Assessing Recent Efforts to Improve Organization of Cancer Care in Poland: What Does the Evidence Tell Us?
Abstract
1. Introduction
2. Key Recent Reforms Aimed at Improving Cancer Care in Poland
2.1. Fast Pathway for Cancer Patients (2015)
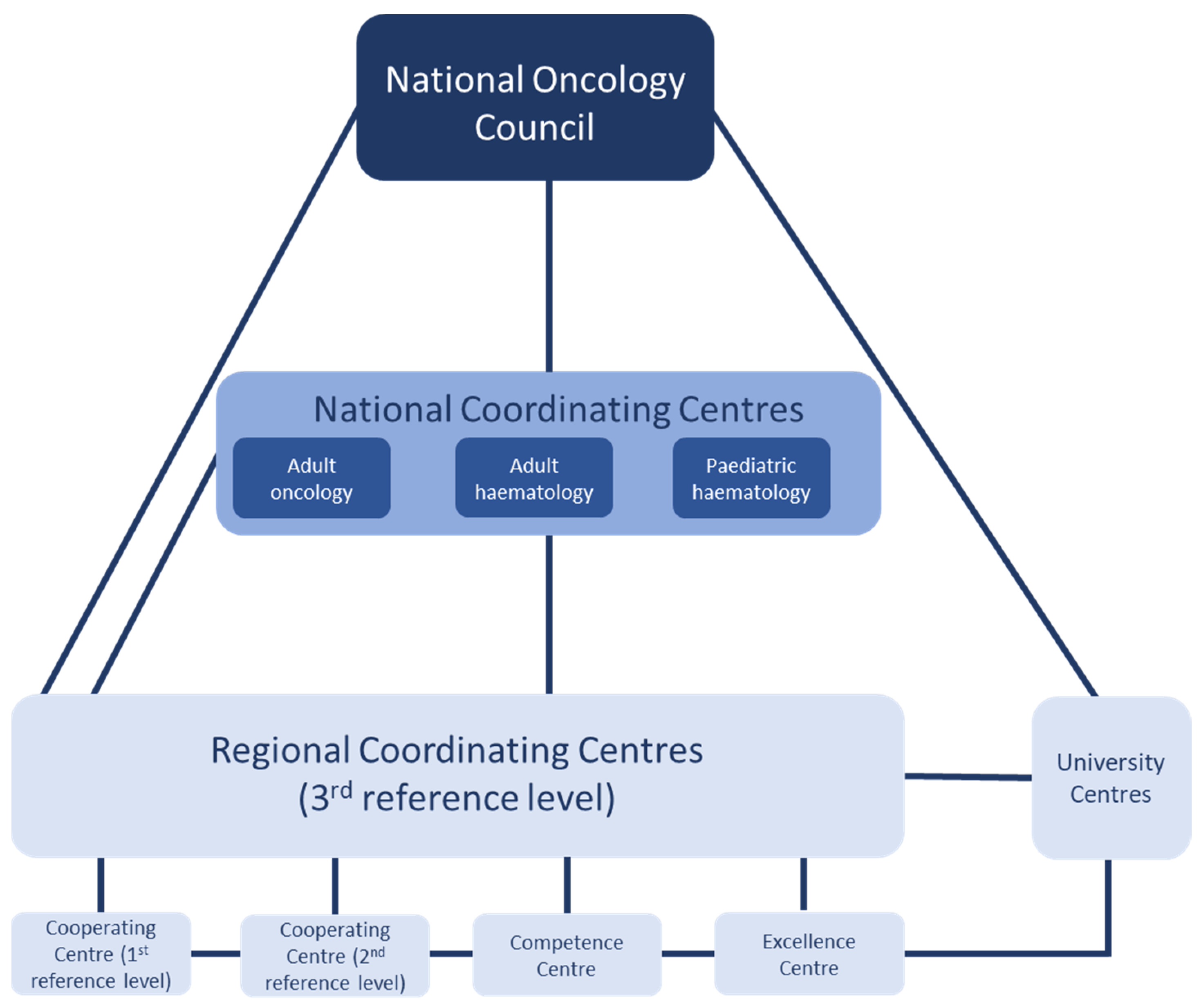
2.2. Pilot of the National Oncology Network (2019–2022)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indicator | |
|---|---|
| 1 | Percentage of deaths within one year from the diagnosis of malignant neoplasm, broken down by cancer stage |
| 2 | Percentage of deaths within 30 days from the date of surgery, broken down by cancer stage |
| 3 | Percentage of deaths within 30 days from the end of chemotherapy, broken down by cancer stage |
| 4 | Percentage of deaths within 30 days from the end of palliative radiotherapy, broken down by stage cancer |
| 5 | Percentage of patients requiring hospitalization due to complications after surgical treatment |
| 6 | Percentage of patients requiring hospitalization due to complications after radiotherapy |
| 7 | Percentage of patients requiring hospitalization due to complications after systemic treatment |
| 8 | Percentage of patients who received chemotherapy as inpatients |
| 9 | Percentage of patients with stage III and IV of cancer |
| 10 | Assessment of the completeness of pathological examination |
| 11 | Percentage of patients who were tested for genetic and molecular predictors |
| 12 | Percentage of surgical procedures performed with minimally invasive methods |
| 13 | Median time that has elapsed from the date the patient was issued a referral for a diagnostic (imaging or pathomorphological) examination to the date of obtaining the result of this examination |
| 14 | Percentage of diagnostic tests repeated within 6 weeks (computed tomography, endoscopy, biopsy, pathomorphological assessment, molecular assessment), by provider, type of tumour and type of examination |
| 15 | Percentage of repeated treatments in diagnoses other than breast cancer |
| 16 | Percentage of patients with rectal cancer who received preoperative radiotherapy |
| 17 | Percentage of postoperative histopathological examinations in patients with colorectal cancer in which the number of assessed lymph nodes was at least 12 |
| 18 | Percentage of patients with colon and rectal cancer with anastomotic leakage |
| 19 | Assessment of the number of lymph nodes removed during prostatectomy |
| 20 | Percentage of pelvic lymphadenectomy performed with the division of histopathological material according to anatomical ranges |
| 21 | Number of positive postoperative margins after prostatectomy |
| 22 | Percentage of patients with suspected lung cancer consulted by a pulmonologist within 14 working days from the date of registration of the referral with the service provider |
| 23 | Percentage of patients with mediastinal lymphadenopathy greater than 10 mm, who underwent EBUS-TBNA |
| 24 | Percentage of patients with suspected lung cancer and pleural effusion, with diagnosed fluid aetiology |
| 25 | Percentage of patients with stage III non-small cell lung cancer who received simultaneous chemoradiotherapy |
| 26 | Percentage of patients with ovarian cancer treated with primary optimal or suboptimal cytoreduction (leaving no residual mass or <1 cm) |
| 27 | Percentage of patients with ovarian cancer who received neoadjuvant chemotherapy (NACT) |
| 28 | Percentage of patients with ovarian cancer who underwent exploratory laparotomy |
| 29 | Percentage of patients with non-infiltrating neoplasm with a diameter of less than 2 cm (after excluding patients with BRCA1 and BRCA2 mutations) undergoing breast-sparing treatment |
| 30 | Percentage of patients with infiltrating neoplasm with a diameter not exceeding 3 cm (total size, including DCIS component; after excluding patients with BRCA1 and BRCA2 mutations) undergoing breast-sparing treatment |
| 31 | Percentage of patients with non-infiltrating neoplasm with a diameter of not more than 2 cm (after excluding patients with BRCA1 and BRCA2 mutations) undergoing breast-sparing treatment |
| 32 | Percentage of DCIS patients who have not had the contents of the armpit removed |
| 33 | Percentage of patients with infiltrating cancer without lymph node metastases (pN0), in whom the lymphatic system of the armpit was not removed |
| 34 | Percentage of patients with hormone-sensitive infiltrating cancer who received hormonal treatment |
| 35 | Percentage of patients with inflammatory neoplasm or locally advanced, unresectable ER-expressing breast cancer who underwent induction chemotherapy |
| Region (Number of Included Patients *) | Median Time from Registration for Diagnostic to Obtaining Results | Percentage of Patients with Genetic and Molecular Tests | Percentage of Patients Who Needed to Be Hospitalised Du to Post-Surgery Complications |
|---|---|---|---|
| Dolnośląskie (11,688) | ↓ Decrease from 19 to 13 days | → Unchanged at 95% | ↓ from 3% to 2% |
| Podlaskie (1827) | ↑ Increase from 6 to 8 days | ↑ Increase from 75% to 97% | ↓ from 3% to 2% |
| Pomorskie (3025) | ↓ Decrease from 18 to 11 days | n.a. | ↓ from 10% to 7% |
| Świętokrzyskie (3984) | ↓ Decrease from 13 to 12 days | ↓ Decrease from 100% to 92% | ↓ from 3% to 2% |
References
- EC. Causes of Death—Deaths by Country of Residence and Occurrence; European Commission (Eurostat): Luxembourg, 2022. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- EC. Poland Country Profile—2021. In State of Health in EU; European Commission (Eurostat): Luxembourg, 2021. [Google Scholar]
- Ministry of Health of the Republic of Poland. Ustawa z dnia 1 lipca 2005 r. o ustanowieniu programu wieloletniego ”Narodowy program zwalczania chorób nowotworowych”. Dz. U. (J. Laws) 2005, 143, 1200. [Google Scholar]
- RM. Uchwała nr 208 Rady Ministrów z Dnia 3 Listopada 2015 r. w Sprawie Ustanowienia Programu Wieloletniego na Lata 2016–2024 pod nazwą "Narodowy Program Zwalczania Chorób Nowotworowych"; Rada Ministrów: Warsaw, Poland, 2015. [Google Scholar]
- NIK. Przygotowanie i Wdrożenie Pakietu Onkologicznego; Najwyższa Izba Kontroli (Supreme Audit Office): Warsaw, Poland, 2017. [Google Scholar]
- Ministry of Health of the Republic of Poland. Program Wieloletni pn. Narodowa Strategia Onkologiczna na Lata 2020–2030; Ministerstwo Zdrowia: Warsaw, Poland, 2020. [Google Scholar]
- Chrostowski, S.; Jassem, J.; Łuczak, J.; Meder, J.; Nasierowska-Guttmejer, A.; Radziewic-Winnicki, I.; Składowski, K.; Wardyn, K.; Wysocki, P.; Rutkowski, P. Strategia Walki z Rakiem w Polsce 2015–2024. 2014, Rada Projektu Strategii Walki z Rakiem w Polsce, 2015–2024. Available online: https://ligawalkizrakiem.pl/images/content/Strategia-Walki-z-Rakiem-w-Polsce/Strategia_wersja_2017.pdf (accessed on 26 July 2022).
- Kalbarczyk, W.P.; Gujski, M.; Brzozowski, S.; Tytko, Z.; Ścibek, A. Walka z Nowotworami i Opieka Onkologiczna w Polsce Wobec Wyzwań Demograficznych i Epidemiologicznych—Propozycje Rozwiązań; Instytut Ochrony Zdrowia: Warsaw, Poland, 2015. [Google Scholar]
- Warzocha, K. Koncepcje zmian systemowych w opiece onkologicznej w Polsce u progu 2015 roku. Hematologia 2014, 5, 179–192. [Google Scholar]
- Kowalska, I.; Sagan, A.; Mokrzycka, A.; Zabdyr-Jamróz, M. The first attempt to create a national strategy for reducing waiting times in Poland: Will it succeed? Health Policy 2015, 119, 258–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NFZ. Coraz Więcej Pieniędzy Przeznaczamy na Leczenie Nowotworów i Leki Onkologiczne; Narodowy Fundusz Zdrowia: Warsaw, Poland, 2020. [Google Scholar]
- Holecki, T.; Romaniuk, P. The oncological package: A new source of concern in Poland’s health system. Lancet Oncol. 2015, 16, E104. [Google Scholar] [CrossRef]
- Dela, R. Pakiet onkologiczny—Reorganizacja i finansowanie świadczeń zdrowotnych. Ogólnopolski Przegląd Med. 2017, 10, 61–65. [Google Scholar]
- Sutkowski, D.M. Pacjent Onkologiczny Pod Opieką Lekarza Rodzinnego; Hematoonkologia.pl: Lublin, Poland, 2020. [Google Scholar]
- Lejcyk–Łoka, M. Instytucja konsylium onkologicznego w Polskim systemie ochrony zdrowia. Teka Kom. Prawniczej PAN Oddział W Lub. 2020, 13, 267–276. [Google Scholar]
- Czauderna, P.; Maciejczyk, A.; Fijuth, J.; Góźdź, S.; Krzakowski, M.; Lech-Marańda, E.; Markiewicz, M.; Szczepański, T.; Walewski, J. Koncepcja Organizacji i Funkcjonowania Krajowej Sieci Onkologicznej (Tom I); Zespół Ministra Zdrowia ds. Opracowania Projektu Koncepcji Organizacji i Funkcjonowania Narodowego Instytutu Onkologii: Warsaw, Poland, 2018. [Google Scholar]
- Różalska, A.; Czech, M. Koordynowana opieka w onkologii. Manag. Issues 2017, 15, 146–158. [Google Scholar] [CrossRef]
- NFZ. DiLO; Narodowy Fundusz Zdrowia (National Health Fund): Warsaw, Poland, 2022. [Google Scholar]
- WHC. Onkobarometr WHC. Raport na Temat Zmian w Dostępności do Gwarantowanych Świadczeń Zdrowotnych Stosowanych w Walce z Nowotworami w Polsce nr 11/3/10/2017; Foundation Watch Health Care: Cracow, Poland, 2017. [Google Scholar]
- Rutkowski, P.Ł.; Maciejczyk, A.; Krzakowski, M.; Flisiak, R.; Gałązka-Sobotka, M.; Reguła, J.; Zegarsi, W.; Kubiatowski, T.; Rosińska, M. Wpływ Pandemii COVID-19 na System Opieki Onkologicznej; Narodowy Instytut Onkologii: Warsaw, Poland, 2021. [Google Scholar]
- NIK. Dostępność i Efekty Leczenia Nowotworów; Najwyższa Izby Kontroli: Warsaw, Poland, 2017. [Google Scholar]
- NIK. Organizacja, Dostępność i Jakość Diagnostyki Patomorfologicznej; Najwyższa Izba Kontroli: Warsaw, Poland, 2020. [Google Scholar]
- Mender, J. Wprowadzenie Referencyjności Szpitali Onkologicznych to Konieczność; Rynek Zdrowia (Online). Available online: https://www.rynekzdrowia.pl/Uslugi-medyczne/Wprowadzenie-referencyjnosci-szpitali-onkologicznych-to-koniecznosc,114732,8.html (accessed on 9 December 2011).
- NFZ. Lecznie Zabiegowe Nowotworów Złośliwych w 2020r; Narodowy Fundusz Zdrowia: Warsaw, Poland, 2018. [Google Scholar]
- Pochrzęst-Motyczyńska, A. Trudno Ocenić Pilotaż Sieci Onkologicznej, bo Dane Niepełne. 2020. Available online: https://www.prawo.pl/zdrowie/pilotaz-krajowej-sieci-onkologicznej-trudno-ocenic-bo-dane-sa,497174.html (accessed on 14 January 2020).
- EC. Europe’s Beating Cancer Plan; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Sobczyk, K.; Wróblewski, M.; Holecki, T.; Szałabska, D. Ocena trafności prognoz map potrzeb zdrowotnych w kontekście zarządzania podażą wybranych usług w ochronie zdrowia. Studia I Pr. Wydziału Nauk. Ekon. I Zarządzania 2018, 51, 265–276. [Google Scholar]
- Ministry of Health of the Republic of Poland. Informacja Pilotaż Krajowej Sieci Onkologicznej; Ministerstwo Zdrowia: Warsaw, Poland, 2021. [Google Scholar]
- Ministry of Health of the Republic of Poland. Rozporządzenie Ministra Zdrowia z Dnia 13 Grudnia 2018 r. w Sprawie Programu Pilotażowego Opieki Nad Świadczeniobiorcą w Ramach Sieci Onkologicznej; Dziennik Ustaw Rzeczypospolitej Polskiej: Warsaw, Poland, 2018. [Google Scholar]
- Maciejczyk, A. Wskaźniki jakości w onkologii – pilotaż sieci onkologicznej [Quality indicators in oncology—Pilot of the oncological network]. In Proceedings of the 15th International Evidence-Based Health Care Symposium pt, “From Evidence to Action”, Online, 5–7 October 2020. [Google Scholar]
- Maciejczyk, A. Pilotaż/Krajowa Sieć Onkologiczna Debata [Pilot/National Oncology Network Debate]. In Proceedings of the Expert parliamentary Debate, Lower House of the Parliament, Warsaw, Poland, 21–23 February 2022. [Google Scholar]
- Badora-Musiał, K.; Sagan, A.; Domagała, A.; Kowalska-Bobko, I. Testing the 2017 PHC reform through pilots: Strengthening prevention and chronic care coordination. Health Policy 2021, 125, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Holecki, T.; Romaniuk, P.; Woźniak-Holecka, J.; Szromek, A.; Syrkiewicz-Świtała, M. Mapping Health Needs to Support Health System Management in Poland. Front. Public Health 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Republic of Poland. Ustawa z Dnia 29 Września 2017 r. o Zmianie Ustawy o Świadczeniach Opieki Zdrowotnej Finansowanych ze Środków Publicznych oraz Niektórych Innych Ustaw; Dz. U.; Ministry of Health of the Republic of Poland: Warsaw, Poland, 2017. [Google Scholar]
- WHO Regional Office for Europe. Situation Analysis on Evidence-Informed Health Policy-Making: Poland; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
| Measures | Key Shortcomings |
|---|---|
| Fast access to diagnostics and treatment | |
|
|
|
|
| Comprehensiveness of diagnostics and treatment | |
|
|
|
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagan, A.; Kowalska-Bobko, I.; Gałązka-Sobotka, M.; Holecki, T.; Maciejczyk, A.; McKee, M. Assessing Recent Efforts to Improve Organization of Cancer Care in Poland: What Does the Evidence Tell Us? Int. J. Environ. Res. Public Health 2022, 19, 9369. https://doi.org/10.3390/ijerph19159369
Sagan A, Kowalska-Bobko I, Gałązka-Sobotka M, Holecki T, Maciejczyk A, McKee M. Assessing Recent Efforts to Improve Organization of Cancer Care in Poland: What Does the Evidence Tell Us? International Journal of Environmental Research and Public Health. 2022; 19(15):9369. https://doi.org/10.3390/ijerph19159369
Chicago/Turabian StyleSagan, Anna, Iwona Kowalska-Bobko, Małgorzata Gałązka-Sobotka, Tomasz Holecki, Adam Maciejczyk, and Martin McKee. 2022. "Assessing Recent Efforts to Improve Organization of Cancer Care in Poland: What Does the Evidence Tell Us?" International Journal of Environmental Research and Public Health 19, no. 15: 9369. https://doi.org/10.3390/ijerph19159369
APA StyleSagan, A., Kowalska-Bobko, I., Gałązka-Sobotka, M., Holecki, T., Maciejczyk, A., & McKee, M. (2022). Assessing Recent Efforts to Improve Organization of Cancer Care in Poland: What Does the Evidence Tell Us? International Journal of Environmental Research and Public Health, 19(15), 9369. https://doi.org/10.3390/ijerph19159369






