Indoor Household Exposures and Associated Morbidity and Mortality Outcomes in Children and Adults in South Africa
Abstract
:1. Introduction
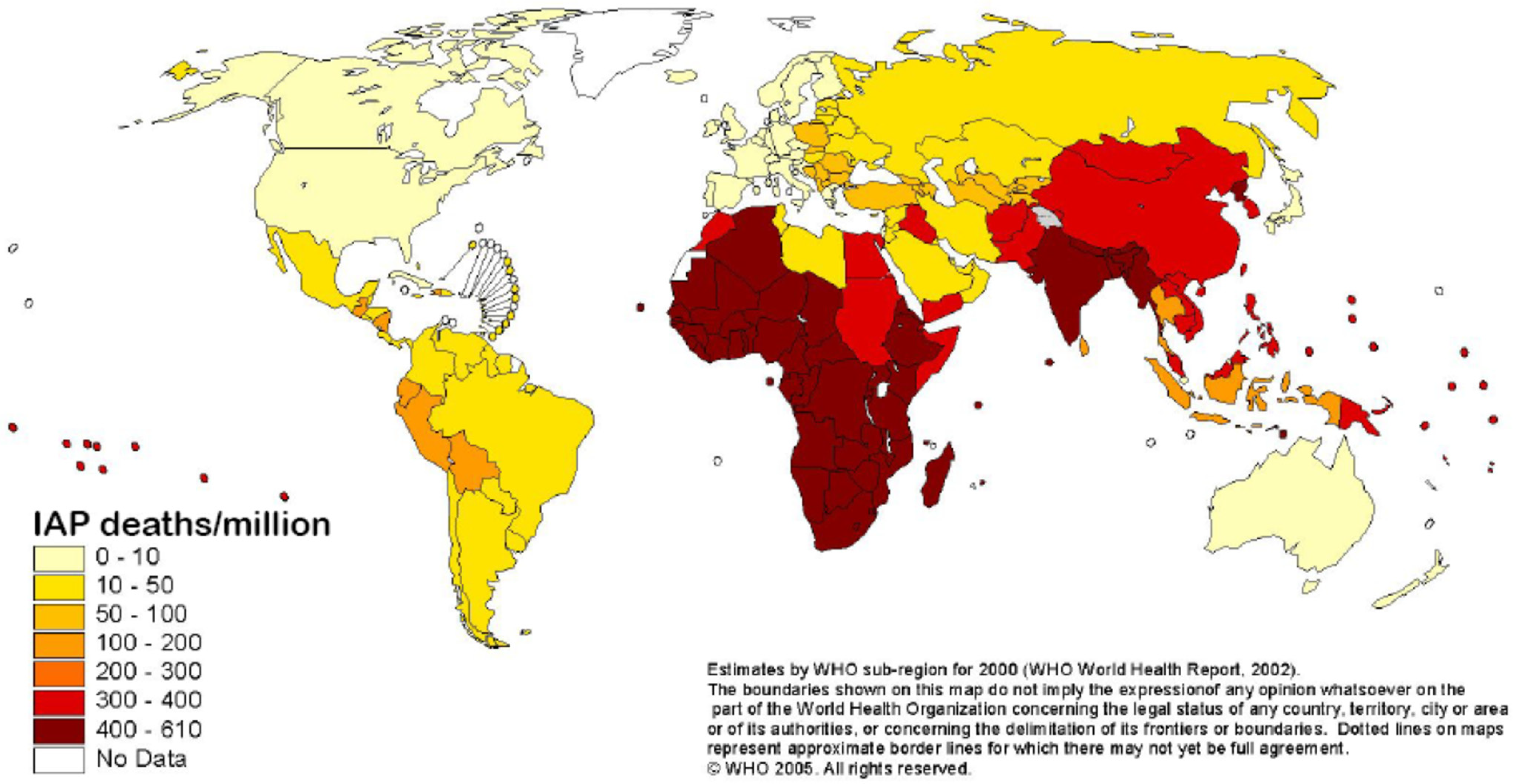
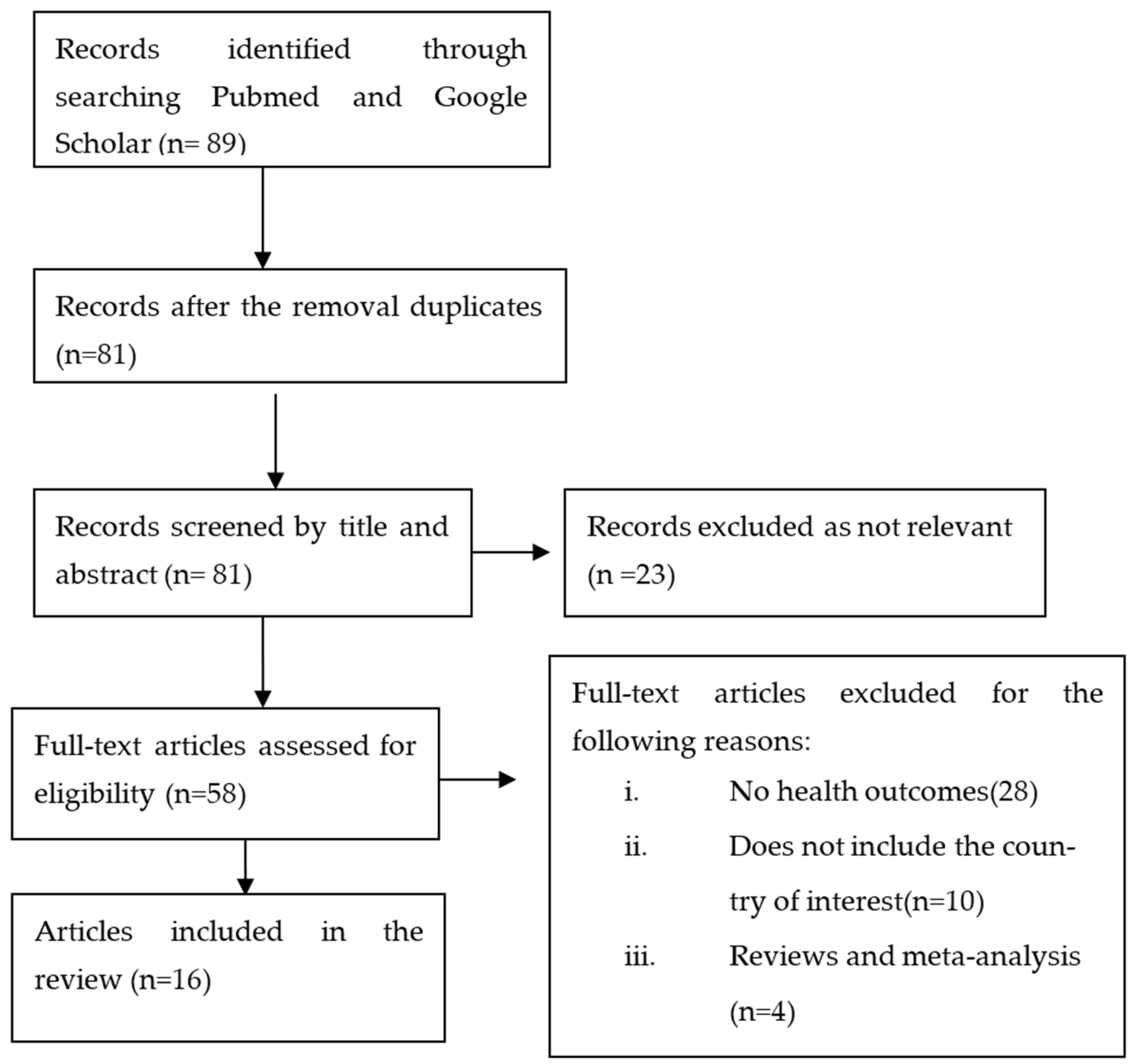
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
3. Results and Discussion
3.1. Study Characteristics
3.2. Bioaerosols, Allergens Exposure and Health Outcomes
3.3. Household/Tobacco Smoking and Dental Caries
3.4. Household/Tobacco Smoking and Respiratory Outcomes
3.5. Household Cooking and Heating Fuels and Adverse Health Outcomes
3.6. Particulate Matter, Gaseous Pollutants and Adverse Health Outcomes
3.7. Indoor Spray Residue and Adverse Health Outcomes
3.8. Household Dampness and Respiratory Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braubach, M.; Fairburn, J. Social inequities in environmental risks associated with housing and residential location—A review of evidence. Eur. J. Public Health 2010, 20, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, R.J. Housing and health: From interdisciplinary principles to transdisciplinary research and practice. Futures 2004, 36, 487–502. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauh, V.A.; Landrigan, P.J.; Claudio, L. Housing and health: Intersection of poverty and environmental exposures. Ann. N. Y. Acad. Sci. 2008, 1136, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Eskenazi, B.; Bornman, R.; Gasper, F.; Crause, M.; Obida, M.; Chevrier, J. Exposure to DDT and Hypertensive Disorders of Pregnancy Among South African Women from an Indoor Residual Spraying Region: The VHEMBE Study. Environ. Res. 2018, 162, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jafta, N.; Barregard, L.; Jeena, P.M.; Naidoo, R.N. Indoor air quality of low and middle income urban households in Durban, South Africa. Environ. Res. 2017, 156, 47–56. [Google Scholar] [CrossRef]
- Hargreaves, M.; Parappukkaran, S.; Morawska, L.; Hitchins, J.; He, C.; Gilbert, D. A pilot investigation into associations between indoor airborne fungal and non-biological particle concentrations in residential houses in Brisbane, Australia. Sci. Total Environ. 2003, 312, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Dannemiller, K.C.; Gent, J.F.; Leaderer, B.P.; Peccia, J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air 2016, 26, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar]
- WHO. Tobacco. World Health Organization. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 18 October 2019).
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- WHO. Tobacco: Key Facts. 2020. Available online: https://www.who.int/news-room/factsheets/detail/tobacco (accessed on 20 August 2020).
- Besaratinia, A.; Pfeifer, G.P. Second-hand smoke and human lung cancer. Lancet Oncol. 2008, 9, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.C.; Glantz, S.A. Evidence secondhand smoke causes breast cancer in 2005 stronger than for lung cancer in 1986. Prev. Med. 2008, 46, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, R.X.; Thrift, A.G.; McNeil, J.J.; Davis, S.M.; Donnan, G.A. Ischemic stroke risk and passive exposure to spouses’ cigarette smoking. Melbourne stroke risk factor study (merfs) group. Am. J. Public Health 1999, 89, 572–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnoya, J.; Glantz, S.A. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005, 111, 2684–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, Z.; Manning, P.J.; Holohan, J.; Keogan, S.; Goodman, P.G.; Clancy, L. Second-hand smoke exposure in cars and respiratory health e_ects in children. Eur. Respir. J. 2009, 34, 629–633. [Google Scholar] [CrossRef] [Green Version]
- WHO. Household Air Pollution and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 8 June 2022).
- Burnett, R.T.; Pope, C.A.; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Rahut, D.B.; Behera, B.; Ali, A. Factors determining household use of clean and renewable energy sources for lighting in Sub-Saharan Africa. Renew. Sustain. Energy Rev. 2017, 72, 661–672. [Google Scholar] [CrossRef]
- Kuhn, R.; Rothman, D.S.; Turner, S.; Solórzano, J.; Hughes, B. Beyond attributable burden: Estimating the avoidable burden of disease associated with household air pollution. PLoS ONE 2016, 11, e0149669. [Google Scholar] [CrossRef] [Green Version]
- Pye, A.; Ronzi, S.; Hugo, B.; Ngahane, M.; Puzzolo, E.; Ashu, A.H.; Pope, D. Drivers of the Adoption and Exclusive Use of Clean Fuel for Cooking in Sub-Saharan Africa: Learnings and Policy Considerations from Cameroon. Int. J. Environ. Res. Public Health 2020, 17, 5874. [Google Scholar] [CrossRef]
- Aemro, Y.B.; Moura, P.; de Almeida, A.T. Inefficient cooking systems a challenge for sustainable development: A case of rural areas of Sub-Saharan Africa. Environ. Dev. Sustain. 2021, 23, 14697–14721. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death in the World. Fact Sheet Number 310; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abiyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Worldometer South Africa Population. 2022. Available online: https://www.worldometers.info/world-population/south-africa-population (accessed on 6 July 2022).
- Southern Africa Labour and Development Research Unit. National Income Dynamics Study 2017, Wave 5 Dataset. Version 1.0.0 Pretoria: Department of Planning, Monitoring, and Evaluation Agency; Southern Africa Labour and Development Research Unit; DataFirst Distributor: Cape Town, South Africa, 2018. [Google Scholar]
- Reddy, P.; Zuma, K.; Shisana, O.; Jonas, K.; Sewpaul, R. Prevalence of tobacco use among adults in south africa: Results from the first south african national health and nutrition examination survey. S. Afr. Med. J. 2015, 105, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellios, N.; van Walbeek, C. Determinants of regular smoking onset in South Africa using duration analysis. BMJ Open 2016, 6, e011076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Department of Health. South Africa Demographic and Health Survey 2016; National Department of Health: Pretoria, South Africa, 2019. [Google Scholar]
- Groenewald, P.; Vos, T.; Norman, R.; Laubscher, R.; van Walbeek, C.; Saloojee, U.; Sitas, F.; Bradshaw, D.; South African Comparitive Risk Assessment Collaboration Group. Estimating the burden of disease attributable to smoking in South Africa in 2000. S. Afr. Med. J. 2007, 97, 674–681. [Google Scholar]
- Goodchild, M.; Nargis, N.; D’Espaignet, E.T. Global economic cost of smoking-attributable diseases. Tob. Control 2018, 27, 58–64. [Google Scholar] [CrossRef]
- Boachie, M.K.; Rossouw, L.; Ross, H. The Economic Cost of Smoking in South Africa. In Proceedings of the Biennial Conference of the Economic Society of South Africa, Johannesburg, South Africa, 3–5 September 2019. [Google Scholar]
- Buthelezi, S.A.; Kapwata, T.; Wernecke, B.; Webster, C.; Mathee, A.; Wright, C.Y. Household Fuel Use for Heating and Cooking and Respiratory Health in a Low-Income, South African Coastal Community. Int. J. Environ. Res. Public Health 2019, 16, 550. [Google Scholar] [CrossRef] [Green Version]
- Barnes, B.R.; Mathee, A.; Thomas, L.; Bruce, N. Household energy, indoor air pollution and child respiratory health in South Africa. J. Energy S. Afr. 2009, 20, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Muller, E.; Diab, R.D.; Binedell, M.; Hounsome, R. Health risk assessment of kerosene usage in an informal settlement in Durban, South Africa. Atmos. Environ. 2003, 37, 2015–2022. [Google Scholar] [CrossRef]
- WHO. Global Health Observatory—Data Repository. 2018. Available online: http://www.who.int/gho/database/en (accessed on 23 May 2018).
- United Nations. Report of the Inter-Agency and Expert Group on Sustainable Development Goal Indicators. 2016. Available online: http://ggim.un.org/knowledgebase/KnowledgebaseArticle51479.aspx (accessed on 5 April 2019).
- Ehrlich, R.I.; Jordaan, E.; Du Toit, D.; Volmink, J.A.; Weinberg, E.; Zwarenstein, M. Underrecognition and undertreatment of asthma in Cape Town primary school children. S. Afr. Med. J. 1998, 88, 986–994. [Google Scholar]
- Wichmann and Voyi. Influence of cooking and heating fuel use on 1–59 month old mortality in South Africa. Matern Child Health J. 2006, 10, 553–561. [Google Scholar] [CrossRef]
- Ayo-Yusuf, O.A.; Reddy, P.S.; Wyk, P.J.; Borne, B.W. Household Smoking as a Risk Indicator for Caries in Ado-lescents’ Permanent Teeth. J Adolesc Health 2007, 41, 309–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafta, N.; Batterman, S.A.; Gqaleni, N.; Naidoo, R.N.; Robins, T.G. Characterisation of allergens and airborne fungi in low and middle-income homes of primary school children in Durban, South Africa. Am. J. Ind. Med. 2012, 55, 1110–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirinde, J.; Wichmann, J.; Voyi, K. Association between wheeze and selected air pollution sources in an air pollution priority area in South Africa: A cross-sectional study. Environ. Health 2014, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, P.N.; Wright, C.Y.; Voyi, K.V.V.; Mathee, A. Household fuel use and child respiratory ill health in two towns in Mpumalanga, South Africa. S. Afr. Med. J. 2015, 105, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Elf, J.L.; Eke, O.; Rakgokong, M.; Variava, E.; Baliram, Y.; Motlhaoleng, K.; Lebina, L.; Shapiro, A.E.; Breysse, P.N.; Golub, J.E.; et al. Indoor air pollution from secondhand tobacco smoke, solid fuels, and kerosene in homes with active tuberculosis disease in South Africa. BMC Res. Notes 2017, 10, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumede, S.; Savage, M. Respiratory health effects associated with indoor particulate matter (PM2.5) in children residing near a landfill site in Durban, South Africa. Air Qual. Atmos. Health 2017, 10, 853–860. [Google Scholar] [CrossRef]
- Vanker, A.; Barnett, W.; Workman, L.; Nduru, P.M.; Sly, P.D.; Gie, R.P.; Zar, H.J. Early-life exposure to indoor air pollution or tobacco smoke and lower respiratory tract illness and wheezing in African infants: A longitudinal birth cohort study. Lancet Planet. Health 2017, 1, e328–e336. [Google Scholar] [CrossRef]
- Olaniyan, T.; Dalvie, M.A.; Roosli, M.; Naidoo, R.; Kunzli, N.; Hoogh, K.; Parker, B.; Leaner, J.; Jeebhay, M. Asthma-related outcomes associated with indoor air pollutants among schoolchildren from four informal settlements in two municipalities in the Western Cape Province of South Africa. Indoor Air 2018, 29, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Longnecker, M.P.; Dionisio, K.L.; Bornman, R.M.S.; Travlos, G.S.; Brar, S.; Whitworth, K.W. Household fuel use and biomarkers of inflammation and respiratory illness among rural South African Women. Environ. Res. 2018, 166, 112–116. [Google Scholar] [CrossRef]
- Bidassey-Manilal, S.; Mbonane, T.; Rathebe, P.C.; Senekane, M.F. Household Fuel Use and Prevalence of Self-reported Allergic Rhinitis in Rural Areas of Mpumalanga, South Africa. In Proceedings of the 2019 Open Innovations (OI), Cape Town, South Africa, 2–4 October 2019; pp. 241–246. [Google Scholar]
- Jafta, N.; Jeena, P.M.; Barregard, L.; Naidoo, R.N. Association of childhood pulmonary tuberculosis with expo-sure to indoor air pollution: A case control study. BMC Public Health 2019, 19, 275. [Google Scholar] [CrossRef] [Green Version]
- Vanker, A.; Nduru, P.M.; Barnett, W.; Dube, F.S.; Sly, P.D.; Gie, R.P.; Nicol, M.P.; Zar, H.J. Indoor air pollution and tobacco smoke exposure: Impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Reponen, T.; Vesper, S.; Levin, L.; Johansson, E.; Ryan, P.; Burkle, J.; Grinshpun, S.A.; Zheng, S.; Bernstein, D.I.; Lockey, J.; et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann. Allergy Asthma Immunol. 2011, 107, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Bibby, K.; Qian, J.; Hospodsky, D.; Rismani-Yazdi, H.; Nazaroff, W.; Peccia, J. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J. 2012, 6, 1801–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madureira, J.; Aguiar, L.; Pereira, C.; Mendes, A.; Querido, M.M.; Neves, P.; Teixeira, J.P. Indoor exposure to bioaerosol particles: Levels and implications for inhalation dose rates in schoolchildren. Air Qual. Atmos. Health 2018, 11, 955–964. [Google Scholar] [CrossRef]
- Zukiewicz-Sobczak, W.; Krasowska, E.; Zwoliński, J.; Sobczak, P.; Chmielewska-Badora, J.; Wróblewska, P.; Wojtyła, A. Allergic diseases-current state of knowledge. Adv. Dermatol. Allergol. 2012, 29, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Lindsley, W.G.; Green, B.J.; Blachere, F.M.; Martin, S.B.; Law, B.F.; Jensen, P.A.; Schafer, M.P. Sampling and Characterization of Bioaerosols. In NIOSH Manual of Analytical Methods, 5th ed.; CDC: Atlanta, GA, USA, 2017; pp. 1–115. [Google Scholar]
- Carlsten, C.; Dimich-Ward, H.; Becker, A.B.; Ferguson, A.C.; Chan, H.W.; DyBuncio, A.; Chan-Yeung, M. Indoor allergen exposure, sensitization, and development of asthma in a high-risk birth cohort. Pediatr. Allergy Immunol. 2010, 21, e740–e746. [Google Scholar] [CrossRef]
- Olmedo, O.; Goldstein, I.F.; Acosta, L.; Divjan, A.; Rundle, A.G.; Chew, G.L.; Mellins, R.B.; Hoepner, L.; Andrews, H.; Lopez-Pintado, S.; et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J. Allergy Clin. Immunol. 2011, 128, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Kistnasamy, E.J.; Robins, T.G.; Naidoo, R.; Batterman, S.; Mentz, G.B.; Jack, C.; Irusen, E. The relationship between asthma and ambient air pollutants among primary school students in Durban, South Africa. Int. J. Environ. Health 2008, 2, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Edelstein, B.L. The dental caries pandemic and disparities problem. BMC Oral Health 2006, 6, S2. [Google Scholar] [CrossRef] [Green Version]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- GonzaÂlez-Valero, L.; Montiel-Company, J.M.; Bellot-ArcõÂs, C.; Almerich-Torres, T.; Iranzo-CorteÂs, J.E.; Almerich-Silla, J.M. Association between passive tobacco exposure and caries in children and adolescents. A systematic review and meta-analysis. PLoS ONE 2018, 13, e0202497. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Wada, K.; Konishi, K.; Uji, T.; Koda, S.; Mizuta, F.; Yamakawa, M.; Watanabe, K.; Ando, K.; Ueyama, J.; et al. Association between exposure to household smoking and dental caries in preschool children: A cross-sectional study Environ. Health Prev. Med. 2019, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Nayani, A.A.; Iqbal, R.; Azam, S.I.; Khan, F.R.; Khan, A.H.; Janjua, N.; Hussain, A. Association between environmental tobacco smoke and dental caries amongst 5–14 years old children in Karachi, Pakistan. J. Pak. Med. Assoc. 2018, 68, 203–209. [Google Scholar] [PubMed]
- Tanaka, S.; Shinzawa, M.; Tokumasu, H.; Seto, K.; Tanaka, S.; Kawakami, K. Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: Population based retrospective cohort study. BMJ 2015, 351, h5397. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.G.; Bromage, T.G. Effects of fetal exposure to nicotine on dental development of the laboratory rat. Anat. Rec. 2000, 258, 397–405. [Google Scholar] [CrossRef]
- Heikkinen, T.; Alvesalo, L.; Osborne, R.H.; Tienari, J. Maternal smoking and tooth formation in the foetus. III. Thin mandibular incisors and delayed motor development at 1 year of age. Early Hum. Dev. 1997, 47, 327–340. [Google Scholar] [CrossRef]
- Avşar, A.; Darka, Ö.; Topaloğlu, B.; Bek, Y. Association of passive smoking with caries and related salivary biomarkers in young children. Arch. Oral Biol. 2008, 53, 969–974. [Google Scholar] [CrossRef]
- Sakki, T.; Knuuttila, M. Controlled study of the association of smoking with lactobacilli, mutans streptococci and yeasts in saliva. Eur. J. Oral Sci. 1996, 104, 619–622. [Google Scholar] [CrossRef]
- Strauss, R.S. Environmental tobacco smoke and serum vitamin C levels in children. Pediatrics 2001, 107, 540–542. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, W.; Zhang, K.; Zhou, X.; Ren, B.; He, J.; Xu, X.; Cheng, L.; Li, M. Nicotine enhances interspecies relationship between Streptcoccus mutans and Candida albicans. Biomed. Res. Int. 2017, 2017, 7953920. [Google Scholar] [CrossRef] [Green Version]
- Vanker, A.; Barnett, W.; Brittain, K.; Gie, R.P.; Koen, N.; Myers, B.; Stein, D.J.; Zar, H.J. Antenatal and early life tobacco smoke exposure in an African birth cohort study. Int. J. Tuberc. Lung Dis. 2016, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Hussey, S.J.K.; Purves, J.; Allcock, N.; Fernandes, V.E.; Monks, P.S.; Ketley, J.M.; Andrew, P.W.; Morrissey, J.A. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ. Microbiol. 2017, 19, 1868–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rylance, J.; Kankwatira, A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gao, X.; Dong, Q.; Sodergren, E.; Weinstock, G.M.; et al. Household air pollution and the lung microbiome of healthy adults in Malawi: A cross-sectional study. BMC Microbiol. 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanker, A.; Barnett, W.; Nduru, P.M.; Gie, R.P.; Sly, P.D.; Zar, H.J. Home environment and indoor air pollution exposure in an African birth cohort study. Sci. Total Environ. 2015, 536, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, P.; Chaloupka, F. Curbing the Epidemic: Governments and the Economics of Tobacco Control; The World Bank: Washington, DC, USA, 1999. [Google Scholar]
- Kadir, M.M.; Mcclure, E.M.; Goudar, S.S.; Garces, A.L.; Moore, J.; Onyamboko, M.; Kaseba, C.; Althabe, F.; Castilla, E.E.; Freire, S.; et al. Exposure of pregnant women to indoor air pollution: A study from nine low and middle income countries. Acta Obstet. Gynecol. Scand. 2010, 89, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Wipfli, H.; Avila-Tang, E.; Navas-Acien, A.; Kim, S.; Onicescu, G.; Yuan, J.; Breysse, P.; Samet, J.M. Secondhand smoke exposure among women and children: Evidence from 31 countries. Am. J. Public Health 2008, 98, 672–679. [Google Scholar] [CrossRef]
- Kasznia-Kocot, J.; Kowalska, M.; Górny, R.; Niesler, A.; Wypych-Slusarska, A. Environmental risk factors for respiratory symptoms and childhood asthma. Ann. Agric. Environ. Med. 2010, 17, 221–229. [Google Scholar]
- Zar, H.J.; Ehrlich, R.I.; Workman, L.; Weinberg, E.G. The changing prevalence of asthma, allergic rhinitis and atopic eczema in African adolescents from 1995 to 2002. Pediatr. Allergy Immunol. 2007, 18, 560–565. [Google Scholar] [CrossRef]
- Adegbola, R.A.; DeAntonio, R.; Hill, P.C.; Roca, A.; Usuf, E.; Hoet, B.; Grenwood, B.M. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE 2014, 9, e103293. [Google Scholar]
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; for the Pneumococcal Carriage Group (PneumoCarr). The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [Green Version]
- Vissing, N.H.; Chawes, B.L.K.; Bisgaard, H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care. Med. 2013, 188, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, K.; Collaco, J.M.; McGrath-Morrow, S.A. Impact of tobacco smoke and nicotine exposure on lung development. Chest 2016, 149, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongtrakool, C.; Roser-Page, S.; Rivera, H.N.; Roman, J. Nicotine alters lung branching morphogenesis through the α7 nicotinic acetylcholine receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L611–L618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Gundavarapu, S.; Peña-Philippides, J.C.; Rir-Sima-Ah, J.; Mishra, N.C.; Wilder, J.A.; Langley, R.J.; Smith, K.R.; Sopori, M.L. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J. Immunol. 2011, 187, 4542–4552. [Google Scholar] [CrossRef] [PubMed]
- Ngobese, S.P.; Egbe, C.O.; Londani, M.; Ayo-Yusuf, O.A. Non-Smoker’s Exposure to Second-Hand Smoke in South Africa during 2017. Int. J. Environ. Res. Public Health 2020, 17, 8112. [Google Scholar] [CrossRef]
- Wang, M.P.; Ho, S.Y.; Lam, T.H. Parental smoking, exposure to secondhand smoke at home, and smoking initiation among young children. Nicotine Tob. Res. 2011, 13, 827–832. [Google Scholar] [CrossRef]
- Ayo-Yusuf, O.A.; Olufajo, O.; Agaku, I.T. Exposure to secondhand smoke and voluntary adoption of smoke-free home and car rules among non-smoking South African adults. BMC Public Health 2014, 14, 580. [Google Scholar] [CrossRef] [Green Version]
- South African Government. NO. 23 of 2007: Tobacco Product Control Amenment Act, 2007; South African Government: Pretoria, South Africa, 2007. [Google Scholar]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull. World Health Organ. 2000, 78, 1078–1092. [Google Scholar]
- Smith, K.R.; Mehta, S.; Maeusezahl-Feuz, M. Indoor air pollution from household use of solid fuels. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004; Volume 2, pp. 1435–1493. [Google Scholar]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef]
- Maluleke, K.R.; Worku, Z. Environmental determinants of asthma among school children aged 13–14 in and around Polokwane, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2009, 6, 2354–2374. [Google Scholar] [CrossRef] [Green Version]
- Hawley, B.; Volckens, J. Proinflammatory effects of cook stove emissions on human bronchial epithelial cells. Indoor Air 2013, 23, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonlokke, J.H.; Riddervold, I.S.; Grønborg, T.K.; Skogstrand, K.; Hougaard, D.M.; Barregard, L.; Sigsgaard, T. Systemic effects of wood smoke in a short-term experimental exposure study of atopic volunteers. J. Occup. Environ. Med. 2014, 56, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghio, A.J.; Soukup, J.M.; Case, M.; Dailey, L.A.; Richards, J.; Berntsen, J.; Devlin, R.B.; Stone, S.; Rappold, A. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup. Environ. Med. 2012, 69, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Karottki, D.G.; Christensen, J.M.; Bønløkke, J.H.; Sigsgaard, T.; Glasius, M.; Loft, S.; Moller, P. Biomarkers of oxidative stress and inflammation after wood smoke exposure in a reconstructed Viking Age house. Environ. Mol. Mutagen. 2014, 55, 652–661. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- WHO. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2006. WHO/SDE/PHE/OEH/06.02, Geneva. Available online: http://www.euro.who.int/Document/E87950.pdf (accessed on 19 November 2013).
- Ostro, B.; Lipsett, M.; Reynolds, P.; Goldberg, D.; Hertz, A.; Garcia, C.; Henderson, K.D.; Bernstein, L. Long-term exposure to constituents of fine particulate air pollutants and mortality: Results from the California teachers study. Environ. Health Perspect. 2010, 118, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Brauer, M.; Amann, M.; Burnett, R.T.; Cohen, A.; Dentener, F.; Ezzati, M.; Henderson, S.B.; Krzyzanowski, M.; Martin, R.V.; Van Dingenen, R.; et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ. Sci. Technol. 2012, 46, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Gurley, E.S.; Homaira, N.; Salje, H.; Ram, P.K.; Haque, R.; Petri, W.; Bresee, J.; Moss, W.J.; Breysse, P.; Luby, S.P.; et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: A birth cohort study in urban Bangladesh. Indoor Air 2013, 23, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Horne, B.D.; Joy, E.A.; Hofmann, M.G.; Gesteland, P.H.; Cannon, J.B.; Lefler, J.S.; Blagev, D.P.; Korgenski, E.K.; Torosyan, N.; Hansen, G.I.; et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am. J. Respir. Crit. Care Med. 2018, 198, 759–766. [Google Scholar] [CrossRef]
- Gascon, A.L.; Brodeur, J. Influence of microsomal enzyme inducers on the reactivity of the isolated guinea pig seminal vesicle to angiotensin and tyramine. Can. J. Physiol. Pharmacol. 1969, 47, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredhult, C.; Sahlin, L.; Olovsson, M. Gene expression analysis of human endometrial endothelial cells exposed to op′-DDT. Mol. Hum. Reprod. 2008, 14, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Maldonado, I.N.; Herrera, C.; Batres, L.E.; González-Amaro, R.; Barriga, F.D.; Yáñez, L. DDT-induced oxidative damage in human blood mononuclear cells. Environ. Res. 2005, 98, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Falcon, M.; Oliva, J.; Osuna, E.; Barba, A.; Luna, A. Hch and ddt residues in human placentas in murcia (spain). Toxicology 2004, 195, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Home dampness, current allergic diseases, and respiratory infections among young adults. Thorax 2001, 56, 462–467. [Google Scholar] [CrossRef]
- Norbäck, D.; Lu, C.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sundell, J.; Deng, Q. Common cold among pre-school children in China—Associations with ambient PM10 and dampness, mould, cats, dogs, rats and cockroaches in the home environment. Environ. Int. 2017, 103, 13–22. [Google Scholar] [CrossRef]
- Newton, R.C. Dampness as a factor in tuberculosis. Trans. Am. Climatol. Assoc. 1900, 16, 15–28. [Google Scholar]
- Kuhn, D.M.; Ghannoum, M.A. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: Infectious disease perspective fungal organisms in damp buildings. Clin. Microbiol. Rev. 2003, 16, 144–172. [Google Scholar] [CrossRef] [Green Version]
| SN | Author, Year | Study Design | Study Population | Sample Size | Study Location | Exposure | Morbidity or Mortality Outcome |
|---|---|---|---|---|---|---|---|
| 1. | Ehrlich et al. [39] | Cross-sectional | Children of ages 7–11 years with reported asthma or multiple asthma symptoms | 249 children | Cape Town, South Africa | Household environmental tobacco smoke | Reduced lung function |
| 2. | Wichmann and Voyi [40] | Cross-sectional | Under-five children | 3556 under-five children living in 2828 households | Nine provinces in South Africa | Exposure to cooking and space heating smoke | Under-five mortality. Children in households using polluting fuels are 2.22 times (95% CI = 1.22–4.04; p = 0.009) at risk of dying than those using clean fuels (OR = 1.95, 95% CI = 1.04–3.68; p = 0.039) |
| 3. | Ayo-Yusuf et al. [41] | Cross-sectional | High school students | 1873 8th-graders | 21 randomly selected schools in the most rural of the nine provinces in South Africa | Household tobacco smoke | Dental caries. Secondhand smoke exposure is linked to caries in permanent teeth in teenagers, regardless of sugar consumption. |
| 4. | Jafta et al. [42] | Case-control design | School children (grades 3–6) with known or probable persistent asthma | 81 children | Durban, South Africa | Allergens—dust mite (Der p1 and Der f1), fungi allergens (Asp f1) and cockroach allergen (Bla g1) | Asthma |
| 5. | Shirinde et al. [43] | Cross-sectional | Children between the ages of 13 and 14 years | 3468 | Ekurhuleni Metropolitan Municipality, namely Tembisa and Kempton Park, South Africa | Environmental Tobacco Smoke, Gas and Paraffin for residential heating | Wheeze |
| 6. | Albers et al. [44] | Cross-sectional | Children between the ages of 9 and 11 years | 627 children | Mpumalanga Province, South Africa | Fuel used for cooking (electricity, gas, paraffin, wood, charcoal) | Respiratory health outcomes: phlegm on the chest, chest cough, bronchitis, wheezing and asthma |
| 7. | Elf et al. [45] | Cross-sectional | All adults (≥18 years of age) and children between seven and 17 years of age living in the same household as the index Tuberculosis case, including the index case themselves | 96 adults and 28 children in 53 households were included | Matlosana district townships surrounding Klerksdorp, South Africa | Secondhand tobacco smoke, use of solid fuels, and kerosene | Tuberculosis |
| 8. | Gumede and Savage [46] | Cross-sectional | Children aged 6 to 12 years | 23 children | Clare Estate community in Durban, South Africa | PM2.5 | Lung function A significant association was observed between the percent predicted forced vital capacity (FVC) and indoor PM2.5 concentration levels (p < 0.002). Impaired lung function was recorded among children. |
| 9. | Vanker et al. [47] | Cohort study | Mother and infant pairs | 1137 mothers with 1143 livebirths | Paarl, South Africa | Particulate matter, nitrogen dioxide, sulphur dioxide, carbon monoxide, and volatile organic compounds benzene and toluene | Exposure to particulate matter was significantly associated with LRTI (OR = 1.43, 95% CI: 1.06–1.95; p = 0.008). wheezing was associated with maternal passive smoke exposure (1.70, 1.25–2.31; p = 0.001) and with any household member smoking (1.55, 1.17–2.06; p = 0.002). |
| 10. | Olaniyan et al. [48] | Cross-sectional | Children between the ages of 9 and 11 years | 590 children | Khayelitsha, Marconi-Beam, Masiphumulele and Oudtshoorn in the Western Cape Province of South Africa | Dampness, presence of visible mold growth, pets in the home, smokers in the home, and the use of paraffin for cooking and heating. | Rhinitis, doctor-diagnosed asthma, ocular-nasal symptoms, wheezing and other respiratory symptoms. Paraffin use was associated with a twofold increased likelihood of having significant airway inflammation (aOR: = 2.31, 95% CI: 1.05–5.06) and an increased risk of rhinitis (aOR = 1.69, 95% CI: 1.05–2.70). Having a smoker in the home significantly increased the odds of current wheeze (aOR = 1.79, 95% CI: 1.02–3.15) Dampness in the home was associated with a twofold increased odds of current wheeze (aOR = 2.60, 95% CI: 1.18–5.71). An association was observed between rhinitis and household dampness (aOR = 3.00 95% CI: 1.47–6.13) and visible mold growth (aOR = 3.37, 95% CI: 1.69–6.71). |
| 11. | Misra et al. [49] | Cross-sectional | reproductive-aged women 20–30 years | 415 women | Women from eight villages in the Thulamela Municipality of the Vhembe district in the Limpopo Province of South Africa. | Cooking fuel (wood and electricity) | Biomarkers of inflammation, respiratory symptoms (breathlessness, wheezing/chest tightness) and illnesses (tuberculosis, pneumonia, and asthma), and blood pressure. Increased odds (aOR = 1.41; 95% CI = 0.72–2.77) of self-reported wheezing/chest tightness among women who cook with wood, An increased odds of both breathlessness (aOR = 1.29, 95% CI = 0.65, 2.56, p > 0.05) and pre-hypertension/hypertension (aOR = 1.29, 95% CI: 0.80, 2.09) among women who reported cooking with wood mostly indoors Wood for cooking has effect on blood pressure (systolic β = −0.33, 95% CI: −2.37, 1.71; diastolic β = −0.21, 95% CI = −1.77, 1.35) |
| 12. | Murray et al. [5] | Cohort | Women participating in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) study | 733 women | Rural Vhembe District of Limpopo Province, South Africa | dichlorodiphenyl trichloroethane (DDT), dichlorodiphenyl dichloroethylene (DDE) | Hypertension, preeclampsia, or eclampsia. DDT was associated with Hypetensive disorder of pregnancy (HDP) based on self-report (OR = 1.50, 95% CI = 1.10–2.03) and medical records (OR = 1.32, 95% CI = 0.99–1.75), respectively. DDE was associated with HDP based on self-report (OR = 1.58, 95% CI = 1.09–2.28) and medical records (OR = 1.47, 95% CI = 1.03, 2.09), respectively. DDE was also associated with gestational hypertension (OR = 1.44, 95% CI = 1.00–2.07). |
| 13. | Bidassey-Manilal et al. [50] | Cross-sectional | Adult above 18 years | 167 households | Mpumalanga Province, South Africa | Coal, wood, kerosene, charcoal animal dung | Allergic rhinitis Children living in households that primarily utilized wood, coal, and kerosene were at risk of developing rhinitis ever (OR = 1.21, 95% CI: 1.05–1.46), current rhinitis (OR = 1.26, 95% CI: 1.01–1.40), and hay fever (OR = 1.11, 95% CI: 1.21–1.48). In the presence of children, cooking with wood, coal, or kerosene increased the risk of contracting rhinitis (OR = 1.31, 95% CI: 1.04–1.69), hay fever (OR = 1.21, 95% CI: 1.07–1.81). Heating homes using kerosene, wood, or coal leads to rhinitis ever, current rhinitis, and current rhinoconjuctiitis (OR = 0.65, 95% CI: 0.53–0.81) and hay fever (OR 0.65 95% CI: 0.53–0.81). |
| 14. | Buthelezi et al. [34] | Cross-sectional | Men and women living in selected households in the study area. | 245 | Umlazi Township in the City of eThekwini, KwaZulu-Natal province, South Africa | Electric (electricity) and non-electric (wood, coal, gas, paraffin) | Upper Respiratory Tract Infections (URTI) and Lower Respiratory Tract Infections (LRTIs) Non-electric sources for heating (aOR = 3.6, 95% CI: 1.2–10.1, p < 0.05) and cooking (aOR = 2.9, 95% CI: 1.1–7.9, p < 0.05) was significantly associated with high prevalence of URTIs. Electric sources for heating was associated with prevalence of LRTIs (aOR = 2.7, 95% CI: 1.1–6.4, p < 0.05). |
| 15. | Jafta et al. [51] | Case-control | Children aged 0–14 years diagnosed with pulmonary Tuberculosis (PTB) and without pulmonary Tuberculosis | 234 children, 107 cases and 127 controls | Durban, South Africa | Dampness, secondhand smoke, PM10, NO2 | Dampness (OR = 1.8, 95% CI: 1.01–3.1), cooking fuel type (OR = 2.6, 95% CI: 1.1–6.4), and SHS (OR = 1.7, 95% CI: 0.98–2.8), and PM10 (OR = 1.4, 95% CI: 0.8–2.3) were positively associated with PTB in children in the unadjusted analysis. In the adjusted analysis, visible dampness was significantly associated with PTB (aOR = 2.4, 95% CI: 1.1–5.0). However, the risk of PTB was lower for increase in NO2 concentration (aOR = 0.4; 95% CI: 0.2–0.8) and not significantly associated with increase in PM10 (aOR = 0.9; 95% CI: 0.5–1.8). |
| 16. | Vanker et al. [52] | Longitudinal study | Pregnant women and infants | 982 pregnant women and 986 infants | Mbekweni and Newman, South Africa | Particulate matter, carbon monoxide, nitrogen dioxide, volatile organic compounds. | Antenatal exposure to NO2 above ambient standards was associated with increased maternal nasopharyngeal carriage of M. catarrhalis when adjusted for clinical covariates as well as the other pollutants (aRR = 3.69, 95% CI = 1.27–10.73); Benzene exposure was associated with maternal H. influenzae carriage when adjusted for clinical covariates (aRR = 2.06, 95% CI: = 1.18–3.59) and tobacco smoke exposure almost doubled the risk of S. pneumoniae carriage in mothers (aRR = 1.73, 95% CI: 1.03–2.92); PM10 was associated with an increased risk of H. influenzae at 6 months, (aRR = 1.60, 95% CI: 1.04–2.46) and M. catarrhalis at 12 months (aRR = 1.39, 95% CI: 1.02–1.90), and NO2 with Gram-negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morakinyo, O.M.; Mokgobu, M.I. Indoor Household Exposures and Associated Morbidity and Mortality Outcomes in Children and Adults in South Africa. Int. J. Environ. Res. Public Health 2022, 19, 9471. https://doi.org/10.3390/ijerph19159471
Morakinyo OM, Mokgobu MI. Indoor Household Exposures and Associated Morbidity and Mortality Outcomes in Children and Adults in South Africa. International Journal of Environmental Research and Public Health. 2022; 19(15):9471. https://doi.org/10.3390/ijerph19159471
Chicago/Turabian StyleMorakinyo, Oyewale Mayowa, and Matlou Ingrid Mokgobu. 2022. "Indoor Household Exposures and Associated Morbidity and Mortality Outcomes in Children and Adults in South Africa" International Journal of Environmental Research and Public Health 19, no. 15: 9471. https://doi.org/10.3390/ijerph19159471






