Quadrivalent Vaccines for the Immunization of Adults against Influenza: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy and Data Source
2.3. Studies Selection
- Evaluated any QIV compared to placebo or TIV or another QIV in adults aged 18–64 years;
- Adopted a randomized controlled trial (RCT) study design that compared two or more groups, one of which receiving any QIV as intervention;
- Issued results on immunogenicity, in terms of seroconversion (SCR) or seroprotection rate (SPR), and efficacy, in terms of laboratory-confirmed influenza.
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
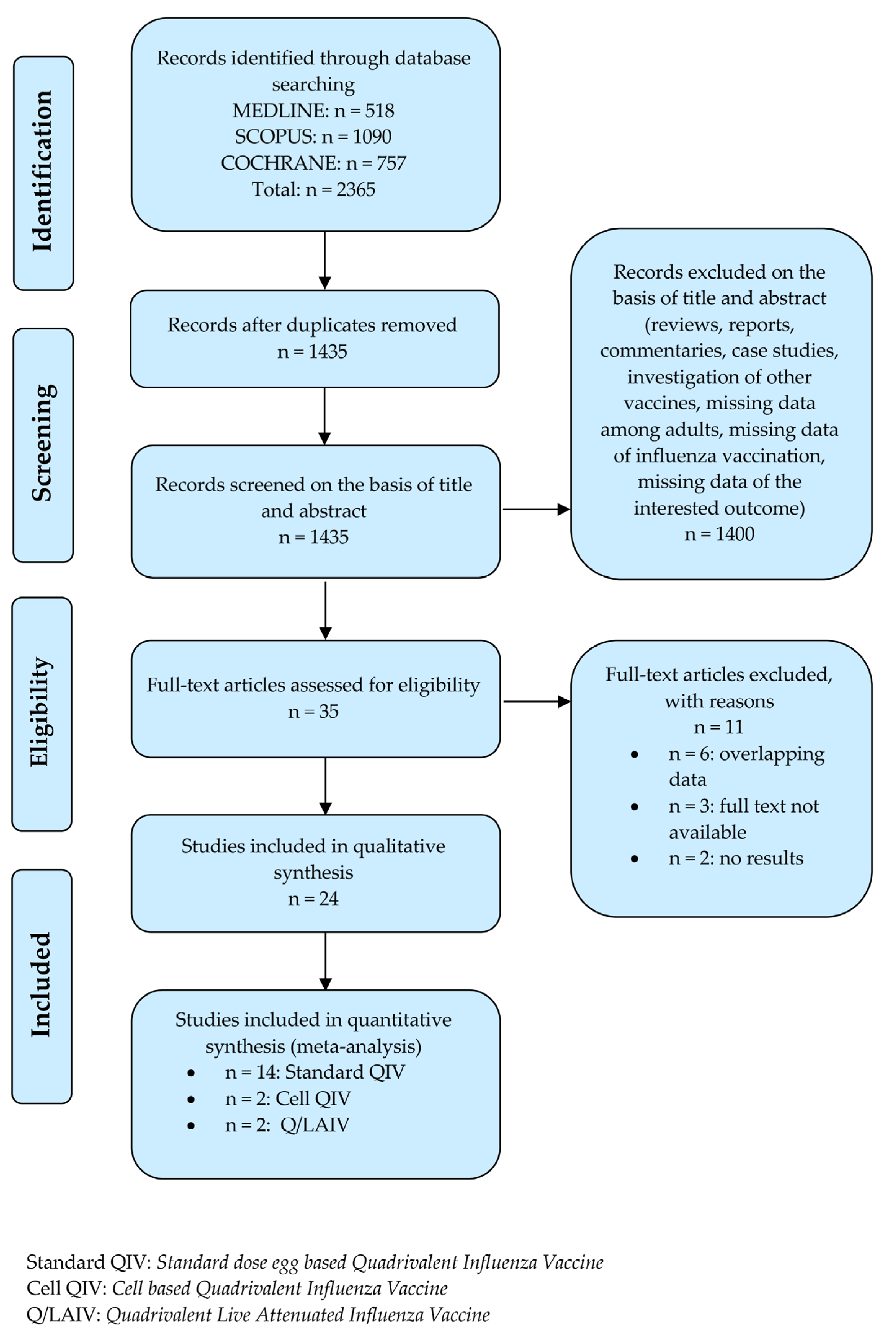
3.1. Studies Selection
3.2. Characteristics of Included RCTs
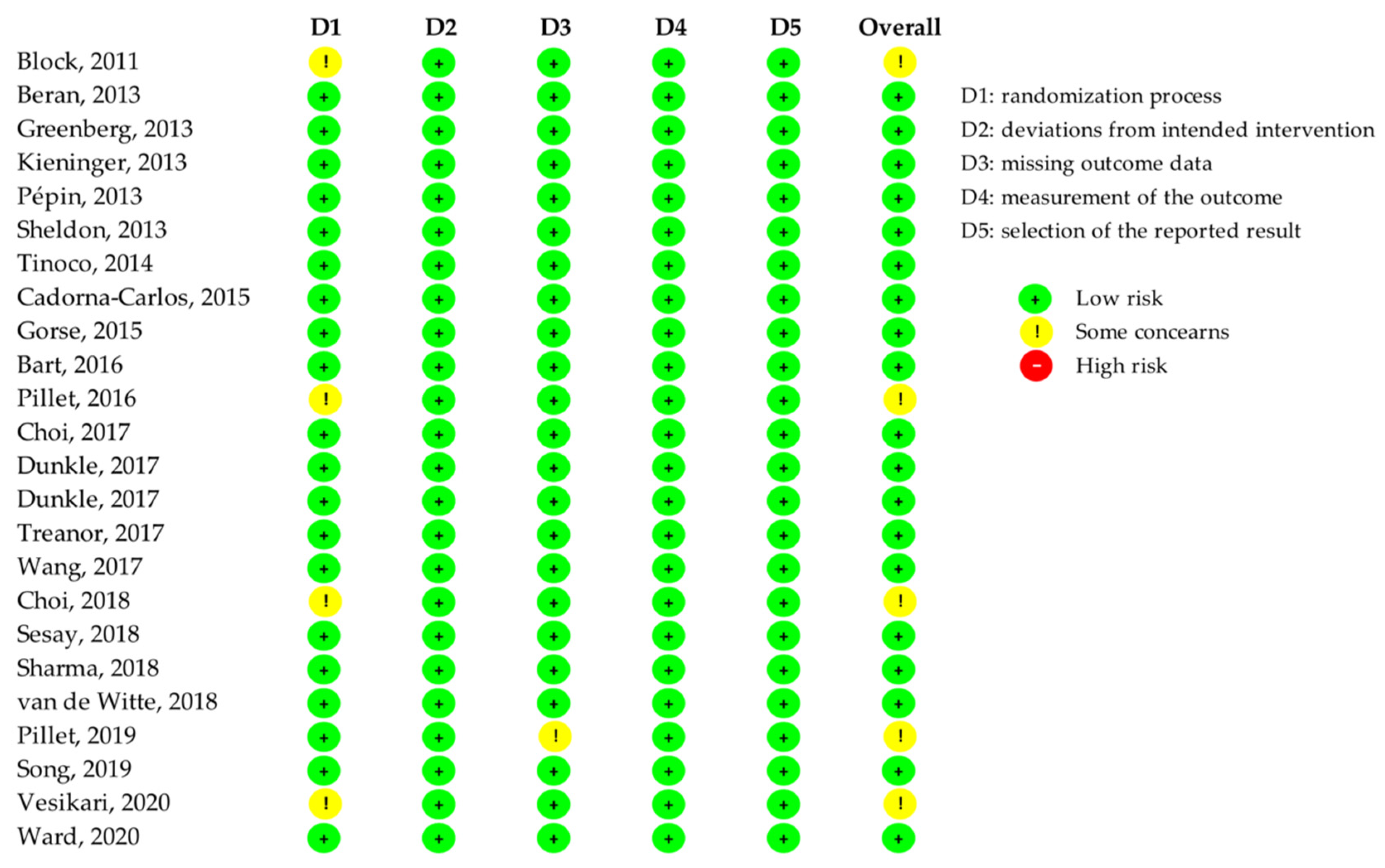
3.3. Quality Assessment of Included RCTs
3.4. Synthesis of Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassini, A.; Colzani, E.; Pini, A.; Mangen, M.-J.J.; Plass, D.; McDonald, S.A.; Maringhini, G.; van Lier, A.; Haagsma, J.A.; Havelaar, A.H.; et al. Impact of Infectious Diseases on Population Health Using Incidence-Based Disability-Adjusted Life Years (DALYs): Results from the Burden of Communicable Diseases in Europe Study, European Union and European Economic Area Countries, 2009 to 2013. Eurosurveillance 2018, 23, 17–00454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Fact Sheet: Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 5 July 2021).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J. The Rationale for Quadrivalent Influenza Vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. Types of Seasonal Influenza Vaccine. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/types-of-seasonal-influenza-vaccine (accessed on 5 July 2021).
- European Medicine Agency. European Medicines Agency Issues Recommendations for 2012/2013 Seasonal Flu Vaccine Composition. Available online: https://www.ema.europa.eu/en/news/european-medicines-agency-issues-recommendations-20122013-seasonal-flu-vaccine-composition (accessed on 5 July 2021).
- Barberis, I.; Tisa, V.; Faccio, V.; Paganino, C.; Trucchi, C.; Martini, M.; Ansaldi, F. Quadrivalent Influenza Vaccine: A New Opportunity to Reduce the Influenza Burden. J. Prev. Med. Hyg. 2016, 57, E28–E33. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and Effectiveness of Influenza Vaccines: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Chuang, J.-H.; Kuo, H.-W.; Huang, W.-T.; Hsu, Y.-F.; Liu, M.-T.; Chen, C.-H.; Huang, H.-H.; Chang, C.-H.; Chou, J.-H.; et al. Surveillance and Vaccine Effectiveness of an Influenza Epidemic Predominated by Vaccine-Mismatched Influenza B/Yamagata-Lineage Viruses in Taiwan, 2011−12 Season. PLoS ONE 2013, 8, e58222. [Google Scholar] [CrossRef] [Green Version]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Kieke, B.A.; Gaglani, M.; Murthy, K.; Piedra, P.A.; Zimmerman, R.K.; Nowalk, M.P.; Raviotta, J.M.; et al. Influenza Vaccine Effectiveness in the United States During 2012-2013: Variable Protection by Age and Virus Type. J. Infect. Dis. 2015, 211, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing Seasonal Influenza—The Need for a Universal Influenza Vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef]
- Bansal, A.; Trieu, M.-C.; Mohn KG, I.; Cox, R.J. Safety, Immunogenicity, Efficacy and Effectiveness of Inactivated Influenza Vaccines in Healthy Pregnant Women and Children Under 5 Years: An Evidence-Based Clinical Review. Front. Immunol. 2021, 12, 744774. [Google Scholar] [CrossRef]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for Preventing Influenza in the Elderly. Cochrane Database Syst. Rev. 2018, 2021, CD004876. [Google Scholar] [CrossRef] [Green Version]
- Moa, A.M.; Chughtai, A.A.; Muscatello, D.J.; Turner, R.M.; MacIntyre, C.R. Immunogenicity and Safety of Inactivated Quadrivalent Influenza Vaccine in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Vaccine 2016, 34, 4092–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Ades, A.E. Combination of Direct and Indirect Evidence in Mixed Treatment Comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE Guidelines: 7. Rating the Quality of Evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Block, S.L.; Yi, T.; Sheldon, E.; Dubovsky, F.; Falloon, J. A Randomized, Double-Blind Noninferiority Study of Quadrivalent Live Attenuated Influenza Vaccine in Adults. Vaccine 2011, 29, 9391–9397. [Google Scholar] [CrossRef]
- Beran, J.; Peeters, M.; Dewé, W.; Raupachová, J.; Hobzová, L.; Devaster, J.-M. Immunogenicity and Safety of Quadrivalent versus Trivalent Inactivated Influenza Vaccine: A Randomized, Controlled Trial in Adults. BMC Infect. Dis. 2013, 13, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, D.P.; Robertson, C.A.; Noss, M.J.; Blatter, M.M.; Biedenbender, R.; Decker, M.D. Safety and Immunogenicity of a Quadrivalent Inactivated Influenza Vaccine Compared to Licensed Trivalent Inactivated Influenza Vaccines in Adults. Vaccine 2013, 31, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, D.; Sheldon, E.; Lin, W.-Y.; Yu, C.-J.; Bayas, J.M.; Gabor, J.J.; Esen, M.; Fernandez Roure, J.L.; Narejos Perez, S.; Alvarez Sanchez, C.; et al. Immunogenicity, Reactogenicity and Safety of an Inactivated Quadrivalent Influenza Vaccine Candidate versus Inactivated Trivalent Influenza Vaccine: A Phase III, Randomized Trial in Adults Aged ≥18 Years. BMC Infect. Dis. 2013, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Pépin, S.; Donazzolo, Y.; Jambrecina, A.; Salamand, C.; Saville, M. Safety and Immunogenicity of a Quadrivalent Inactivated Influenza Vaccine in Adults. Vaccine 2013, 31, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, E.A.; Jeanfreau, R.; Sliman, J.A.; Charenkavanich, S.; Rousculp, M.D.; Dubovsky, F.; Mallory, R.M. Immunogenicity of a Quadrivalent Ann Arbor Strain Live Attenuated Influenza Vaccine Delivered Using a Blow-Fill-Seal Device in Adults: A Randomized, Active-Controlled Study*. Influenza Other Respir. Viruses 2013, 7, 1142–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinoco, J.C.; Pavia-Ruz, N.; Cruz-Valdez, A.; Aranza Doniz, C.; Chandrasekaran, V.; Dewé, W.; Liu, A.; Innis, B.L.; Jain, V.K. Immunogenicity, Reactogenicity, and Safety of Inactivated Quadrivalent Influenza Vaccine Candidate versus Inactivated Trivalent Influenza Vaccine in Healthy Adults Aged ≥18 Years: A Phase III, Randomized Trial. Vaccine 2014, 32, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Cadorna-Carlos, J.B.; Nolan, T.; Borja-Tabora, C.F.; Santos, J.; Montalban, M.C.; de Looze, F.J.; Eizenberg, P.; Hall, S.; Dupuy, M.; Hutagalung, Y.; et al. Safety, Immunogenicity, and Lot-to-Lot Consistency of a Quadrivalent Inactivated Influenza Vaccine in Children, Adolescents, and Adults: A Randomized, Controlled, Phase III Trial. Vaccine 2015, 33, 2485–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorse, G.J.; Falsey, A.R.; Ozol-Godfrey, A.; Landolfi, V.; Tsang, P.H. Safety and Immunogenicity of a Quadrivalent Intradermal Influenza Vaccine in Adults. Vaccine 2015, 33, 1151–1159. [Google Scholar] [CrossRef]
- Bart, S.; Cannon, K.; Herrington, D.; Mills, R.; Forleo-Neto, E.; Lindert, K.; Abdul Mateen, A. Immunogenicity and Safety of a Cell Culture-Based Quadrivalent Influenza Vaccine in Adults: A Phase III, Double-Blind, Multicenter, Randomized, Non-Inferiority Study. Hum. Vaccin. Immunother. 2016, 12, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Pillet, S.; Aubin, É.; Trépanier, S.; Bussière, D.; Dargis, M.; Poulin, J.-F.; Yassine-Diab, B.; Ward, B.J.; Landry, N. A Plant-Derived Quadrivalent Virus like Particle Influenza Vaccine Induces Cross-Reactive Antibody and T Cell Response in Healthy Adults. Clin. Immunol. 2016, 168, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.S.; Noh, J.Y.; Song, J.Y.; Cheong, H.J.; Wie, S.-H.; Lee, J.S.; Lee, J.; Kim, S.-W.; Jeong, H.W.; Jung, S.-I.; et al. Immunogenicity and Safety of a Cell Culture-Derived Inactivated Quadrivalent Influenza Vaccine (NBP607-QIV): A Randomized, Double-Blind, Multi-Center, Phase III Clinical Trial in Adults and Elderly Subjects. Hum. Vaccin. Immunother. 2017, 13, 1653–1660. [Google Scholar] [CrossRef] [Green Version]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.A.; Goldenthal, K.L.; Muse, D.; Cox MM, J. Randomized Comparison of Immunogenicity and Safety of Quadrivalent Recombinant Versus Inactivated Influenza Vaccine in Healthy Adults 18–49 Years of Age. J. Infect. Dis. 2017, 216, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox MM, J. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.T.; Albano, F.R.; Sawlwin, D.C.; Graves Jones, A.; Airey, J.; Formica, N.; Matassa, V.; Leong, J. Immunogenicity and Safety of a Quadrivalent Inactivated Influenza Vaccine Compared with Two Trivalent Inactivated Influenza Vaccines Containing Alternate B Strains in Adults: A Phase 3, Randomized Noninferiority Study. Vaccine 2017, 35, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Liu, S.-Z.; Chu, K.; Zhao, Y.; Zhu, F.-C.; Hu, Y.-M.; Meng, F.-Y.; Li, J.-X.; Luo, L.; Yang, J.-Y.; et al. Immunogenicity and Safety of an Inactivated Quadrivalent Influenza Vaccine Candidate versus Inactivated Trivalent Influenza Vaccines in Participants >/=3 Years of Age: A Double-Blind, Randomized, Parallel-Controlled Phase III Clinical Trial in China. Expert. Rev. Vaccines 2017, 16, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Noh, J.Y.; Lee, J.; Choi, J.Y.; Lee, J.-S.; Kim, M.S.; Kim, H.S.; Bang, J.; Lavis, N.; Kim, W.J. Immunogenicity and Safety of a Split-Virion Quadrivalent Influenza Vaccine in Adults 18–60 Years of Age in the Republic of Korea. Hum. Vaccin. Immunother. 2018, 14, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesay, S.; Brzostek, J.; Meyer, I.; Donazzolo, Y.; Leroux-Roels, G.; Rouzier, R.; Astruc, B.; Szymanski, H.; Toursarkissian, N.; Vandermeulen, C.; et al. Safety, Immunogenicity, and Lot-to-Lot Consistency of a Split-Virion Quadrivalent Influenza Vaccine in Younger and Older Adults: A Phase III Randomized, Double-Blind Clinical Trial. Hum. Vaccin. Immunother. 2018, 14, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Singh, V.B.; Kumar, S.; Prajapati, V.; Patel, J.; Vukkala, R.; Jangid, S.K.; Sanmukhani, J.; Gupta, G.; Patel, P.; et al. Immunogenicity and Safety of the First Indigenously Developed Indian Tetravalent Influenza Vaccine (Split Virion) in Healthy Adults ≥ 18 Years of Age: A Randomized, Multicenter, Phase II/III Clinical Trial. Hum. Vaccin. Immunother. 2018, 14, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Van de Witte, S.; Nauta, J.; Montomoli, E.; Weckx, J. A Phase III Randomised Trial of the Immunogenicity and Safety of Quadrivalent versus Trivalent Inactivated Subunit Influenza Vaccine in Adult and Elderly Subjects, Assessing Both Anti-Haemagglutinin and Virus Neutralisation Antibody Responses. Vaccine 2018, 36, 6030–6038. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and Safety of a Quadrivalent Plant-Derived Virus like Particle Influenza Vaccine Candidate—Two Randomized Phase II Clinical Trials in 18 to 49 and ≥50 Years Old Adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Lee, J.; Woo, H.J.; Wie, S.-H.; Lee, J.S.; Kim, S.W.; Kim, T.H.; Jung, S.-I.; Noh, J.Y.; Choi, W.S.; et al. Immunogenicity and Safety of an Egg-Based Inactivated Quadrivalent Influenza Vaccine (GC3110A) versus Two Inactivated Trivalent Influenza Vaccines with Alternate B Strains: A Phase Ⅲ Randomized Clinical Trial in Adults. Hum. Vaccin. Immunother. 2019, 15, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Vesikari, T.; Virta, M.; Heinonen, S.; Eymin, C.; Lavis, N.; Chabanon, A.L.; Gresset-Bourgeois, V. Immunogenicity and Safety of a Quadrivalent Inactivated Influenza Vaccine in Pregnant Women: A Randomized, Observer-Blind Trial. Hum. Vaccin. Immunother. 2020, 16, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, Immunogenicity, and Safety of a Plant-Derived, Quadrivalent, Virus-like Particle Influenza Vaccine in Adults (18–64 Years) and Older Adults (≥65 Years): Two Multicentre, Randomised Phase 3 Trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Influenza (Flu): Past Seasons Estimated Influenza Disease Burden Averted by Vaccination. Available online: https://www.cdc.gov/flu/vaccines-work/past-burden-averted-est.html (accessed on 7 April 2022).
- Preaud, E.; Durand, L.; Macabeo, B.; Farkas, N.; Sloesen, B.; Palache, A.; Shupo, F.; Samson, S.I. Annual Public Health and Economic Benefits of Seasonal Influenza Vaccination: A European Estimate. BMC Public Health 2014, 14, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregoning, J.S. First Human Efficacy Study of a Plant-Derived Influenza Vaccine. Lancet 2020, 396, 1464–1465. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The Role of Serum Haemagglutination-Inhibiting Antibody in Protection against Challenge Infection with Influenza A2 and B Viruses. Epidemiol. Infect. 1972, 70, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Cox, R. Correlates of Protection to Influenza Virus, Where Do We Go from Here? Hum. Vaccin. Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between Haemagglutination-Inhibiting Antibody Titres and Clinical Protection against Influenza: Development and Application of a Bayesian Random-Effects Model. BMC Med. Res. Methodol. 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, C.M.; Montomoli, E. Influenza Immunology Evaluation and Correlates of Protection: A Focus on Vaccines. Expert Rev. Vaccines 2016, 15, 967–976. [Google Scholar] [CrossRef]
- Bandell, A.; Woo, J.; Coelingh, K. Protective Efficacy of Live-Attenuated Influenza Vaccine (Multivalent, Ann Arbor Strain): A Literature Review Addressing Interference. Expert Rev. Vaccines 2011, 10, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Wijnans, L.; Voordouw, B. A Review of the Changes to the Licensing of Influenza Vaccines in Europe. Influenza Other Respir. Viruses 2016, 10, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Mardanova, E.S.; Ravin, N.V. Plant-Produced Recombinant Influenza A Vaccines Based on the M2e Peptide. Curr. Pharm. Des. 2018, 24, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted Influenza Vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Montagne, J.R.; Fauci, A.S. Intradermal Influenza Vaccination—Can Less Be More? N. Engl. J. Med. 2004, 351, 2330–2332. [Google Scholar] [CrossRef] [PubMed]
- Divino, V.; Ruthwik Anupindi, V.; DeKoven, M.; Mould-Quevedo, J.; Pelton, S.I.; Postma, M.J.; Levin, M.J. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines During the 2019–2020 Influenza Season in the United States. Open Forum Infect. Dis. 2022, 9, ofab604. [Google Scholar] [CrossRef]
| Type of Vaccine [ref] | SCR (95%CI) {n} | |||
|---|---|---|---|---|
| H1N1 | H3N2 | B Yamagata | B Victoria | |
| Standard QIV [23,24,25,26,28,29,36,37,38,39,40,41,43,44] | 65% (58–72%) {14} * | 65% (58–72%) {14} * | 63% (58–68%) {14} * | 63% (59–67%) {14} * |
| Low Dose Adjuvanted QIV [23] | 58% (48–67%) {1} | 66% (57–75%) {1} | 79% (70–86%) {1} | 65% (56–74%) {1} |
| Cell based QIV [31,33] | 58% (47–68%) {2} * | 51% (47–56%) {2} * | 48% (40–57%) {2} * | 53% (50–56%) {2} * |
| Plant-derived QIV [45] | 37% (32–43%) {1} | 46% (40–52%) {1} | 32% (26–37%) {1} | 18% (14–23%) {1} |
| Recombinant QIV [35] | 56% (49–63%) {1} | 63% (56–69%) {1} | 43% (36–50%) {1} | 26% (20–33%) {1} |
| Intradermal QIV [30] | 58% (55–61%) {1} | 59% (56–61%) {1} | 56% (53–59%) {1} | 50% (47–53%) {1} |
| Live attenuated QIV [22,27] | 5% (4–6%) {2} ° | 5% (4–6%) {2} ° | 9% (7–11%) {2} ° | 10% (6–15%) {2} * |
| Type of Vaccine | SPR (95%CI) {n} | |||
|---|---|---|---|---|
| H1N1 | H3N2 | B Yamagata | B Victoria | |
| Standard QIV [23,24,25,26,28,29,36,37,38,39,40,41,43,44] | 65% (58–72%) {14} * | 94% (88–98%) {14} * | 96% (93–99%) {14} * | 81% (68–91%) {14} * |
| Low Dose Adjuvanted QIV [23] | 88% (81–94%) {1} | 98% (94–100%) {1} | 95% (90–98%) {1} | 97% (92–99%) {1} |
| Cell based QIV [31,33] | 98% (97–99%) {2} ° | 99% (98–99%) {2} ° | 99% (98–99%) {2} ° | 98% (93–100%) {2} * |
| Plant-derived QIV [45] | 75% (69–80%) {1} | 85% (81–89%) {1} | 76% (71–91%) {1} | 41% (35–47%) {1} |
| Recombinant QIV [35] | 91% (88–94%) {1} | 100% (99–100%) {1} | 68% (63–73%) {1} | 50% (44–56%) {1} |
| Intradermal QIV | N.A. | N.A. | N.A. | N.A. |
| Live attenuated QIV [27] | 25% (23–28%) {1} | 26% (23–28%) {1} | 79% (76–81%) {1} | 56% (53–58%) {1} |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannocci, A.; Pellacchia, A.; Millevolte, R.; Chiavarini, M.; de Waure, C. Quadrivalent Vaccines for the Immunization of Adults against Influenza: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 9425. https://doi.org/10.3390/ijerph19159425
Mannocci A, Pellacchia A, Millevolte R, Chiavarini M, de Waure C. Quadrivalent Vaccines for the Immunization of Adults against Influenza: A Systematic Review of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(15):9425. https://doi.org/10.3390/ijerph19159425
Chicago/Turabian StyleMannocci, Alice, Andrea Pellacchia, Rossella Millevolte, Manuela Chiavarini, and Chiara de Waure. 2022. "Quadrivalent Vaccines for the Immunization of Adults against Influenza: A Systematic Review of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 15: 9425. https://doi.org/10.3390/ijerph19159425







