Effect of Humic Acid on the Growth and Metabolism of Candida albicans Isolated from Surface Waters in North-Eastern Poland
Abstract
:1. Introduction
2. Material and Methods
2.1. Material and Growth Conditions
2.2. Determination of Proteins in Biomass and Secretion of Candida albicans
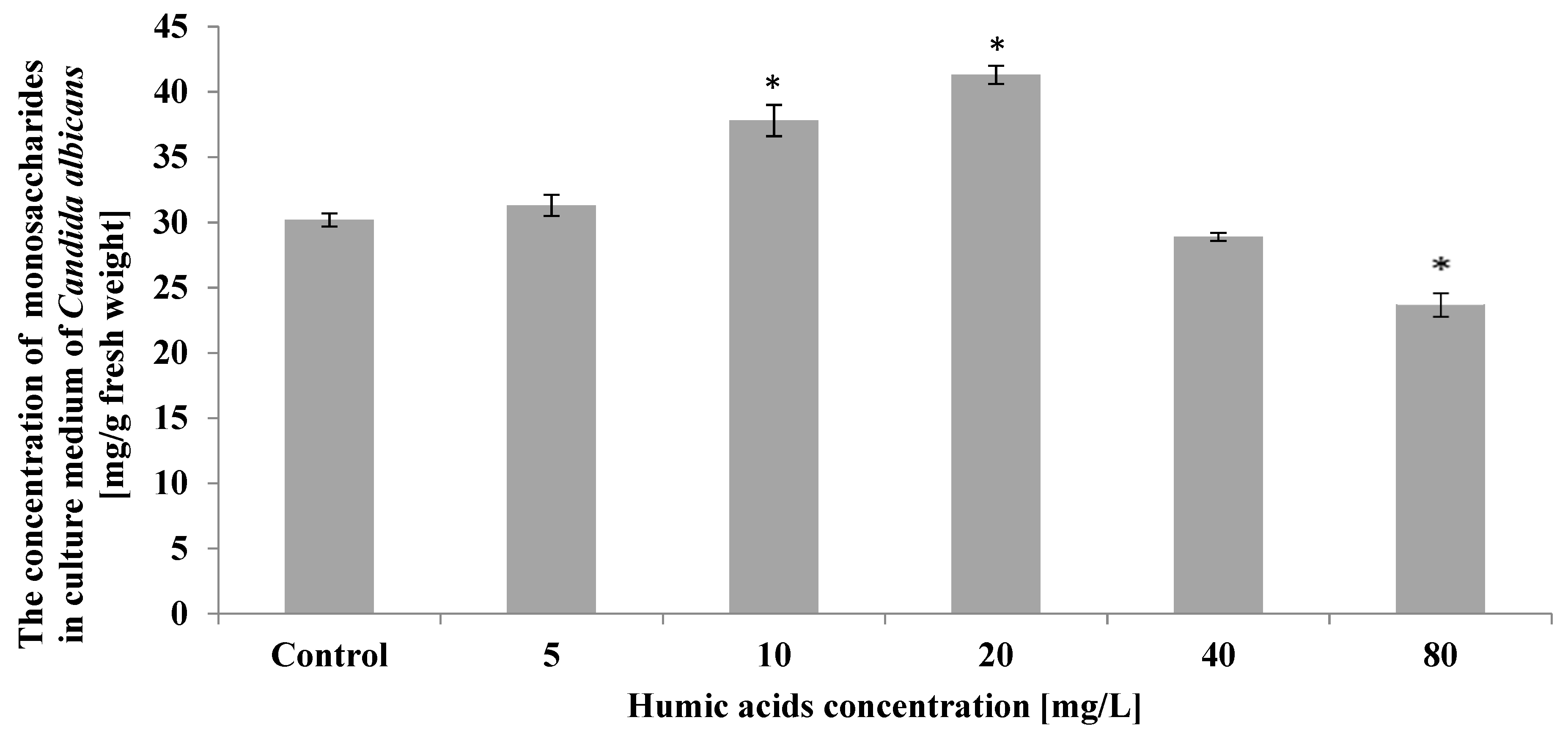
2.3. Determination of Monosaccharides in the Biomass and Secretions of Candida albicans
2.4. Determination of Catalase (CAT) Activity in Candida albicans Biomass
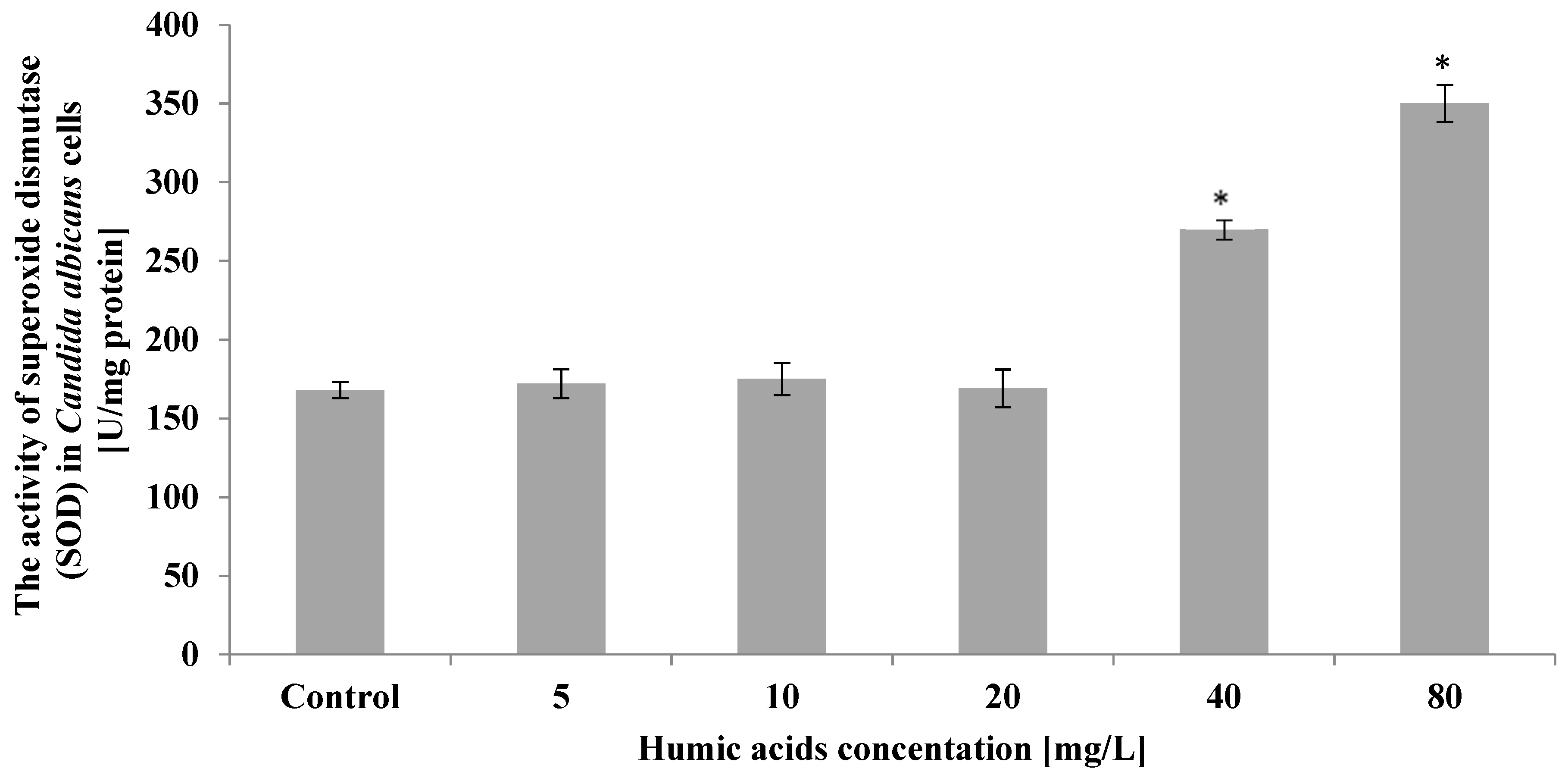
2.5. Determination of Superoxide Dismutase (SOD) Activity in Candida albicans Biomass
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bährs, H.; Steinberg, C.E.W. Impact of two different humic substances on selected coccal green algae and cyanobacteria—Changes in growth and photosynthetic performance. Environ. Sci. Pollut. Res. 2012, 19, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ghabbour, E.A.; Davies, G.; Ghali, N.K.; Mulligan, M.D. The effect of temperature on tight metal binding by peat and soil derived solid humic acids. Can. J. Soil Sci. 2001, 81, 331–336. [Google Scholar] [CrossRef]
- Peňa-Mẻndez, E.M.; Havel, J.; Patoćka, J. Humic substances-coumpounds of still unknown structure: Application in agriculture, industry, environment and biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, C.E.; Meinelt, T.; Timofeyev, M.A.; Bittner, M.; Menzel, R. Humic substances. Part 2: Interactions with organisms. Environ. Sci. Pollut. Res. Int. 2008, 15, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hax, C.L.; Golladay, S.W. Macroinvertebrate colonization and biofilm development on leaves and wood in a boreal river. Freshw. Biol. 1993, 29, 79–87. [Google Scholar] [CrossRef]
- Műnster, U. Studies on phosphatase activities in humic lakes. Environ. Intern. 1994, 20, 49–60. [Google Scholar] [CrossRef]
- Kamiyama, T.; Itakura, S.; Nagasaki, K. Changes in microbial loop components: Effects of a harmful algal bloom formation and its decay. Aquat. Microb. Ecol. 2000, 21, 21–30. [Google Scholar] [CrossRef]
- Siuda, W.; Chrust, R.J. Decomposition and utilization of particulate organic matter by bacteria in lakes of different trophic status. Pol. J. Environ. Stud. 2002, 11, 53–65. [Google Scholar]
- Pascoal, C.; Marvanová, L.; Cássio, F. Aquatic hyphomycete diversity in streams of Northwest Portugal. Fungal Divers. 2005, 19, 109–128. [Google Scholar]
- Krauss, G.J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in freshwaters: Ecology, physiology and biochemical pptetntial. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Chauvet, E.; Barlocher, F.; Graca, M.A.S.; Canhoto, C. Top-down and bottom-up control of litter decomposers in streams. Freshw. Biol. 2014, 59, 2172–2182. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Kapur, M.; Labana, S.; Lal, B.; Sarma, P.; Bhattacharya, D.; Thakur, I. Microbial diversity: Application of microorganisms for the biodegradation of xenobiotics. Curr. Sci. 2005, 89, 101–112. [Google Scholar]
- Junghanns, C.; Moeder, M.; Krauss, G.; Martin, C.; Schlosser, D. Degradation of the xenoestrogen nonylphenol by aquatic fungi and their laccases. Microbiology 2005, 151, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Augustin, T.; Schlosser, D.; Baumbach, R.; Schmidt, J.; Grancharov, K.; Krauss, G.; Krauss, G.J. Biotransformation of 1-Naphthol by a strictly aquatic fungus. Curr. Microbiol. 2006, 52, 216–220. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, C.E.W. Humic substances in the environment with an emphasis on freshwater systems. Environ. Sci. Pollut. Res. 2008, 15, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Krauss, G.; Schlosser, D.; Krauss, G.J. Aquatic fungi in heavy metal and organically polluted habitats. In Biodiversity of Fungi: Their Role in Human Life; Deshmukh, S.K., Rai, M.K., Enfield, N.H., Eds.; Science Publishers, Inc.: New York, NY, USA, 2005; pp. 221–246. [Google Scholar]
- Sridhar, K.R.; Krauss, G.; Bärlocher, F.; Raviraja, N.S.; Wennrich, R.; Baumbach, R.; Krauss, G.J. Decomposition of alder leaves in two heavy metal-polluted strewams in Central Germany. Aquat. Microb. Ecol. 2001, 26, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Biedunkiewicz, A.; Silicki, A.; Mazurkiewicz-Zapałowicz, K. Yeast-like fungi in selected bath of Szczecin. Limnol. Rev. 2007, 3, 3–10. [Google Scholar]
- Biedunkiewicz, A. Selected microfungi postulated as bioindicators in the assessment of waters purity in Poland. In Innovation Processes in ICSTI Community. Production of Clean Water: Challenges and Innovative Solutions, 4th ed.; Kodola, V., Ed.; International Centre for Scientific and Technical Information: Paris, France, 2011; pp. 115–124. [Google Scholar]
- Cudowski, A.; Pietryczuk, A.; Hauschild, T. Quantitative and qualitative diversity of aquatic fungi in relation to the physical and chemical parameters of water quality in the Augustów Canal. Fungal Ecol. 2015, 13, 193–204. [Google Scholar] [CrossRef]
- Cudowski, A.; Pietryczuk, A. Biodiversity of mycoplankton in the profile of eutrophic lakes with varying water quality. Fungal Ecol. 2020, 48, 100978. [Google Scholar] [CrossRef]
- McDonough, L.K.; Santos, I.R.; Andersen, M.S.; O’Carroll, D.M.; Rutlidge, H.; Meredith, K.; Oudone, P.; Bridgeman, J.; Gooddy, D.C.; Sorensen, J.P.R.; et al. Changes in global groundwater organic carbon driven by climate change and urbanization. Nat. Commun. 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, B.R. Spectrophotometric determination of protein in alkaline solution. Nature 1958, 182, 246. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Samogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Samogyi, M. Notes on sugar determination. J. Biol. Chem. 1954, 195, 19–23. [Google Scholar] [CrossRef]
- Aeby, H. Catalase in vitro. Methods Enzymol. 1984, 105, 125–212. [Google Scholar]
- Beauchamp, C.; Fridovich, I. SOD improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 444, 276–287. [Google Scholar] [CrossRef]
- Pietryczuk, A.; Cudowski, A.; Hauschild, T. Effect of lakes with varied trophic status on the species diversity and abundance of fungi. Ecotoxicol. Environ. Saf. 2014, 109, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Pietryczuk, A.; Cudowski, A.; Hauschild, T.; Świsłocka, M.; Więcko, A.; Karpowicz, M. Abudance and species diversity of fungi in rivers with various contaminations. Curr. Microbiol. 2017, 75, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Wurzbacher, C.; Bärlocher, F.; Grossart, H.P. Fungi in lake ecosystem. Aquat. Microb. Ecol. 2010, 58, 125–149. [Google Scholar] [CrossRef] [Green Version]
- Biedunkiewicz, A.; Baranowska, E. Yeasts and yeast-like fungi as an element of purity assessment off surface waters. Pol. J. Environ. Stud. 2011, 20, 267–274. [Google Scholar]
- Wurzbacher, C.; Rösel, S.; Rychła, A.; Grossart, H.P. Importance of saprotrophic freshwater fungi for pollen degradation. PLoS ONE 2014, 9, e94643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Fryar, S.; Tian, Q.; Bahkali, A.H.; Xu, J. Lignicolous freshwater fungi along a north-south latitudinal gradient in the Asia/Australian region; can we predict th impact of global warming on biodiversity and function? Fungal Ecol. 2015, 19, 190–200. [Google Scholar] [CrossRef]
- Heinrich, A. Differential Sensitivity of a Coccal Green Algal and a Cyanobacterial Species to Dissolved Natural Organic Matter (NOM). Environ. Sci. Pollut. Res. Int. 2007, 14, 11–18. [Google Scholar]
- Gim, G.H.; Kim, S.W. Growth factors in oceanic sediment significantly stimulate the biomass and lipid production of two oleaginous microalgae. J. Appl. Phycol. 2019, 31, 49–59. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Stepanova, E.V.; Koroleva, O.V. Mitigating activity of humic substances: Direct influence on biota. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice, Proceedings of the NATO Adanced Research Workshop on Use of Humates to Remediate Polluted Environments: From Theory to Practice, Zvenigorod, Russia, 23-29 September 2002; Perminova, I.V., Hatfield, K., Hertkorn, N., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 285–309. [Google Scholar]
- Meshcheryakova, O.V.; Gruzdev, A.I.; Nemova, N.N. Comparative Evaluation of Carbohydrate Metabolism in Perch (Perca fluviatilis L.) from Water Bodies with Different Contents of Humic Acids. Biol. Bull. Russ. Acad. Sci. 2004, 31, 15–20. [Google Scholar] [CrossRef]
- Menzel, R.; Stürzenbaum, K.; Kulas, J.; Bärenwaldt, A.; Steinberg, C.E.W. Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2005, 39, 8324–8332. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, J.; Große, S.; Marx, C.; Siwczak, F.; Stengel, S.; Bruns, T.; Bauer, R.; Kiehntopf, M.; Williams, D.L.; Wang, Z.Q.; et al. Candida albicans β-Glucan Differentiates Human Monocytes into a Specific Subset of Macrophages. Front. Immunol. 2018, 30, 2818. [Google Scholar] [CrossRef]
- Ding, J.; Huang, X.; Zhang, L.; Zhao, N.; Yang, D.; Zhang, K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.L. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 87, 829–845. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antyoxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- González-Párraga, P.; Hernández, J.A.; Argüelles, J.C. Role of antioxidant enzymatic defences against oxidative stress (H2O2) and the acquisition of oxidative tolerance in Candida albicans. Yeast 2003, 20, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Herrero-de-Dios, C.; Belmonte, R.; Budge, S.; Lopez Garcia, A.; Kolmogorova, A.; Lee, K.K.; Martin, B.D.; Ribeiro, A.; Bebes, A.; et al. Elevated catalase expression in a fungal pathogen is a double-edged sword of iron. PLOS Pathog. 2017, 13, e1006405. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cudowski, A.; Pietryczuk, A.; Górniak, A. Effect of Humic Acid on the Growth and Metabolism of Candida albicans Isolated from Surface Waters in North-Eastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 9408. https://doi.org/10.3390/ijerph19159408
Cudowski A, Pietryczuk A, Górniak A. Effect of Humic Acid on the Growth and Metabolism of Candida albicans Isolated from Surface Waters in North-Eastern Poland. International Journal of Environmental Research and Public Health. 2022; 19(15):9408. https://doi.org/10.3390/ijerph19159408
Chicago/Turabian StyleCudowski, Adam, Anna Pietryczuk, and Andrzej Górniak. 2022. "Effect of Humic Acid on the Growth and Metabolism of Candida albicans Isolated from Surface Waters in North-Eastern Poland" International Journal of Environmental Research and Public Health 19, no. 15: 9408. https://doi.org/10.3390/ijerph19159408





