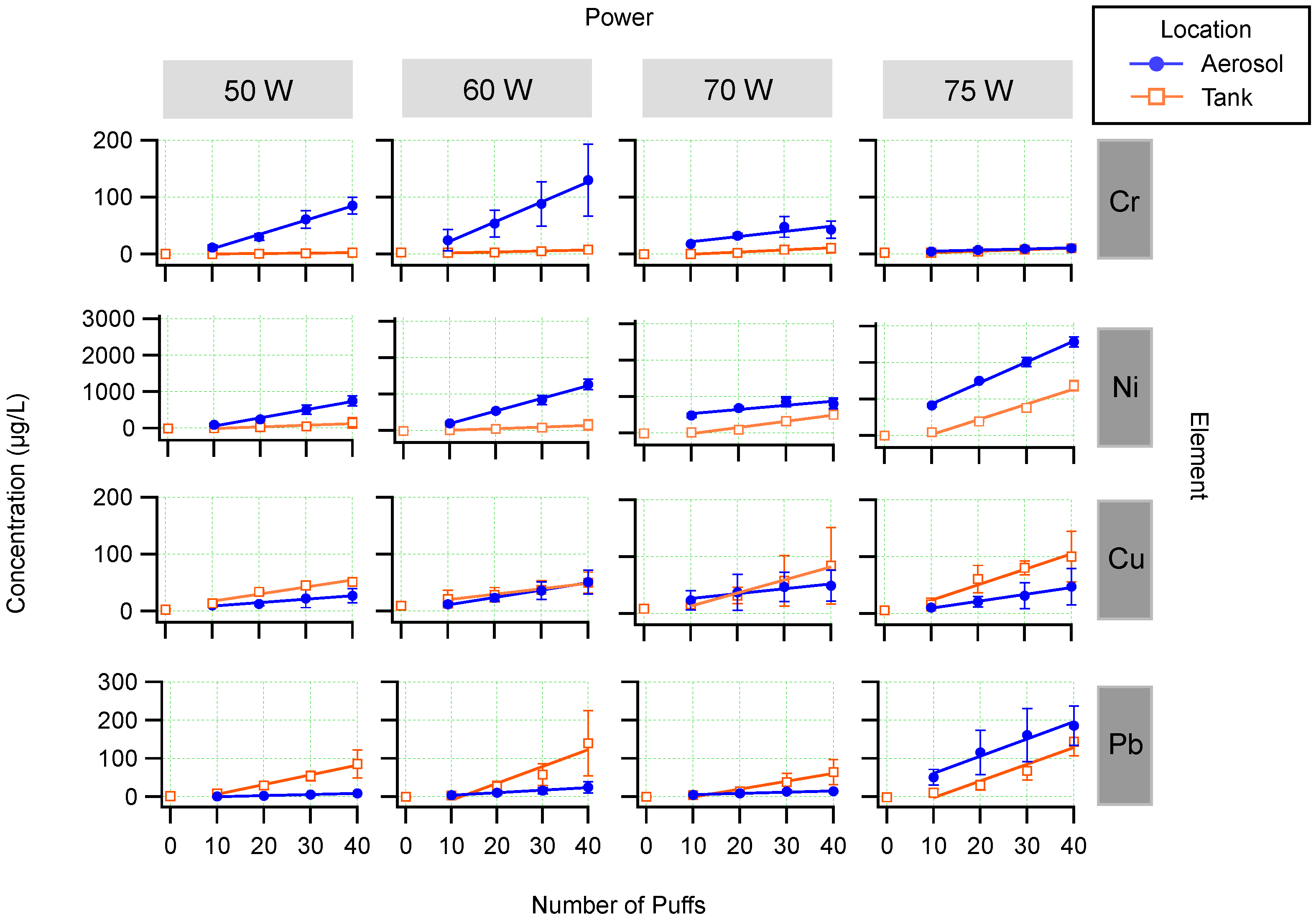
3.1. Influence of Number of Puffs
3.2. Influence of Power
3.3. Aerosol/Tank Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cr | Chromium |
| Ni | Nickel |
| Cu | Copper |
| Pb | Lead |
| GFAAS | Graphite Furnace Atomic Absorption Spectrometer |
| HEPA | High-Efficiency Particulate Air |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
Appendix A
Appendix A.1. Instrument Parameters and Control Experiments
Appendix A.2. Measurement Quality Control and Repeatability
Appendix A.3. Limits of Detection and Quantitation
| Element | Instrument LOD () | Instrument LOQ () | Dilution Factor | Aerosol or Tank Sample LOD () | Aerosol or Tank Sample LOQ () |
|---|---|---|---|---|---|
| Cr | 0.4 | 1 | 2 | 0.9 | 3 |
| Ni | 1 | 3 | 10 | 10 | 30 |
| Cu | 0.3 | 0.9 | 2 | 0.5 | 2 |
| Pb | 0.9 | 3 | 2 | 2 | 6 |
Appendix A.4. Metal Concentrations in Unfired E-Liquid
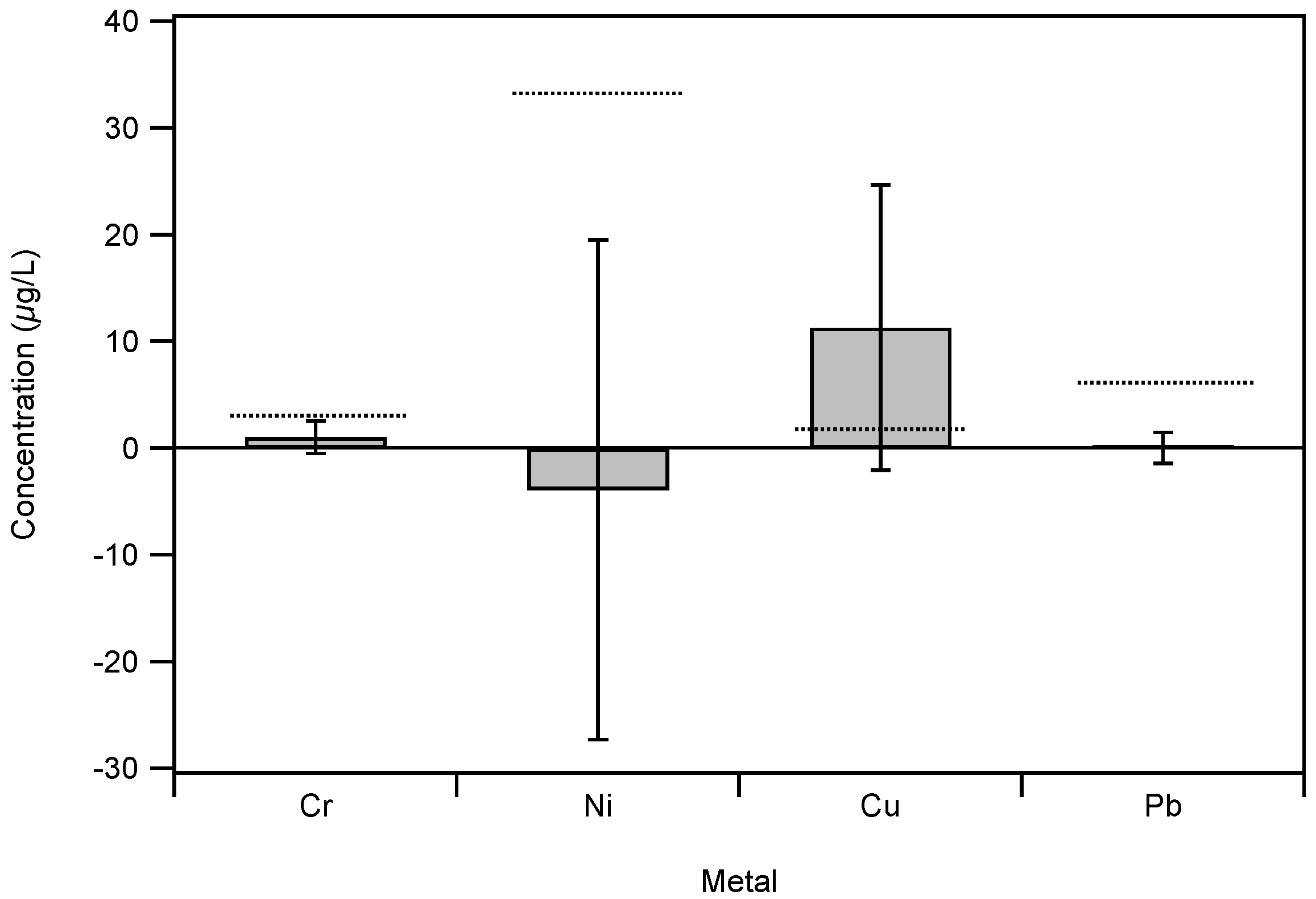
References
- Marques, P.; Piqueras, L.; Sanz, M.J. An updated overview of e-cigarette impact on human health. Respir. Res. 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; St. Helen, G.; Liakoni, E. Clinical Pharmacology of Electronic Nicotine Delivery Systems (ENDS): Implications for Benefits and Risks in the Promotion of the Combusted Tobacco Endgame. J. Clin. Pharmacol. 2021, 61, S18–S36. [Google Scholar] [CrossRef] [PubMed]
- Bonner, E.; Chang, Y.; Christie, E.; Colvin, V.; Cunningham, B.; Cunningham, B.; Elson, D.S.; Ghetu, C.C.; Huizenga, J.; Hutton, S.J.; et al. The chemistry and toxicology of vaping. Pharmacol. Ther. 2021, 225, 107837. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Summers, J.; Ouakrim, D.A.; Hoek, J.; Edwards, R.; Blakely, T. Improving on estimates of the potential relative harm to health from using modern ENDS (vaping) compared to tobacco smoking. BMC Public Health 2021, 21, 2038. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, J.A.; Rosas-Salazar, C.; Moore, P.E. E-cigarette and vaping product use-associated lung injury in the pediatric population: A critical review of the current literature. Pediatr. Pulmonol. 2021, 56, 1857–1867. [Google Scholar] [CrossRef]
- Singh, T.; Kennedy, S.; Marynak, K.; Persoskie, A.; Melstrom, P.; King, B.A. Characteristics of electronic cigarette use among Middle and High School students-United States, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1425–1429. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Arrazola, R.A.; Corey, C.G.; Husten, C.G.; Neff, L.J.; Homa, D.M.; King, B.A. Tobacco use among Middle and High School Students-United States, 2011–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 361–367. [Google Scholar] [CrossRef]
- Yan, R.; Chen, X.L.; Xu, Y.M.; Xu, Y.M.; Lau, A.T.Y. Epimutational effects of electronic cigarettes. Environ. Sci. Pollut. Res. 2021, 28, 17044–17067. [Google Scholar] [CrossRef]
- Becker, T.D.; Rice, T. Youth vaping: A review and update on global epidemiology, physical and behavioral health risks, and clinical considerations. Eur. J. Pediatr. 2021, 181, 453–462. [Google Scholar] [CrossRef]
- Gaiha, S.M.; Cheng, J.; Halpern-Felsher, B. Association Between Youth Smoking, Electronic Cigarette Use, and COVID-19. J. Adolesc. Health 2020, 67, 519–523. [Google Scholar] [CrossRef]
- Chen, D.T.H.; Kyriakos, C.N. Cigarette and E-Cigarettes Dual Users, Exclusive Users and COVID-19: Findings from Four UK Birth Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 3935. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.D.; Bornstein, S.L.; Vassallo, R.; Salonen, B.R.; Bhuiyan, M.N.; Schroeder, D.R.; Croghan, I.T. Symptoms COVID 19 Positive Vapers Compared to COVID 19 Positive Non-vapers. J. Prim. Care Community Health 2022, 13, 21501319211062672. [Google Scholar] [CrossRef]
- Das, K.; Das, S.; Dhundasi, S. Nickel, its adverse health effects and oxidative stress. Indian J. Med Res. 2008, 128, 412–425. [Google Scholar] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, Z.; Rehman, M.Y.A.; Khan, H.K.; Malik, R.N. Health risk assessment associated with heavy metals through fractioned dust from coal and chromite mines in Pakistan. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef]
- Custodio, M.; Peñaloza, R.; Ochoa, S.; Cuadrado, W. Human risk associated with the ingestion of artichokes grown in soils irrigated with water contaminated by potentially toxic elements, Junin, Peru. Saudi J. Biol. Sci. 2021, 28, 5952–5962. [Google Scholar] [CrossRef]
- Sarah, R.; Tabassum, B.; Idrees, N.; Hashem, A.; Abd_Allah, E.F. Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (U.P.), India. Saudi J. Biol. Sci. 2019, 26, 979–984. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Mishra, B.K.; Gupta, S.K. Metal pollution in water environment and the associated human health risk from drinking water: A case study of Sukinda chromite mine, India. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1433–1455. [Google Scholar] [CrossRef]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ. Sci. Pollut. Res. 2021, 28, 33042–33059. [Google Scholar] [CrossRef]
- Mohanta, V.L.; Naz, A.; Mishra, B.K. Distribution of heavy metals in the water, sediments, and fishes from Damodar river basin at steel city, India: A probabilistic risk assessment. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 406–429. [Google Scholar] [CrossRef]
- Adimalla, N.; Manne, R.; Zhang, Y.; Qian, H. Appraisal of vulnerable zones of non-carcinogenic and carcinogenic causing health risks associated with exposure of potentially toxic elements in soils of India: A meta-analysis. Geocarto Int. 2022, 1–17. [Google Scholar] [CrossRef]
- Re, D.B.; Hilpert, M.; Saglimbeni, B.; Strait, M.D.; Ilievski, V.; Coady, M.; Talayero, M.; Wilmsen, K.; Chesnais, H.; Balac, O.; et al. Exposure to e-cigarette aerosol over two months induces accumulation of neurotoxic metals and alteration of essential metals in mouse brain. Environ. Res. 2021, 202, 111557. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, E.A.; Eshraghian, E.A.; Al-Delaimy, W.K.; Al-Delaimy, W.K. A review of constituents identified in e-cigarette liquids and aerosols. Tob. Prev. Cessat. 2021, 7, 10. [Google Scholar] [CrossRef]
- Olmedo, P.; Rodrigo, L.; Grau-Perez, M.; Hilpert, M.; Navas-Acien, A.; Tellez-Plaza, M.; Tellez-Plaza, M.; Pla, A.; Gil, F. Metal exposure and biomarker levels among e-cigarette users in Spain. Environ. Res. 2021, 202, 111667. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, C.; Mallampati, S.R.; Wise, A.R. Metals in Cannabis Vaporizer Aerosols: Sources, Possible Mechanisms, and Exposure Profiles. Chem. Res. Toxicol. 2021, 34, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Domingo-Relloso, A.; Kleiman, N.J.; Navas-Acien, A.; Rule, A.M.; Hilpert, M. Effects of e-liquid flavor, nicotine content, and puff duration on metal emissions from electronic cigarettes. Environ. Res. 2022, 204, 112270. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, A.; Sobczak, A.; Szdzuj, J.; Grygoyć, K.; Kośmider, L. Metal Concentration Assessment in the Urine of Cigarette Smokers Who Switched to Electronic Cigarettes: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 1877. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Ventura, J.; Loza, A.; Wang, Y.; Talbot, P. Chemical Elements in Electronic Cigarette Solvents and Aerosols Inhibit Mitochondrial Reductases and Induce Oxidative Stress. Nicotine Tob. Res. 2020, 22, S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, D.; Zhao, D.; Aravindakshan, A.; Hilpert, M.; Olmedo, P.; Rule, A.M.; Navas-Acien, A.; Navas-Acien, A.; Aherrera, A. Metal/Metalloid Levels in Electronic Cigarette Liquids, Aerosols, and Human Biosamples: A Systematic Review. Environ. Health Perspect. 2020, 128, 036001. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Navas-Acien, A.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Adria-Mora, B.; Domingo-Relloso, A.; Aherrera, A.; Kleiman, N.J.; Rule, A.M.; et al. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ. Res. 2019, 174, 125–134. [Google Scholar] [CrossRef]
- Manyanga, J.; Ganapathy, V.; Bouharati, C.; Mehta, T.; Sadhasivam, B.; Acharya, P.; Acharya, P.; Zhao, D.; Queimado, L. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Ganapathy, V.; Manyanga, J.; Brame, L.; McGuire, D.; Sadhasivam, B.; Floyd, E.; Rubenstein, D.A.; Ramachandran, I.; Wagener, T.; Queimado, L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS ONE 2017, 12, e0177780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aherrera, A.; Olmedo, P.; Grau-Perez, M.; Tanda, S.; Goessler, W.; Jarmul, S.; Chen, R.; Cohen, J.E.; Rule, A.M.; Navas-Acien, A. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ. Res. 2017, 159, 313–320. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Brinkman, M.C.; Granville, C.A.; Gordon, S.M.; Clark, P.I. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res. 2016, 18, 1895–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical composition of aerosol from an e-cigarette: A quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, F.C.; Buonanno, G.; Stabile, L.; Vigo, P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014, 184, 523–529. [Google Scholar] [CrossRef]
- Son, Y.; Mainelis, G.; Delnevo, C.; Wackowski, O.A.; Schwander, S.; Meng, Q. Investigating e-cigarette particle emissions and human airway depositions under various e-cigarette-use conditions. Chem. Res. Toxicol. 2020, 33, 343–352. [Google Scholar] [CrossRef]
- Farsalinos, K.; Voudris, V.; Poulas, K. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int. J. Environ. Res. Public Health 2015, 12, 5215–5232. [Google Scholar] [CrossRef] [Green Version]
- Gaur, S.; Agnihotri, R. Health Effects of Trace Metals in Electronic Cigarette Aerosols—A Systematic Review. Biol. Trace Elem. Res. 2019, 188, 295–315. [Google Scholar] [CrossRef]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A.; et al. Metal concentrations in e-cigarette liquid and aerosol samples: The contribution of metallic coils. Environ. Health Perspect. 2018, 126, 027010. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Bozhilov, K.; Ghai, S.; Talbot, P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 2017, 12, e0175430. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; To, A.; Bozhilov, K.; Talbot, P. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS ONE 2015, 10, e0138933. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C.H. Toxic Metal-Containing Particles in Aerosols from Pod-Type Electronic Cigarettes. J. Anal. Toxicol. 2021, 45, 337–347. [Google Scholar] [CrossRef]
- Omaiye, E.E.; Williams, M.; Bozhilov, K.N.; Talbot, P. Design features and elemental/metal analysis of the atomizers in pod-style electronic cigarettes. PLoS ONE 2021, 16, e0248127. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.H.; Pappas, R.S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2019, 16, 4450. [Google Scholar] [CrossRef] [Green Version]
- Korzun, T.; Lazurko, M.; Munhenzva, I.; Barsanti, K.C.; Huang, Y.; Jensen, R.P.; Escobedo, J.O.; Luo, W.; Peyton, D.H.; Strongin, R.M. E-cigarette airflow rate modulates toxicant profiles and can lead to concerning levels of solvent consumption. ACS Omega 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef]
- Al-Qaysi, W.W.; Abdulla, F.H. Analytical methods for the identification of micro/nano metals in e-cigarette emission samples: A review. Chemical Papers 2021, 75, 6169–6180. [Google Scholar] [CrossRef]
- Duell, A.K.; Pankow, J.F.; Gillette, S.M.; Peyton, D.H. Boiling points of the propylene glycol plus glycerol system at 1 atmosphere pressure: 188.6–292 °C without and with added water or nicotine. Chem. Eng. Commun. 2018, 205, 1691–1700. [Google Scholar] [CrossRef]
- Cooperation Centre for Scientific Research Relative to Tobacco. Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection-Definitions and Standard Conditions; Technical report; CORESTA: Paris, France, 2020. [Google Scholar]
- Mara, A.; Langasco, I.; Deidda, S.; Caredda, M.; Meloni, P.; Meloni, P.; Deroma, M.; Pilo, M.I.; Spano, N.; Sanna, G. ICP-MS Determination of 23 Elements of Potential Health Concern in Liquids of e-Cigarettes. Method Development, Validation, and Application to 37 Real Samples. Molecules 2021, 26, 6680. [Google Scholar] [CrossRef]
- Cooperation Centre for Scientific Research Relative to Tobacco. Metals Analysis Method for E-Liquids; Technical report; CORESTA: Paris, France, 2020. [Google Scholar]
- Dunbar, Z.; Das, A.; O’Connor, R.; Goniewicz, M.; Wei, B.; Travers, M. Brief report: Lead levels in selected electronic cigarettes from Canada and the United States. Int. J. Environ. Res. Public Health 2018, 15, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, C.J.; Jo, S.H.; Kim, K.H.; Sohn, J.R.; Son, Y.S. The transfer characteristics of heavy metals in electronic cigarette liquid. Environ. Res. 2019, 174, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Q.; Hensel, E.; Eddingsaas, N.C.; Robinson, R.J. Effects of Manufacturing Variation in Electronic Cigarette Coil Resistance and Initial Pod Mass on Coil Lifetime and Aerosol Generation. Int. J. Environ. Res. Public Health 2021, 18, 4380. [Google Scholar] [CrossRef] [PubMed]
- Rastian, B. Quantification of the Transfer Mechanism of Potentially Harmful Heavy Metals to the Inhaled Aerosol Particles Generated by an Electronic Cigarette. Master’s Thesis, California State University, Fullerton, CA, USA, 2020. [Google Scholar]


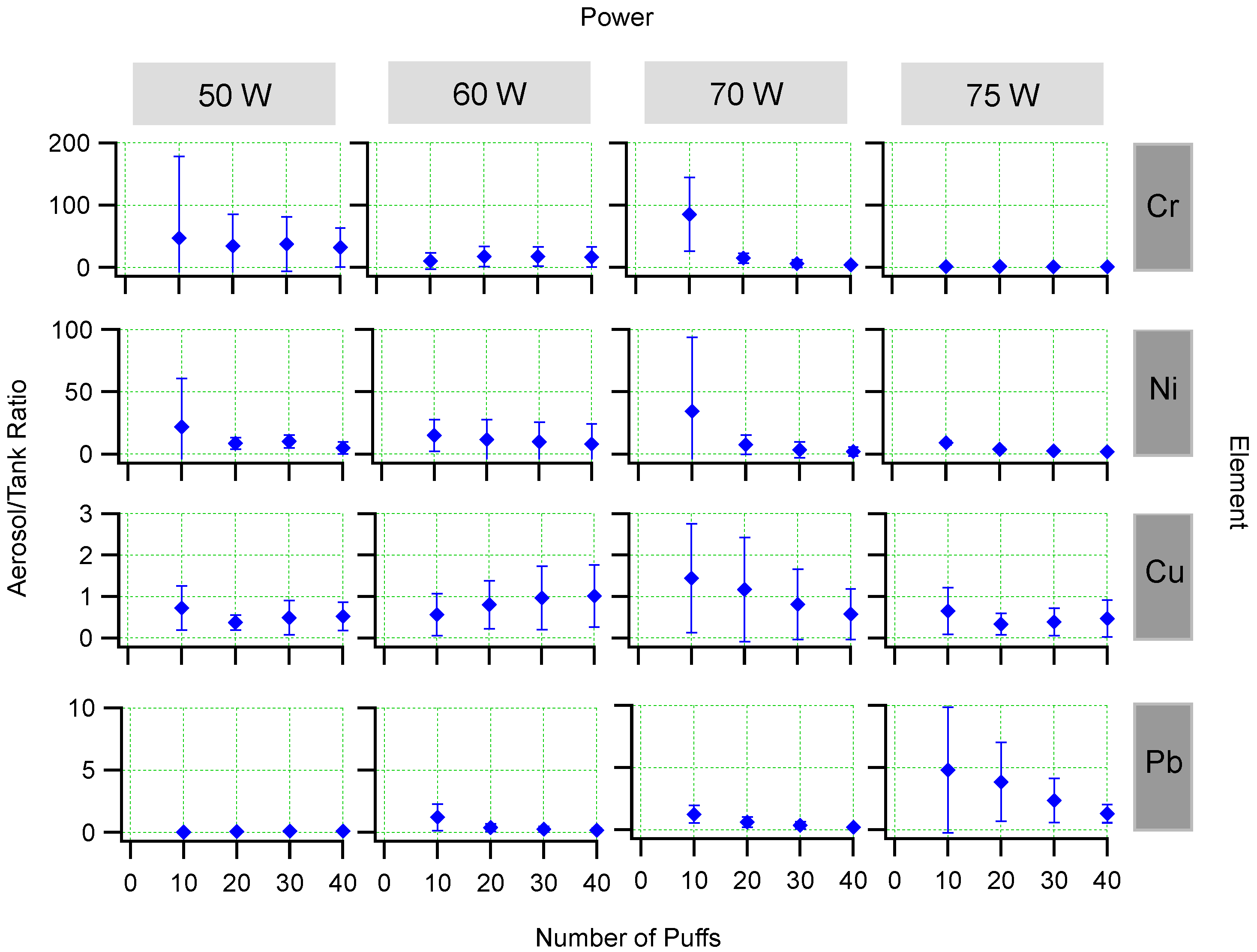
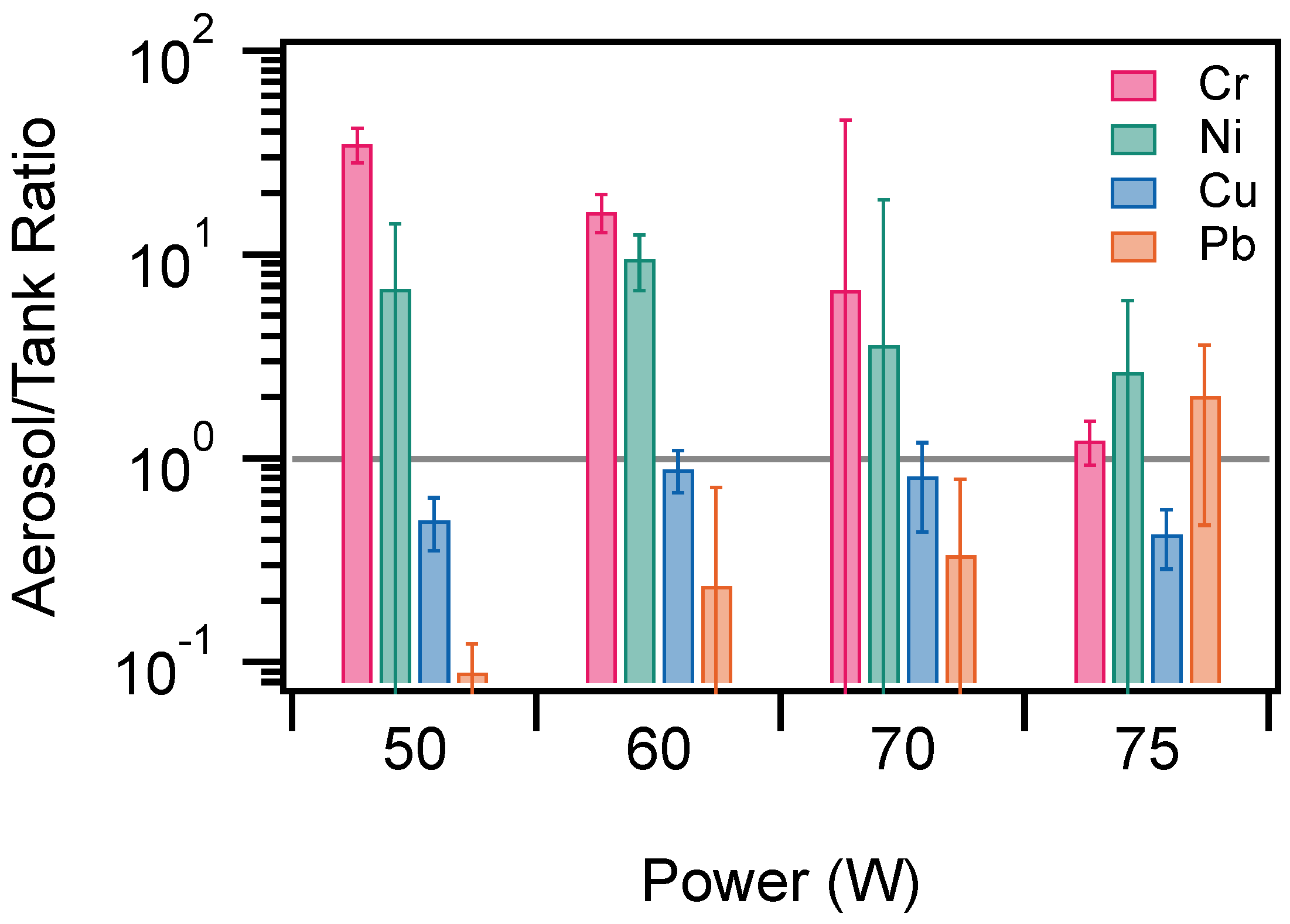
| Element | Location | Power (W) | R | ||
|---|---|---|---|---|---|
| Cr | Aerosol | 50 | |||
| Cr | Aerosol | 60 | |||
| Cr | Aerosol | 70 | |||
| Cr | Aerosol | 75 | 0.9569 | ||
| Cr | Tank | 50 | 0.9879 | ||
| Cr | Tank | 60 | 0.9345 | ||
| Cr | Tank | 70 | 0.9640 | ||
| Cr | Tank | 75 | 0.9957 | ||
| Ni | Aerosol | 50 | 0.9881 | ||
| Ni | Aerosol | 60 | 0.9951 | ||
| Ni | Aerosol | 70 | 0.7683 | ||
| Ni | Aerosol | 75 | 0.9966 | ||
| Ni | Tank | 50 | 0.8535 | ||
| Ni | Tank | 60 | 0.9651 | ||
| Ni | Tank | 70 | 0.9709 | ||
| Ni | Tank | 75 | 0.9721 | ||
| Cu | Aerosol | 50 | 0.9594 | ||
| Cu | Aerosol | 60 | 0.9960 | ||
| Cu | Aerosol | 70 | 0.9119 | ||
| Cu | Aerosol | 75 | 0.9870 | ||
| Cu | Tank | 50 | 0.9424 | ||
| Cu | Tank | 60 | 0.9824 | ||
| Cu | Tank | 70 | 0.9863 | ||
| Cu | Tank | 75 | 0.9520 | ||
| Pb | Aerosol | 50 | 0.9828 | ||
| Pb | Aerosol | 60 | 0.9867 | ||
| Pb | Aerosol | 70 | 0.9288 | ||
| Pb | Aerosol | 75 | 0.9612 | ||
| Pb | Tank | 50 | 0.9901 | ||
| Pb | Tank | 60 | 0.9122 | ||
| Pb | Tank | 70 | 0.9663 | ||
| Pb | Tank | 75 | 0.9206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastian, B.; Wilbur, C.; Curtis, D.B. Transfer of Metals to the Aerosol Generated by an Electronic Cigarette: Influence of Number of Puffs and Power. Int. J. Environ. Res. Public Health 2022, 19, 9334. https://doi.org/10.3390/ijerph19159334
Rastian B, Wilbur C, Curtis DB. Transfer of Metals to the Aerosol Generated by an Electronic Cigarette: Influence of Number of Puffs and Power. International Journal of Environmental Research and Public Health. 2022; 19(15):9334. https://doi.org/10.3390/ijerph19159334
Chicago/Turabian StyleRastian, Brian, Chase Wilbur, and Daniel B. Curtis. 2022. "Transfer of Metals to the Aerosol Generated by an Electronic Cigarette: Influence of Number of Puffs and Power" International Journal of Environmental Research and Public Health 19, no. 15: 9334. https://doi.org/10.3390/ijerph19159334






