Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics
Abstract
:1. Introduction
- -
- Aluminum salts, as chlorohydrates, used as antiperspirant: they form an insoluble aluminum hydroxide gel that physically plugs the sweat ducts, reducing the amount of sweat reaching the skin.
- -
- Lakes colorants: complex molecular structures where the colorant is prepared by reacting it with aluminum oxide in aqueous conditions in order to make it insoluble; they are used in make-up products such as lipsticks.
- -
- Insoluble minerals: mainly used in toothpastes as mild abrasive and to improve rheology.
- -
- As part of natural mineral substances used as colorant or pearl in colored cosmetics products such as eyeshadow, face powders, and nail polish.
- -
- Aluminum fluoride in oral products for fluorine content (Annex III/34);
- -
- Aluminum zirconium chloride hydroxide complexes AlxZr (OH)yClz and the aluminum zirconium chloride hydroxide glycine complexes in antiperspirant for zirconium and anhydrous aluminum zirconium chloride hydroxide content (Annex III/50);
- -
- Trisodium 5-hydroxy-1-(4-sulphophenyl)-4-(4-sulphophenylazo)pyrazole-3-carboxylate and aluminum lake (CI 19140), Benzenemethanaminium, N-ethyl-N-[4-[[4-[ethyl-[(3-sulfophenyl)-methyl]-amino]-phenyl](2-sulfophenyl)methylene]-2,5-cyclohexadien-1-ylidene]-3-sulfo, inner salts, disodium salt and its ammonium and aluminum salts (Acid Blue Acid Blue 9 Ammonium salt; CI 42090), and Trisodium 1-(1-naphthylazo)-2-hydroxynaphthalene-4′,6,8-trisulphonate and aluminum lake (Cl 16255) when used as hair dye substance in nonoxidative hair dye products (Annexes III/189, III/190, III/192).
- -
- when “exposure from non-cosmetic sources of aluminum (food and pharmaceuticals) was aggregated with exposure from cosmetics, food contributed in a similar order of magnitude as cosmetics” [29];
- -
- The use of aluminum compounds is safe up to the following equivalent aluminum concentrations: deo-roll on gel max. 6.18%, Deo Spray antiperspirant max. 3.24%, Deo Spray pump max. 3.24%, Toothpaste max. 3.18%, lipstick max. 14.62%.
2. Materials and Methods
2.1. Chemicals
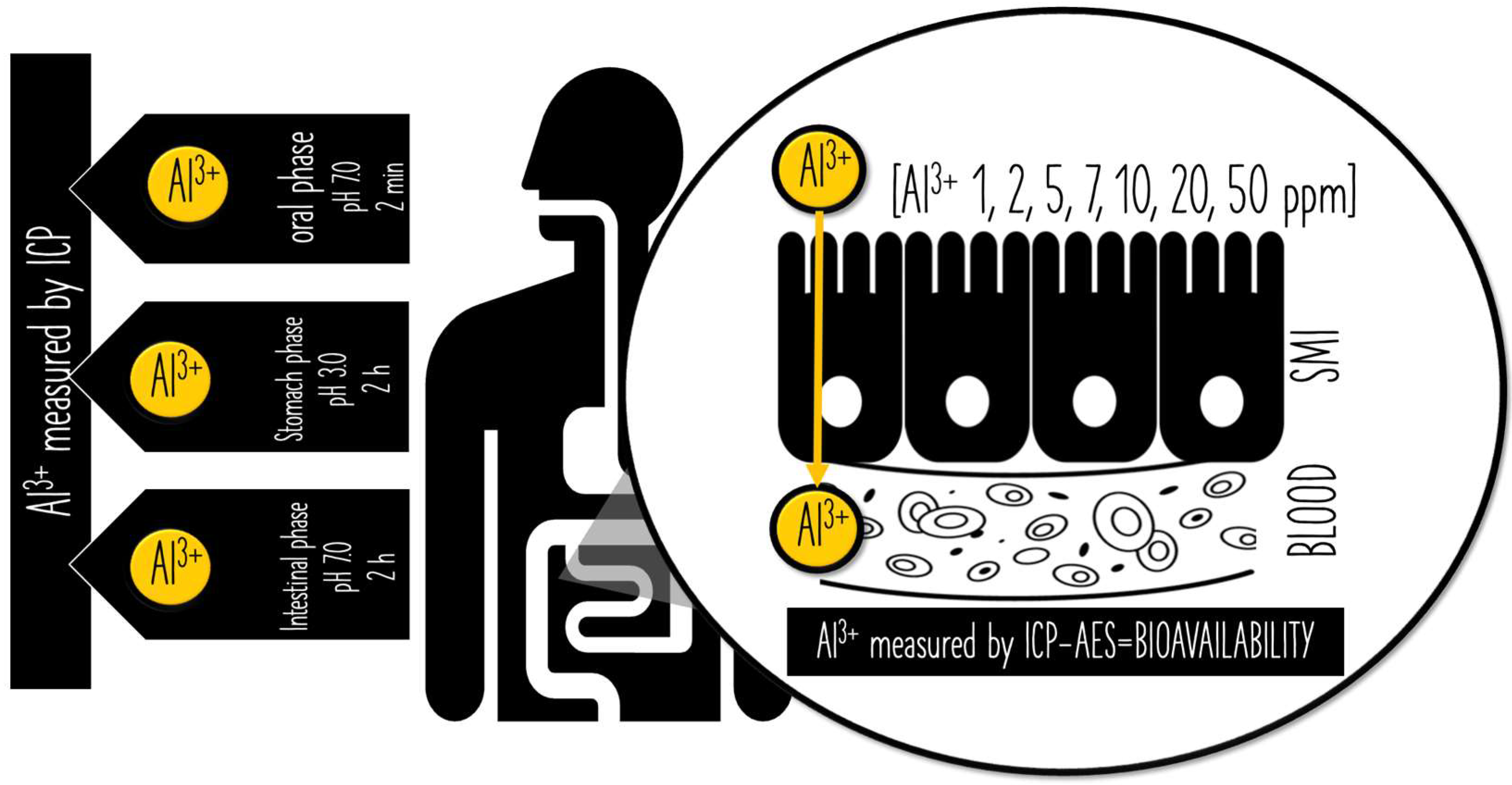
2.2. In Vitro Digestion According to INFOGEST Model
2.3. In Vitro Aluminum Permeability across the Small Intestine
2.4. Inductively Coupled Plasma—Atomic Emission Spectroscopy (ICP-AES) Analyses
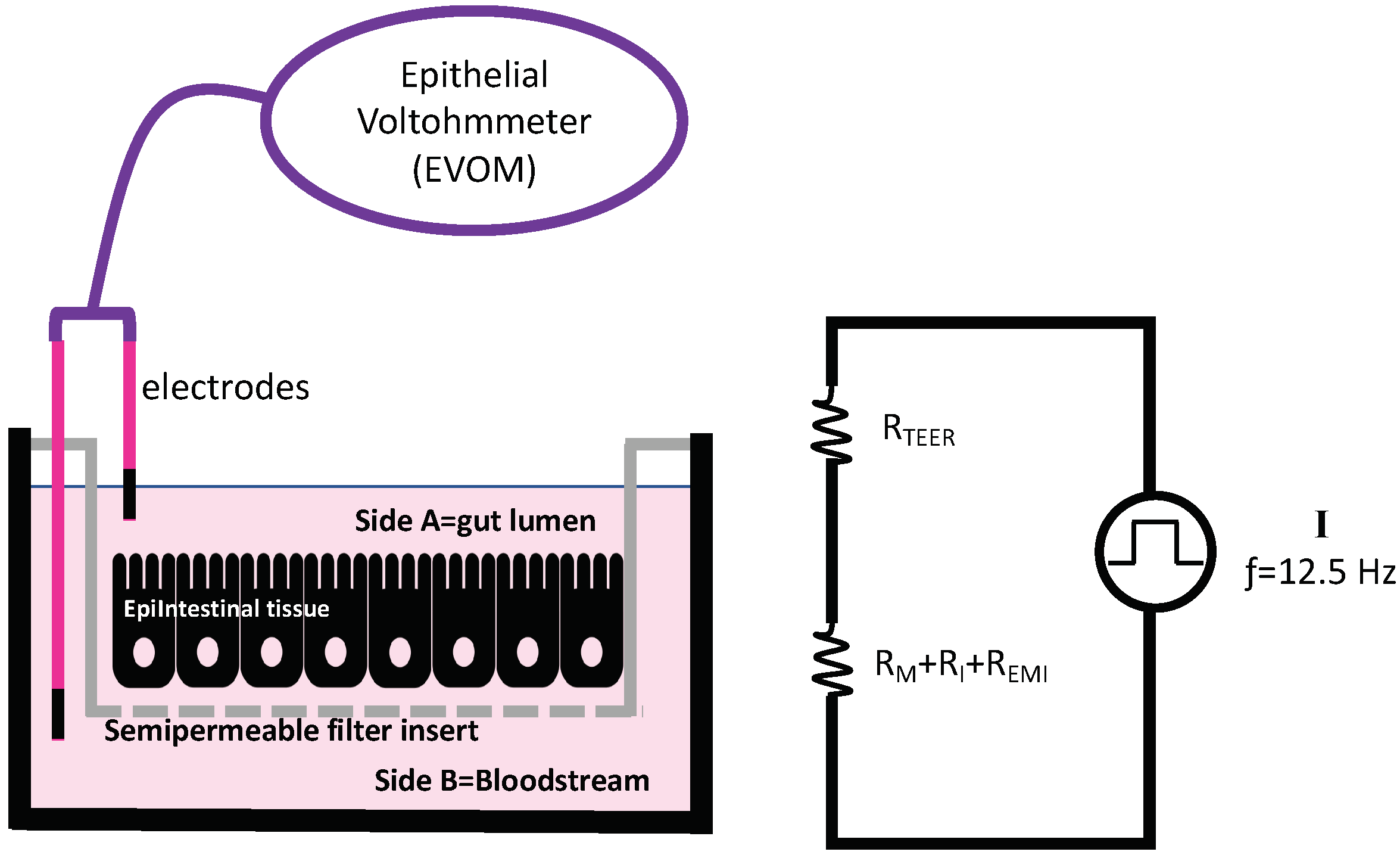
2.5. Transepithelial Electric Resistance (TEER)
2.6. Histological Analyses
2.7. Safety Assessment including Margin of Safety Calculation
3. Results
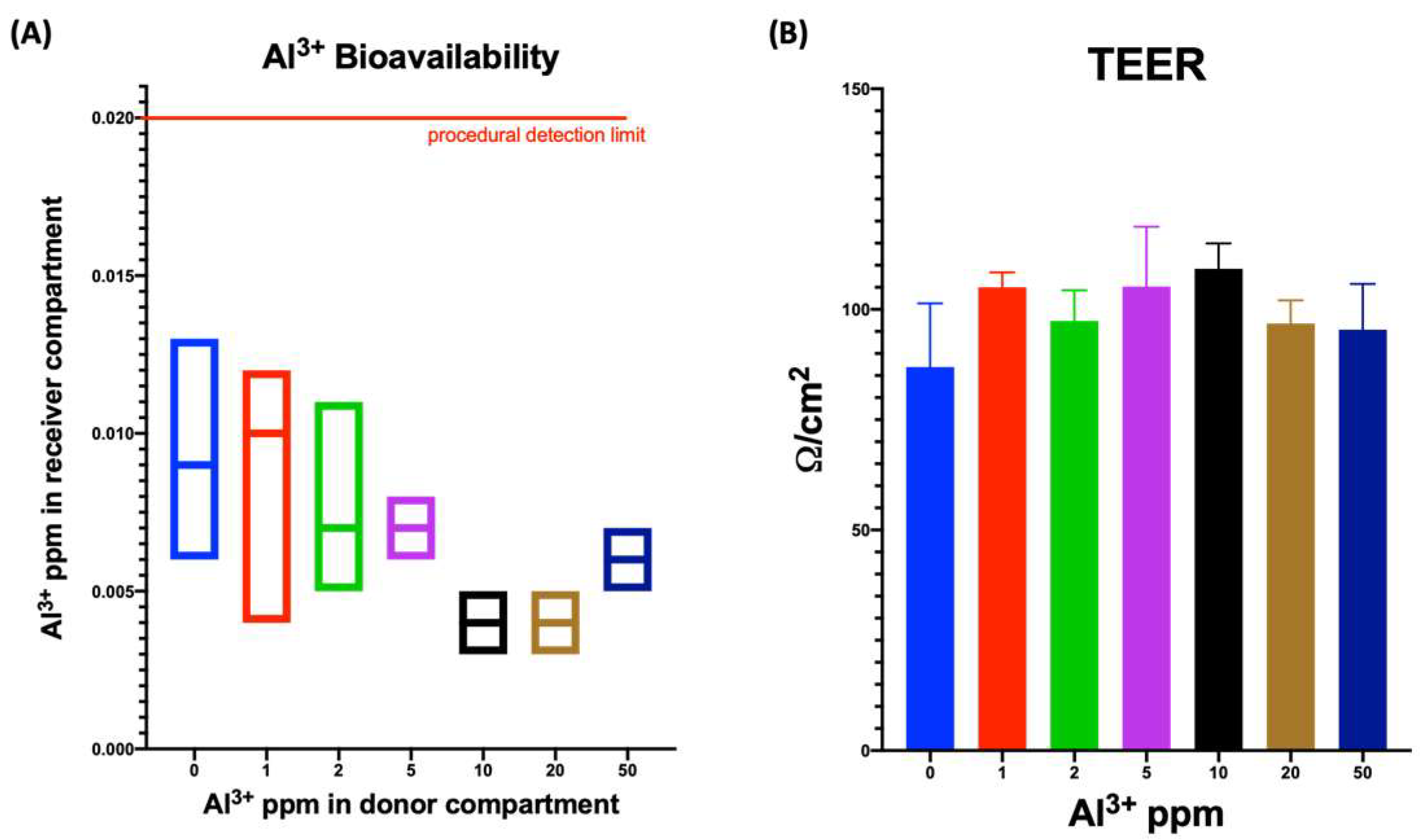
3.1. Aluminum Leach Evaluation throughout Digestive System
3.2. Evaluating Bioavailability by Means of Tridimensional Model of the Human Small Intestine
3.3. Margin of Safety Calculation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gad, S. Aluminum; Wexler, P., Ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium Toxicosis: A Review of Toxic Actions and Effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.; Bargellini, A.; Vinceti, M. Aluminum and Tin: Food Contamination and Dietary Intake in an Italian Population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, X.; Cao, P.; Wang, X.; Gao, P.; Ma, N.; Li, N.; Xu, H. A Preliminary Investigation of Naturally Occurring Aluminum in Grains, Vegetables, and Fruits from Some Areas of China and Dietary Intake Assessment: Aluminum in Grains, Vegetables, and Fruits. J. Food Sci. 2019, 84, 701–710. [Google Scholar] [CrossRef]
- Pillay, V.; Jonnalagadda, S.B. Elemental Uptake by Edible Herbs and Lettuce (Latuca sativa). J. Environ. Sci. Health Part B 2007, 42, 423–428. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Griglione, A.; Falsetti, S.; Curcio, A.; Abete, M.C. Aluminium Occurrence in Plant Feed from Northwestern Italy. J. Trace Elem. Med. Biol. 2021, 68, 126850. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium Is Essential for Root Growth and Development of Tea Plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Ingerman, L.; Jones, D.G.; Keith, S.; Rosemond, Z.A. ATSDR Toxicological Profile for Alumium; CDC Stacks: Atlanta, GA, USA, 2008.
- Gramiccioni, L.; Ingrao, G.; Milana, M.R.; Santaroni, P.; Tomassi, G. Aluminium Levels in Italian Diets and in Selected Foods from Aluminium Utensils. Food Addit. Contam. 1996, 13, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.P.; Steinegger, A.; Schlatter, C. Contribution of Aluminium from Packaging Materials and Cooking Utensils to the Daily Aluminium Intake. Z. Lebensm. Unters. Forsch. 1993, 197, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives Evaluation of Certain Food Additives; World Health Organisation Techical Report Series; WHO: Geneva, Switzerland, 2009.
- Bratakos, S.M.; Lazou, A.E.; Bratakos, M.S.; Lazos, E.S. Aluminium in Food and Daily Dietary Intake Estimate in Greece. Food Addit. Contam. Part B 2012, 5, 33–44. [Google Scholar] [CrossRef] [PubMed]
- El Daouk, S.; Pineau, A.; Taha, M.; Ezzeddine, R.; Hijazi, A.; Al Iskandarani, M. Aluminum Exposure from Food in the Population of Lebanon. Toxicol. Rep. 2020, 7, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Kolbaum, A.E.; Berg, K.; Müller, F.; Kappenstein, O.; Lindtner, O. Dietary Exposure to Elements from the German Pilot Total Diet Study (TDS). Food Addit. Contam. Part A 2019, 36, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of Aluminium sulphates (E 520–523) and Sodium Aluminium Phosphate (E 541) as Food Additives. EFSA J. 2018, 16, e5372. [Google Scholar] [CrossRef] [Green Version]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gürtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M.; et al. Aggregated Aluminium Exposure: Risk Assessment for the General Population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.-H.; Chen, S.-C.; Lin, C.-H.; Chen, Y.-C. Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population. Int. J. Environ. Res. Public Health 2021, 18, 1099. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; et al. Re-evaluation of Sodium Aluminium Silicate (E 554) and Potassium Aluminium Silicate (E 555) as Food Additives. EFSA J. 2020, 18, e6152. [Google Scholar] [CrossRef]
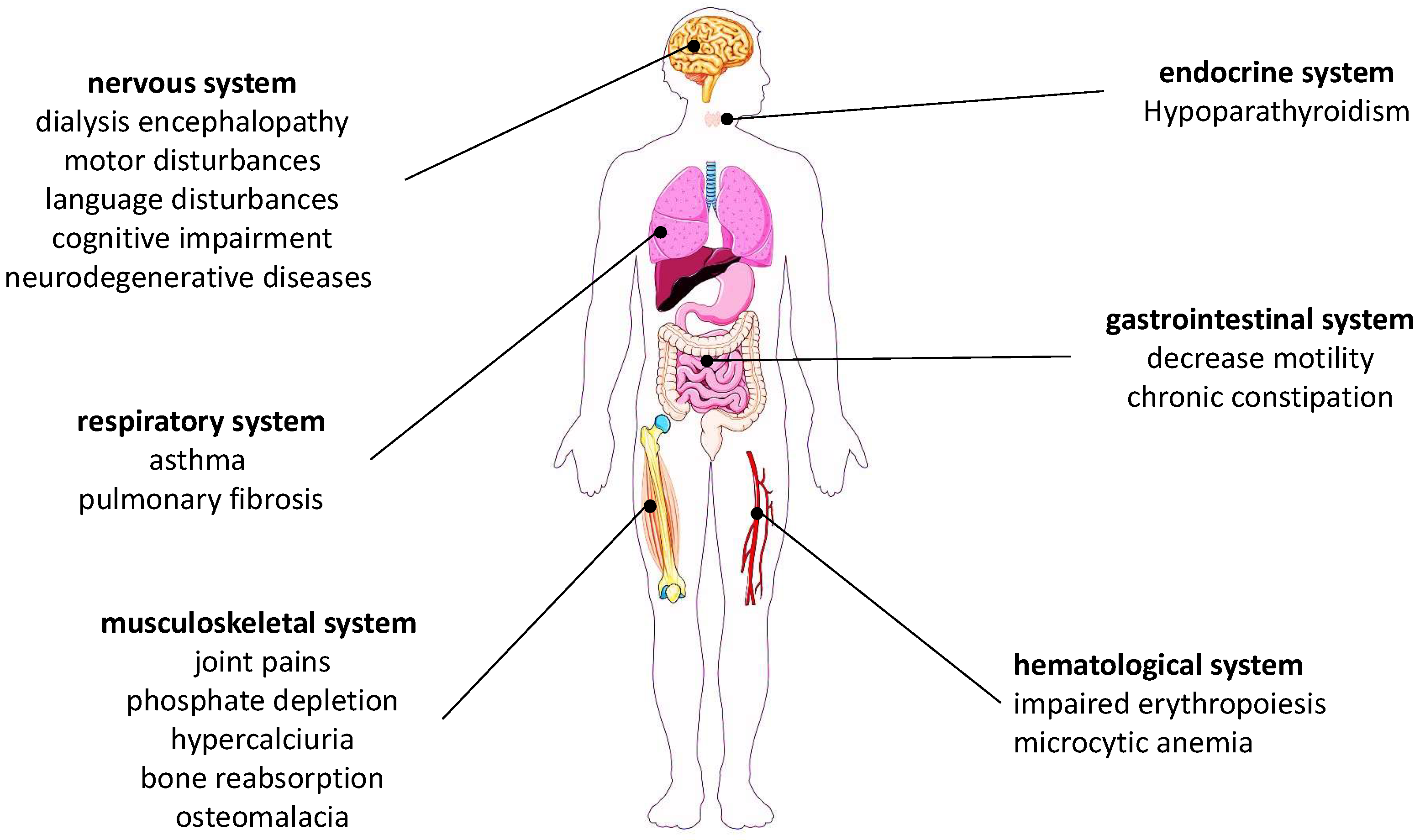
- Crisponi, G.; Fanni, D.; Gerosa, C.; Nemolato, S.; Nurchi, V.M.; Crespo-Alonso, M.; Lachowicz, J.I.; Faa, G. The Meaning of Aluminium Exposure on Human Health and Aluminium-Related Diseases. BioMol. Concepts 2013, 4, 77–87. [Google Scholar] [CrossRef]
- Adani, G.; Filippini, T.; Garuti, C.; Malavolti, M.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Costa, M.; Vinceti, M.; et al. Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 7941. [Google Scholar] [CrossRef] [PubMed]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s Disease. In Neurotoxicity of Metals; Advances in Neurobiology; Aschner, M., Costa, L.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 18, pp. 183–197. ISBN 978-3-319-60188-5. [Google Scholar]
- Lévesque, L.; Mizzen, C.A.; McLachlan, D.R.; Fraser, P.E. Ligand Specific Effects on Aluminum Incorporation and Toxicity in Neurons and Astrocytes. Brain Res. 2000, 877, 191–202. [Google Scholar] [CrossRef]
- Walton, J.R. Chronic Aluminum Intake Causes Alzheimer’s Disease: Applying Sir Austin Bradford Hill’s Causality Criteria. J. Alzheimers Dis. JAD 2014, 40, 765–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Dekant, W. Metal Salts with Low Oral Bioavailability and Considerable Exposures from Ubiquitous Background: Inorganic Aluminum Salts as an Example for Issues in Toxicity Testing and Data Interpretation. Toxicol. Lett. 2019, 314, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; Panteri, E.; et al. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 11th Revision, 30–31 March 2021, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021, 127, 105052. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; Panteri, E.; et al. SCCS Opinion on the Safety of Aluminium in Cosmetic Products—Submission III—SCCS/1644/22—Preliminary Opinion; Scientific Committee for Consumer Safety: Brussels, Belgium, 2022. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.-J. Impedance-Based Cell Monitoring: Barrier Properties and Beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haorah, J.; Schall, K.; Ramirez, S.H.; Persidsky, Y. Activation of Protein Tyrosine Kinases and Matrix Metalloproteinases Causes Blood-Brain Barrier Injury: Novel Mechanism for Neurodegeneration Associated with Alcohol Abuse. Glia 2008, 56, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, P.M.D.; Paterson, J.C.; Thom, G.; Ginman, U.; Lundquist, S.; Webster, C.I. Modelling the Endothelial Blood-CNS Barriers: A Method for the Production of Robust in Vitromodels of the Rat Blood-Brain Barrier and Blood-Spinal Cord Barrier. BMC Neurosci. 2013, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Parlamento Europeo e Consiglio Sui Prodotti Cosmetici. La Commissione Decisione di Esecuzione della Commissione del 25 Novembre 2013 Relativa Alle Linee Guida sull’allegato I Del Regolamento (CE) n. 1223/2009; Parlamento Europeo e Consiglio Sui Prodotti Cosmetici: Brussels, Belgium, 2013. [Google Scholar]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Amiri, R.J.; Pirzadeh, M.; Moghadamnia, A.A. Aluminum Poisoning with Emphasis on Its Mechanism and Treatment of Intoxication. Emerg. Med. Int. 2022, 2022, 1480553. [Google Scholar] [CrossRef] [PubMed]
- Windisch, J.; Keppler, B.K.; Jirsa, F. Aluminum in Coffee. ACS Omega 2020, 5, 15335–15343. [Google Scholar] [CrossRef]
- Bhargava, V.P.; Netam, A.K.; Singh, R.; Sharma, P. Aluminium and Neuro-Degeneration: Mechanism of Pathogenesis and Possible Strategies for Mitigation. Asian J. Pharm. Res. Health Care 2021, 13, 101–114. [Google Scholar] [CrossRef]
- Di Paolo, C.; Reverte, I.; Colomina, M.T.; Domingo, J.L.; Gómez, M. Chronic Exposure to Aluminum and Melatonin through the Diet: Neurobehavioral Effects in a Transgenic Mouse Model of Alzheimer Disease. Food Chem. Toxicol. 2014, 69, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk Factors Associated with the Onset and Progression of Alzheimer’s Disease: A Systematic Review of the Evidence. NeuroToxicology 2017, 61, 143–187. [Google Scholar] [CrossRef]
- Priest, N.D.; Skybakmoen, E.; Jackson, G. The Bioavailability of Ingested 26Al-Labelled Aluminium and Aluminium Compounds in the Rat. NeuroToxicology 2021, 83, 179–185. [Google Scholar] [CrossRef]


| Stoichiometric Ratio | Al(OH)3 | → ← | Al3+ | 3 OH− |
|---|---|---|---|---|
| Molar weight | 78 g/mol | 27 g/mol | 3 × 17 = 51 g/mol | |
| Weight (gram) | 7.6 g | 2.63 g | 4.97 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaria, G.; De Negri Atanasio, G.; Filippini, T.; Robino, F.; Dondero, L.; Soggia, F.; Rispo, F.; Tardanico, F.; Ferrando, S.; Aicardi, S.; et al. Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics. Int. J. Environ. Res. Public Health 2022, 19, 9362. https://doi.org/10.3390/ijerph19159362
Allaria G, De Negri Atanasio G, Filippini T, Robino F, Dondero L, Soggia F, Rispo F, Tardanico F, Ferrando S, Aicardi S, et al. Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics. International Journal of Environmental Research and Public Health. 2022; 19(15):9362. https://doi.org/10.3390/ijerph19159362
Chicago/Turabian StyleAllaria, Giorgia, Giulia De Negri Atanasio, Tommaso Filippini, Federica Robino, Lorenzo Dondero, Francesco Soggia, Francesca Rispo, Francesca Tardanico, Sara Ferrando, Stefano Aicardi, and et al. 2022. "Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics" International Journal of Environmental Research and Public Health 19, no. 15: 9362. https://doi.org/10.3390/ijerph19159362
APA StyleAllaria, G., De Negri Atanasio, G., Filippini, T., Robino, F., Dondero, L., Soggia, F., Rispo, F., Tardanico, F., Ferrando, S., Aicardi, S., Demori, I., Markus, J., Cortese, K., Zanotti-Russo, M., & Grasselli, E. (2022). Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics. International Journal of Environmental Research and Public Health, 19(15), 9362. https://doi.org/10.3390/ijerph19159362










